Summary
This review summarizes emerging concepts related to the roles of dendritic cells and innate immunity in organ transplant rejection. First, it highlights the primary role that recipient, rather than donor, dendritic cells have in rejection and reviews their origin and function in the transplanted kidney. Second, it introduces the novel concept that recognition of allogeneic non-self by host monocytes (referred to here as innate allorecognition) is necessary for initiating rejection by inducing monocyte differentiation into mature, antigen-presenting dendritic cells. Both concepts provide opportunities for preventing rejection by targeting monocytes or dendritic cells.
Keywords: acute rejection, ischemia reperfusion, lymphocytes
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease, but continuous suppression of the recipient’s immune system is required to prevent rejection of the grafted kidney (renal allograft). Despite immunosuppression, long-term renal allograft outcomes remain suboptimal, with ten-year graft survival hovering around 45% and 60% for deceased and living donor kidneys, respectively 1. A more thorough understanding of the mechanisms of graft rejection is therefore needed to improve outcomes without further increasing the burden of immunosuppression.
Allograft rejection is dependent on the activation of recipient T lymphocytes that recognize major or minor histocompatibility antigens expressed by donor but not host tissues (alloantigens) 2. Once activated, T lymphocytes reject the allograft by inflicting direct cytotoxicity on graft cells or by providing help to other cells of the immune system such as B lymphocytes, which differentiate into antibody producing cells, and macrophages, which cause tissue inflammation. Therefore, a central question in transplantation immunology is how T lymphocytes are alerted to the presence of foreign tissue and how that leads to their activation. Here, we will attempt to answer these questions by reviewing the role of the innate immune system in initiating the T lymphocyte response after kidney transplantation, with particular emphasis on dendritic cells (DCs) whose principal functions are to present antigen and provide essential co-stimulatory signals to T lymphocytes.
Innate versus Adaptive
Mammalian immunity has long been defined through the adaptive features of T and B lymphocytes. Lymphocytes express somatically diversified receptors that recognize foreign antigens with high molecular specificity, expand clonally upon sensing antigen, and undergo further differentiation to generate short-lived effector and long-lived memory cells. This form of adaptation (clonal expansion, differentiation, and memory) ensures that the host is protected against microbial pathogens both acutely and in the long-term, earning T and B lymphocyte responses the well-justified moniker “adaptive immunity”. Although clearly essential for survival, adaptive immunity is also the reason why we reject life-saving allografts.
The initial and key requirement for mounting a successful adaptive immune response is activation of the T lymphocyte clone or clones specific for the non-self antigen. Seminal work in the 1980s established that full activation of T lymphocytes requires two molecular signals: one delivered by the T cell receptor for antigen (TCR), which engages antigenic peptides presented in the grooves of major histocompatibility complex (MHC) molecules on activated antigen presenting cells (APCs), namely DCs, and the other delivered by costimulatory and cytokine receptors whose ligands are also expressed by activated DCs 3. An important question that lingered at the time, however, was the nature of the stimulus that induces quiescent DC to acquire antigen presenting and costimulatory functions 4. The answer to this question unfolded rapidly with the discovery of pattern recognition receptors (PRRs), a prime example being Toll-like receptors (TLR), which recognize pathogen-associated molecular patterns (PAMPs) present in microbes but not the host and cause activation of DCs 5. This form of non-self recognition was dubbed “innate immunity” as PRRs are germline-encoded and are evolutionarily conserved, predating the emergence of adaptive immunity, and are responsible for triggering many aspects of the inflammatory response that provides immediate protection against infection. So what role do DCs play in allograft rejection, and what are the innate immune mechanisms that lead to their activation after transplantation?
The Role of Dendritic Cells in Allograft Rejection
On a per cell basis, activated DCs are the most effective APCs in mice and humans 6. They are around 100-fold more potent at inducing the proliferation of allogeneic T cells in a mixed lymphocyte reaction (MLR) and at presenting antigens to self MHC-restricted T cells than their nearest relative, the macrophage. DCs are found in lymphoid and non-lymphoid tissues throughout the body, including the kidney 7, and their numbers increase in the presence of inflammation. Inflammation also triggers the migration of DCs from non-lymphoid tissues to secondary lymphoid organs where they encounter and activate T lymphocytes. Therefore, organ transplants, unlike any other immune challenge, can potentially activate host T lymphocytes via two pathways: one is through alloantigens (usually intact allogeneic MHC molecules) presented “directly” by donor DCs that accompany the transplanted organ, and the second is via alloantigens that have been taken up and processed by recipient DCs - a process referred to as “indirect” allorecognition8,9. Which DC then – donor or recipient – is essential for driving the alloimmune response, where do T lymphocytes encounter activated DCs after transplantation, and what are the consequences of this encounter?
Which DC: donor or recipient?
The precursor frequency of T lymphocytes with direct reactivity to non-self MHC molecules in mice and humans has been estimated to be as high as 5 – 10%, several orders of magnitude greater than that for conventional antigens 10, 11. This high precursor frequency, the presence of a significant number of donor DCs that express non-self MHC molecules within the transplanted organ, and the ability of donor DCs to induce potent proliferation of host T cells in the MLR led to the hypothesis that donor DCs that travel from the allograft to the recipient’s secondary lymphoid tissues after transplantation are the primary drivers of the alloimmune response 12. Support for this hypothesis also derives from classical experiments showing that depletion of “passenger leukocytes” from thyroid, pancreatic islet, or kidney allografts prior to transplantation resulted in their long-term survival in the host without the need for any immunosuppression 13–17. Conversely, injection of donor DCs into the recipient of a DC-depleted kidney allograft restored acute rejection 18, providing a cause-effect relationship between donor DCs and initiation of the alloimmune response.
Later studies, however, using murine heart, skin, and kidney transplantation models showed that donor DCs contribute to but are not essential for rejection. This was initially demonstrated by transplanting allografts from donors that lack MHC or co-stimulatory (CD80 and CD86) molecules 19–22 - thus, rendering donor DCs incapable of activating T cells – and later by depleting grafts of DCs using targeted approaches 23. Cahalan and coworkers demonstrated that donor DCs that migrate out of transplanted organs are quickly surrounded and killed by NK cells in the secondary lymphoid tissues of the recipient 23, 24, suggesting that intact donor DCs are unlikely to play a significant role in priming recipient T lymphocytes. Using the CD11c-DTR mouse model in which DCs can be selectively targeted and killed by diphtheria toxin, they also showed that depleting donor DCs in heart allografts did not delay rejection while depletion of recipient DCs prolonged graft survival significantly 23. The implication of these studies is that donor DCs, unlike what was previously suspected, are not essential for initiating alloimmune responses. Instead, donor and recipient DCs are either equally capable of performing the task or the latter are in fact the more important players.
How can one then reconcile the older data with the newer observations? An evolving concept is that donor DCs transplanted with the graft function as antigen transporting rather than antigen presenting cells that deliver an antigenic cargo of non-self MHC molecules to recipient DCs 8. This concept is supported by in vitro as well as emerging in vivo data that membrane fragments displaying intact MHC molecules are exchanged between DCs, a phenomenon known as “cross-dressing” or “semi-direct” antigen presentation, leading to the stimulation of T lymphocytes that recognize the transferred MHC 25–27. Therefore, it is possible that after transplantation both “directly” and “indirectly” alloreactive T lymphocytes are activated by recipient DCs: the former by recipient DCs that have acquired intact non-self MHC molecules from donor DCs and the latter by recipient DCs that have taken up donor alloantigens and processed them for presentation in the context of self-MHC molecules. Additional in vivo data are still needed to validate this concept, but such data are likely to emerge in the near future.
Where do T lymphocytes encounter activated DCs?
Immunologists have traditionally focused on DC-T lymphocyte encounters in secondary lymphoid organs (the spleen, lymph nodes, and mucosal lymphoid tissues) because these are the key sites where primary immune responses take place. Naïve and a subset of memory T lymphocytes, so-called central memory T lymphocytes, home to secondary lymphoid tissues by virtue of their expression of the chemokine receptor CCR7 28. There they make stable contacts with and are activated by DCs that present the antigens which they recognize. Earlier studies demonstrated that acute allograft rejection in an immunologically naïve animal is indeed dependent on T lymphocyte activation within secondary lymphoid tissues 29. Later studies, however, uncovered exceptions to this rule. First, it was demonstrated that memory T lymphocytes cause allograft rejection in the absence of secondary lymphoid tissues 30, consistent with the ability of both central and effector memory T lymphocytes to home to and proliferate at non-lymphoid sites 31. Second, it was shown that the acute rejection of certain types of allografts, namely lung and full-thickness or vascularized skin transplants, is not dependent on secondary lymphoid organs, even in immunologically naïve recipients 32–34. The latter observations can be explained by the presence of bronchio-alveolar lymphoid tissues (BALT) that readily support naïve T cell activation by DCs in the lung, and to the rapid induction of endothelial peripheral node addressin (PNAd) in neovascularized full-thickness skin grafts that enable naïve T cells to enter the DC-rich dermis 35. Therefore, alloimmune responses are initiated in either host secondary lymphoid tissues or in the graft itself depending on the type of organ transplanted and the type of T lymphocyte (naïve vs memory) involved. The role of memory T lymphocytes in initiating the rejection response is germane to the clinical setting because alloreactivity in humans is not restricted to the naïve T lymphocyte repertoire but is equally represented in the memory pools 10, 36.
DCs in the transplanted kidney: continuous love affair with the T cell
In addition to T lymphocyte-DC interactions within secondary lymphoid tissues, it is now accepted that activated T lymphocytes interact with DCs outside secondary lymphoid organs 37, raising several important question in transplantation: Do memory or effector T cells that migrate to an allograft contact DCs there? If they do, which DCs and what functions do these contacts serve? Intra-vital imaging of lung and skin allografts in the mouse has demonstrated that host T cells make stable contacts with DCs within the graft tissue 32, 38, but did not establish the role of these interactions. Using similar imaging technology, we have recently shown that the majority of anti-donor effector T cells that migrate to a transplanted kidney engage in prolonged, stable contacts with DCs in the graft 39. The contacts occurred within the lumina of post-capillary venules, specifically with the dendrites of perivascular DCs that reach into the bloodstream, and in the interstitium of the renal cortex. Stable contacts between T lymphocytes and graft DCs within vascular lumina caused T lymphocyte arrest and transmigration across the endothelium. This previously unappreciated function of graft DCs is dependent on presentation of cognate antigen by the DC to the T lymphocyte but is independent of chemokine signaling via Gαi-coupled receptors 39. Therefore, graft DCs play a prominent role in mediating the migration of donor antigen-specific T lymphocytes into the transplanted kidney and, quite likely, their subsequent retention in the interstitium (see below). Based on studies in viral infection models 40 and emerging data in a kidney transplantation model (Zheng & Lakkis, unpublished), it is possible that cognate interactions between graft DCs and T lymphocyte are also important for memory T cell recall and further activation of effector T cells within the graft. Therefore, the relationship between DCs and T lymphocytes is not restricted to a one-night stand in secondary lymphoid organs but is one that blossoms into a protracted love affair in the target non-lymphoid tissue. In transplantation, this implies that interrupting T lymphocyte-DC interactions in the graft could provide an opportunity to prevent or reverse rejection in a cognate, donor-specific manner.
Which DC is then responsible for engaging effector and memory T cells within the transplanted kidney? It has long been known that donor DCs that accompany the graft emigrate out of the graft and can be detected in the recipient’s secondary lymphoid organs, at least in the immediate period after transplantation 12, 41. However, the rate and extent by which donor DCs are replaced by recipient DCs and the lineage of the recipient DCs that populate the graft has not been carefully elucidated. Recent work from our laboratory has shown that the majority of donor DCs in mouse kidney and heart grafts are replaced by recipient DCs within one day after transplantation (Zheng & Lakkis, unpublished). In kidney grafts, donor DCs represented less than 10% of all DCs by day seven after transplantation, while in heart grafts the proportion was even lower. DC replacement occurred in both allogeneic and syngeneic grafts, but the absolute number of recipient DCs was approximately 40-fold higher in the former. The vast majority of recipient DCs present in a transplanted kidney or heart were derived from monocytes and had a mature phenotype – they expressed high levels of MHC class II and costimulatory molecules (e.g., CD80). Moreover, effector T cells that infiltrated kidney grafts made stable, cognate interactions with recipient monocyte-derived DCs. Depletion of monocyte-lineage cells in the host at the time of transplantation significantly reduced the T lymphocyte infiltrate, indicating that DCs of recipient origin play an important role in T lymphocyte migration and retention in the graft 42. These findings provide support for targeting recipient DCs that populate the graft or targeting their precursor, the monocyte, as a novel means to prevent rejection.
The Role of the Innate Immune System in Allograft Rejection
The process of transplanting an organ from one individual to another is associated with significant inflammation in the graft caused mainly by ischemia-reperfusion injury. There is ample evidence to indicate that this form of inflammation potentiates the host’s adaptive alloimmune response through myriad molecular and cellular mediators 43, 44. These include small molecules such as free oxygen radicals, uric acid, and nucleic acids; lipid products such as prostaglandins and leukotrienes; protein molecules such as the complement system and HMGB1; and myeloid cells such as neutrophils, macrophages, and DCs. Many of these mediators influence not only the afferent (activation) phase of the adaptive alloimmune response but also its effector arm by enhancing T lymphocyte migration into the graft and the tissue damage that ensues. Therefore, broadly defined to include inflammation, innate immunity is an important contributor to allograft rejection. However, is innate immunity necessary or sufficient for rejection, and if necessary, is the innate immune response to an allograft solely an inflammatory response caused by the transplantation procedure (ischemia-reperfusion injury) or is it a response to non-self determinants present in allogeneic but not self-tissues?
The innate immune system: necessary or sufficient?
That the innate immune system is not sufficient for allograft rejection is well established. Many studies have shown that T lymphocyte-depleted humans or experimental animals do not mount an acute rejection response until T lymphocytes have returned to the circulation 45, 46. A recent analysis of cardiac allografts transplanted to RAG−/− mice, which lack T and B lymphocytes but have an intact if not heightened innate immune system, confirmed that innate immunity alone does not lead to either acute or chronic rejection 47. In the same study, injecting adjuvants to stimulate the innate immune response of RAG−/− mice, or reconstituting the mice with ‘innate’ B-1 lymphocytes to generate natural IgM antibodies, failed to recapitulate rejection. Some mouse studies, however, have suggested that NK cells, which belong to the lymphoid lineage and share adaptive features with lymphocytes, are sufficient for causing chronic rejection if their number and function are enhanced by concomitant viral infection or exogenous cytokines 48, 49. Moreover, a small cohort of patients profoundly depleted of T lymphocytes at the time of kidney transplantation, but not given any maintenance immunosuppression, experienced transient decline in graft function around one month after transplantation - at a time when circulating T cells were present in only very small numbers 50. Graft biopsies in these patients revealed a predominantly monocytic infiltrate. The experimental and human data therefore establish that the innate immune system contributes to rejection but is not sufficient for causing it.
Whether innate immune activation is necessary for allograft rejection is a more difficult question to answer. First, experimental animals that lack an innate immune system but have functional adaptive immunity do not exist (for one, such animals will likely not survive beyond the early neonatal period). Second, the great breadth and redundancy of innate immune mediators preclude testing all of them at once. Nevertheless, emerging data have begun to ascertain whether general components or features of innate immunity play key roles in allograft rejection. These will be reviewed next.
The danger hypothesis
The danger hypothesis was proposed in 1994 by Matzinger as an alternate to Janeway’s PRRs/PAMPs model of innate immunity to account not only for antimicrobial immune responses but also for robust responses that arise in the absence of obvious microbial adjuvants, a prime example being transplantation 51. In its most contemporary iteration, this hypothesis states that innate immune cells recognize danger-associated molecular patterns (DAMPs) released from stressed or dying cells, whether cell stress or death is caused by infection, ischemia, or other forms of injury. Many DAMPs have been identified, all of which induce inflammation and in some cases potentiate adaptive immunity to foreign antigens, including alloantigens 52, 53. Most, if not all, identified DAMPs appear to mediate their inflammatory actions via known PRRs that recognize microbial products, most commonly via TLR4. The role of TLR4 in ischemia-reperfusion injury of transplanted organs has been established in experimental animals and humans 54.
One shortcoming of the danger hypothesis is the possibility that DAMPs, although potent inducers of inflammation and ischemia-reperfusion injury, are not sufficient for triggering robust adaptive immune responses as PAMPs do. Sporri and Reis e Sousa reported that indirect activation by inflammatory mediators generated DCs that supported CD4 T lymphocyte clonal expansion but failed to direct T helper cell differentiation, mainly because the DCs failed to produce IL-12 55. In contrast, exposure to PAMPs resulted in fully activated DCs that produced IL-12 and promoted T helper responses. In transplantation, additional evidence suggests that danger may not be necessary for triggering allograft rejection. For example, the rejection of allografts mismatched with the recipient at major and/or multiple minor histocompatibility antigens occurs without significant delay in the absence of innate signaling pathways or cytokines that mediate the action of DAMPs 56–60. Likewise, allografts parked in T lymphocyte-deficient mice are rejected when the host is replenished with T lymphocytes long after tissue injury has resolved 61–65, implying that danger is not necessary for triggering rejection or that unaccounted for danger or microbial stimuli persist in these recipients. An alternative explanation is that additional innate stimuli that are responsible for “full” DC activation exist in the setting of organ transplantation. If so, what could these stimuli be?
Innate sensing of allogeneic non-self
Examples of ancient allorecognition systems that predate the evolution of adaptive immunity abound in nature 66, 67. This fact has long suggested the possibility that mammalian innate immune systems have retained the ability to recognize allogeneic non-self, presumably to alert the host to harmful non-microbial invaders such as stem cells from the fetus or transmissible tumor cells from another individual 47. Our group has formally tested the possibility that the mouse innate immune system distinguishes between self and allogeneic non-self in a manner analogous to its ability to differentiate between self and microbial non-self 68. Zecher et al showed that injecting allogeneic RAG−/− splenocytes into the ear pinnae of RAG−/− recipinets elicits significantly greater swelling and infiltration of the skin with host myeloid cells than injecting syngeneic splenocytes 69. Depletion and cell transfer experiments established that the response is independent of NK cells and, instead, is mediated by monocytes 69. These studies provided direct evidence that the mouse innate immune system is capable of distinguishing between self and allogeneic non-self. However, they did not establish the biological significance of such innate sensing and what its consequences are for allograft rejection.
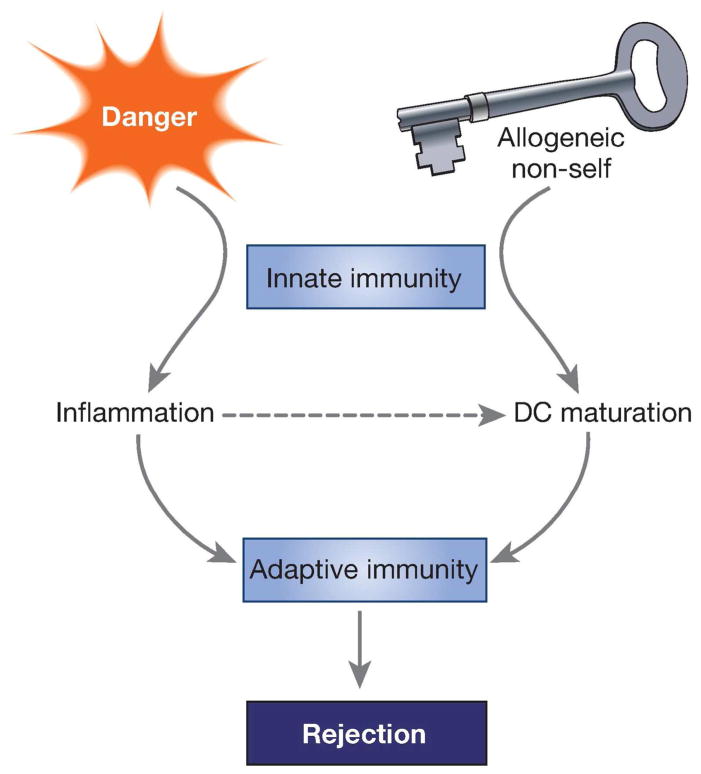
By performing heart, kidney, and bone marrow transplants into RAG−/−γc−/− mice, which lack T, B, NK and innate lymphoid cells, we have now established that innate sensing of allogeneic non-self is necessary for initiating alloimmunity 42. In these experiments, allogeneic grafts elicited persistent differentiation of monocytes to mature DC that expressed IL-12 and stimulate T cell proliferation and IFNγ production. In contrast, syngeneic grafts elicited transient and less pronounced differentiation of monocytes to DC, which neither expressed IL-12 nor stimulate IFNγ production. In a heart transplantation model where T cell recognition is restricted to a single foreign antigen on the graft, rejection occurred only if allogeneic non-self was also sensed by the host’s innate immune system. Therefore, danger alone, which is common to both syngeneic and allogeneic grafts, is not sufficient for inducing “full” DC activation. Instead, innate recognition of allogeneic allogeneic non-self by monocytes is required for this process and for initiating T lymphocyte-dependent alloimmunity. These concepts are summarized schematically in Figure 1. The mechanisms by which monocytes recognize allogeneic non-self and the nature of the allodeterminants that trigger monocyte differentiation to mature DC have not been identified yet. Elucidating these mechanisms should provide the possibility of matching between donors and recipients at innate allodeterminants to improve graft outcomes or of interrupting innate allorecognition pathways to prevent acute or chronic rejection.
Figure 1. Innate allorecognition and danger link innate to adaptive immunity after transplantation.
Recognition of allogeneic non-self by recipient monocytes is key for generating mature DC that drive graft rejection by T lymphocytes. Danger, which causes inflammation in the graft but is not sufficient for driving rejection, is nevertheless essential for potentiating the adaptive alloimmune response.
Concluding Remarks and Therapeutic Prospects
In this review we did not aim at providing a comprehensive review of the literature on the roles of DCs and the innate immune system in kidney transplantation but at emphasizing two emerging concepts in this area. First is the concept that recipient DCs, specifically those derived from monocytes, are increasingly being recognized as key players in allograft rejection. They have a primary role in T lymphocyte activation, migration, and retention in the graft. Second is the novel concept that monocytes distinguish between self and allogeneic non-self and by doing so, trigger allograft rejection and perpetuate it. Danger stimuli, although important in ischemia-reperfusion injury, are not sufficient for initiating alloimmunity.
We believe that both concepts should provide valuable opportunities in the future to inhibit alloimmune responses in a cognate and safe manner. The first provides the prospect that inhibiting recipient monocyte migration to the graft or their differentiation into DCs could interrupt rejection even after T cell priming has already taken place in secondary lymphoid tissues (for example, rejection mediated by memory T cells). The second, the innate allorecognition concept, raises the possibility that identifying the mechanisms by which monocytes sense allogeneic non-self could lead to novel matching schemes between donors or recipients to minimize rejection, especially chronic rejection which becomes manifest long after danger stimuli have subsided. Alternatively, blocking the signaling pathways triggered by the recognition of allogeneic non-self by monocytes would constitute a potentially novel and unexplored therapeutic modality in transplantation.
Footnotes
Disclosures:
There are no interests to disclose.
References
- 1.USRDS. Atlas of ESRD. 2013 http://www.usrds.org/2013/view/Default.aspx.
- 2.Lakkis FG, Lechler RI. Origin and biology of the allogeneic response. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas AK, Janeway CA., Jr Immunology: improving on nature in the twenty-first century. Cell. 2000;100:129–38. doi: 10.1016/s0092-8674(00)81689-x. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PJ, et al. The renal mononuclear phagocytic system. J Am Soc Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli AE. Dendritic cells of myeloid lineage: the masterminds behind acute allograft rejection. Curr Opin Organ Transplant. 2014;19:20–7. doi: 10.1097/MOT.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 9.Gould DS, Auchincloss H. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 10.Macedo C, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–66. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 11.Suchin EJ, et al. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 12.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens: A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafferty KJ, Bootes A, Dart G, Talmage DW. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976;22:138–49. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Talmage DW, Dart G, Radovich J, Lafferty KJ. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976;191:385. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 15.Bowen KM, Andrus L, Lafferty KJ. Successful allotransplantation of mouse pancreatic islets to nonimmunosuppressed recipients. Diabetes. 1980;29 (Suppl 1):98–104. doi: 10.2337/diab.29.1.s98. [DOI] [PubMed] [Google Scholar]
- 16.Batchelor JR, Welsh KI, Maynard A, Burgos H. Failure of long surviving, passively enhanced kidney allografts to provoke T-dependent alloimmunity. I Retransplantation of (AS X AUG)F1 kidneys into secondary AS recipients. J Exp Med. 1979;150:455–64. doi: 10.1084/jem.150.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh KI, Batchelor JR, Maynard A, Burgos H. Failure of long surviving, passively enhanced kidney allografts to provoke T-dependent alloimmunity. II Retransplantation of (AS X AUG)F1 kidneys from AS primary recipients into (AS X WF)F1 secondary hosts. J Exp Med. 1979;150:465–70. doi: 10.1084/jem.150.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelbrot DA, et al. Expression of B7 molecules in recipient, not donor, mice determines the survival of cardiac allografts. J Immunol. 1999;163:3753–7. [PubMed] [Google Scholar]
- 20.Campos L, et al. Survival of MHC-deficient mouse heterotopic cardiac allografts. Transplantation. 1995;59:187–91. [PubMed] [Google Scholar]
- 21.Mannon RB, Griffiths R, Ruiz P, Platt JL, Coffman TM. Absence of donor MHC antigen expression ameliorates chronic kidney allograft rejection. Kidney Int. 2002;62:290–300. doi: 10.1046/j.1523-1755.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 22.Grusby MJ, et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci USA. 1993;90:3913–7. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrod KR, et al. NK cell patrolling and elimination of donor-derived dendritic cells favor indirect alloreactivity. J Immunol. 2010;184:2329–36. doi: 10.4049/jimmunol.0902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrod KR, Wei SH, Parker I, Cahalan MD. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc Natl Acad Sci USA. 2007;104:12081–6. doi: 10.1073/pnas.0702867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrera OB, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–37. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 26.Sivaganesh S, et al. Copresentation of intact and processed MHC alloantigen by recipient dendritic cells enables delivery of linked help to alloreactive CD8 T cells by indirect-pathway CD4 T cells. J Immunol. 2013;190:5829–38. doi: 10.4049/jimmunol.1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 30.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2002;99:6175–6180. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obhrai JS, et al. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 32.Gelman AE, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–73. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, et al. Secondary lymphoid organs are important but not absolutely required for allograft responses. Am J Transplant. 2003;3:259–66. doi: 10.1034/j.1600-6143.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 34.Kant CD, et al. Primary vascularization of allografts governs their immunogenicity and susceptibility to tolerogenesis. J Immunol. 2013;191:1948–56. doi: 10.4049/jimmunol.1202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasr IW, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7:1071–9. doi: 10.1111/j.1600-6143.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 36.Heeger PS, et al. Pretransplant frequency of donor-specific, IFN-g-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 37.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–44. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 38.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–9. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 39.Walch JM, et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest. 2013;123:2663–2671. doi: 10.1172/JCI66722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 41.Larsen CP, et al. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberbarnscheidt MH, et al. Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 2014 Jun 1; doi: 10.1172/JCI74370. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberbarnscheidt MH, Zecher D, Lakkis FG. The innate immune system in transplantation. Semin Immunol. 2011;23:264–72. doi: 10.1016/j.smim.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori DN, Kreisel D, Fullerton JN, Gilroy DW, Goldstein DR. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev. 2014;258:132–44. doi: 10.1111/imr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall BM, Dorsch S, Roser B. The cellular basis of allograft rejection in vivo. I The cellular requirements for first-set rejection of heart grafts. J Exp Med. 1978;148:878–89. doi: 10.1084/jem.148.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall BM. Cells mediating allograft rejection. Transplantation. 1991;51:1141–51. doi: 10.1097/00007890-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Zecher D, et al. Innate immunity alone is not sufficient for chronic rejection but predisposes healed allografts to T cell-mediated pathology. Transpl Immunol. 2012;26:113–118. doi: 10.1016/j.trim.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham JA, et al. Viral infection induces de novo lesions of coronary allograft vasculopathy through a natural killer cell-dependent pathway. Am J Transplant. 2009;9:2479–84. doi: 10.1111/j.1600-6143.2009.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroemer A, et al. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol. 2008;180:7818–26. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 50.Kirk AD, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 51.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 52.Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28:229–48. doi: 10.1615/critrevimmunol.v28.i3.40. [DOI] [PubMed] [Google Scholar]
- 53.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruger B, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 56.Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant. 2004;4:1429–39. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 57.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur J Immunol. 2006;36:1994–2002. doi: 10.1002/eji.200636249. [DOI] [PubMed] [Google Scholar]
- 58.Hutton MJ, et al. Islet allograft rejection is independent of toll-like receptor signaling in mice. Transplantation. 2009;88:1075–80. doi: 10.1097/TP.0b013e3181bd3fe2. [DOI] [PubMed] [Google Scholar]
- 59.Oberbarnscheidt MH, et al. Type I interferons are not critical for skin allograft rejection or the generation of donor-specific CD8+ memory T cells. Am J Transplant. 2010;10:162–7. doi: 10.1111/j.1600-6143.2009.02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H, et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol. 2011;186:230–41. doi: 10.4049/jimmunol.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bingaman AW, et al. Vigorous allograft rejection in the absence of danger. J Immunol. 2000;164:3065–3071. doi: 10.4049/jimmunol.164.6.3065. [DOI] [PubMed] [Google Scholar]
- 62.Anderson C, et al. Testing time-, ignorance-, and danger-based models of tolerance. J Immunol. 2001;166:3663–3671. doi: 10.4049/jimmunol.166.6.3663. [DOI] [PubMed] [Google Scholar]
- 63.Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat Med. 2001;7:80–7. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 64.Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zecher D, et al. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. J Immunol. 2010;184:6649–57. doi: 10.4049/jimmunol.0903729. [DOI] [PubMed] [Google Scholar]
- 66.Burnet FM. “Self-recognition” in colonial marine forms and flowering plants in relation to the evolution of immunity. Nature. 1971;232:230–235. doi: 10.1038/232230a0. [DOI] [PubMed] [Google Scholar]
- 67.Rosengarten RD, Nicotra ML. Model systems of invertebrate allorecognition. Curr Biol. 2011;21:R82–92. doi: 10.1016/j.cub.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 68.Oberbarnscheidt MH, Lakkis FG. Innate allorecognition. Immunol Rev. 2014;258:145–9. doi: 10.1111/imr.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zecher D, van Rooijen N, Rothstein D, Shlomchik W, Lakkis F. An Innate Response to Allogeneic Nonself Mediated by Monocytes. J Immunol. 2009;183:7810–7816. doi: 10.4049/jimmunol.0902194. [DOI] [PubMed] [Google Scholar]