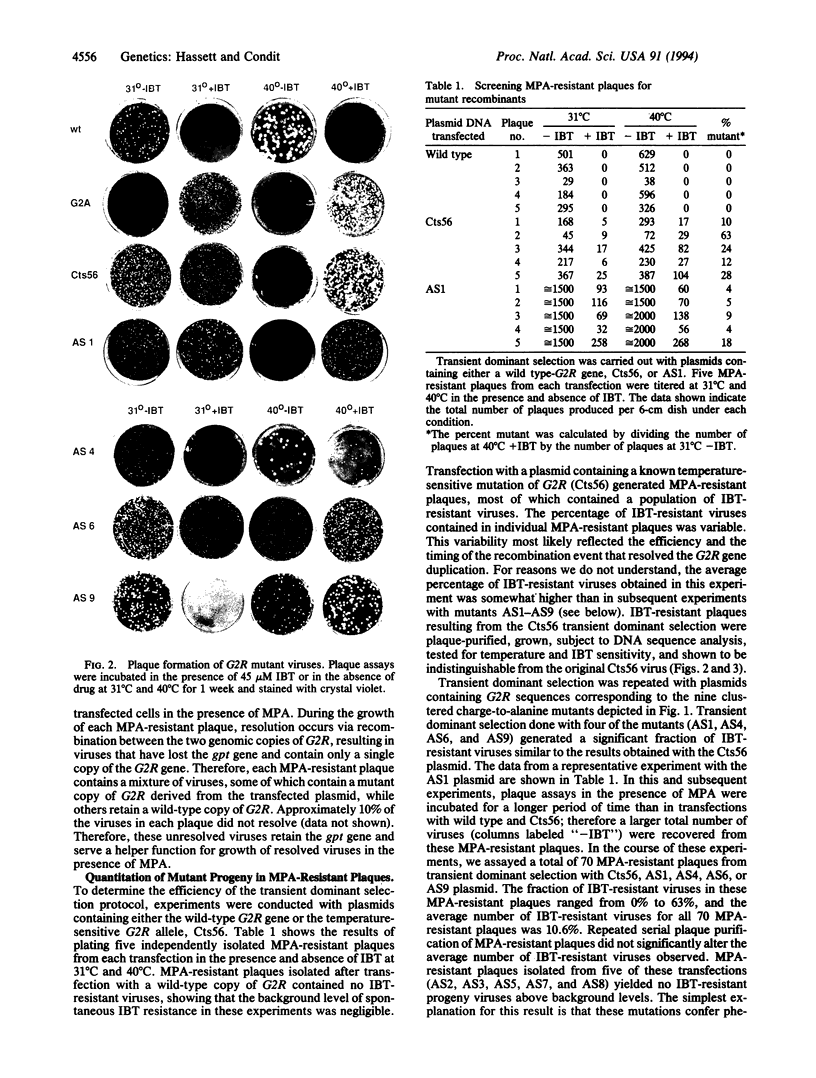
Abstract
The feasibility of using "clustered charge-to-alanine" mutagenesis (replacement by alanine of two or more charged residues clustered in a five- or six-amino acid sequence) to create temperature-sensitive, conditionally lethal mutations in vaccinia virus was examined by creating nine mutations in the vaccinia virus gene G2R. G2R was chosen for this analysis because mutations in this gene confer selectable phenotypes. Specifically, vaccinia viruses that contain a wild-type copy of G2R nare sensitive the effects of the anti-poxvirus drug isatin-beta-thiosemicarbazone (IBT), while mutations in G2R that completely abolish the function of the G2R protein product confer dependence upon IBT for growth. A previously isolated mutant carrying a temperature-sensitive mutation that maps to G2R (Cts56) is resistant to IBT at the permissive temperature and dependent upon IBT at the restrictive temperature. Nine clustered charge-to-alanine mutants were examined. Four of the these mutants (AS1, AS4, AS6, and AS9) display some degree of temperature sensitivity in the function of the G2R gene product. AS1 is temperature sensitive for growth in both a plaque assay and in a one-step growth experiment. AS6 and AS9 form small plaques at the nonpermissive temperature and are temperature sensitive for growth in a one-step growth experiment. AS4 manifests its temperature sensitivity as temperature-dependent IBT resistance. Five of the mutations (AS2, AS3, AS5, AS7, and AS8) appeared to confer phenotypes indistinguishable from that of wild-type vaccinia. These results demonstrate that temperature-sensitive conditionally lethal mutants can be created in vaccinia virus by altering the charge characteristics of essential viral proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. F., Paoni N. F., Keyt B. A., Botstein D., Jones A. J., Presta L., Wurm F. M., Zoller M. J. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J Biol Chem. 1991 Mar 15;266(8):5191–5201. [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Niles E. G. Orthopoxvirus genetics. Curr Top Microbiol Immunol. 1990;163:1–39. doi: 10.1007/978-3-642-75605-4_1. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Diamond S. E., Kirkegaard K. Clustered charged-to-alanine mutagenesis of poliovirus RNA-dependent RNA polymerase yields multiple temperature-sensitive mutants defective in RNA synthesis. J Virol. 1994 Feb;68(2):863–876. doi: 10.1128/jvi.68.2.863-876.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Transient dominant selection of recombinant vaccinia viruses. J Virol. 1990 Jun;64(6):3108–3111. doi: 10.1128/jvi.64.6.3108-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Meis R. J., Condit R. C. Genetic and molecular biological characterization of a vaccinia virus gene which renders the virus dependent on isatin-beta-thiosemicarbazone (IBT). Virology. 1991 Jun;182(2):442–454. doi: 10.1016/0042-6822(91)90585-y. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Condit R. C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986 Apr 15;150(1):10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G., Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992 Oct;132(2):337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]