Abstract
Buprenorphine maintenance therapy (BMT) is increasingly the preferred opioid maintenance agent due to its reduced toxicity and availability in an office-based setting in the United States. Although BMT has been shown to be highly efficacious, it is often discontinued soon after initiation. No current systematic review has yet investigated providers’ or patients’ reasons for BMT discontinuation or the outcomes that follow. Hence, provider and patient perspectives associated with BMT discontinuation after a period of stable buprenorphine maintenance and the resultant outcomes were systematically reviewed with specific emphasis on pre-buprenorphine-taper parameters predictive of relapse following BMT discontinuation. Few identified studies address provider or patient perspectives associated with buprenorphine discontinuation. Within the studies reviewed providers with residency training in BMT were more likely to favor long term BMT instead of detoxification, and providers were likely to consider BMT discontinuation in the face of medication misuse. Patients often desired to remain on BMT because of fear of relapse to illicit opioid use if they were to discontinue BMT. The majority of patients who discontinued BMT did so involuntarily, often due to failure to follow strict program requirements, and 1 month following discontinuation, rates of relapse to illicit opioid use exceeded 50% in every study reviewed. Only lower buprenorphine maintenance dose, which may be a marker for attenuated addiction severity, predicted better outcomes across studies. Relaxed BMT program requirements and frequent counsel on the high probability of relapse if BMT is discontinued may improve retention in treatment and prevent the relapse to illicit opioid use that is likely to follow BMT discontinuation.
Keywords: buprenorphine, opioid dependence, treatment cessation, provider perspectives, patient perspectives
1. Introduction
Opioid maintenance therapy (OMT) with methadone or buprenorphine is the current gold standard treatment for opioid use disorders (Mattick, Breen, Kimber, & Davoli, 2009; Mattick, Kimber, Breen, & Davoli, 2008; Thomas et al., 2014). In addition to reducing illicit opioid use (Mattick et al., 2008; 2009), OMT can be associated with reductions in mortality (Clausen, Anchersen, & Waal, 2008; Degenhardt et al., 2011), criminal activity (Bates & Pemberton, 1996; Dolan et al., 2005; Mattick et al., 2009), and high-risk behavior associated with transmission of Human Immunodeficiency Virus (Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011). Further, OMT increases quality of life (Giacomuzzi et al., 2003; Nosyk et al., 2011; Ponizovsky & Grinshpoon, 2007; Winklbaur, Jagsch, Ebner, Thau, & Fischer, 2008), and adherence to OMT significantly reduces overall healthcare costs (Tkacz, Volpicelli, Un, & Ruetsch, 2013).
Despite known efficacy of OMT, the majority of opioid-dependent patients in the United States are not currently being treated with OMT (Kleber, 2008; SAMHSA, 2011). Financial barriers, restrictive legislation, patient preference, physician ambivalence and non-evidence-based approaches to addiction treatment all contribute to low rates of OMT (Appel, Ellison, Jansky, & Oldak, 2004; Gryczynski et al., 2013; Nosyk et al., 2013), and these rates persist despite the significantly enhanced availability of OMT afforded through the Drug Addiction Treatment Act of 2000, which allows for buprenorphine to be prescribed in a less restrictive office-based setting (Jaffe & O’Keeffe, 2003).
Buprenorphine OMT (BMT), because of its blunted toxicity (Walsh, Preston, Bigelow, & Stitzer, 1995; Walsh, Preston, Stitzer, Cone, & Bigelow, 1994) and increased accessibility (Jaffe & O’Keeffe, 2003), offers some advantages to methadone OMT. Buprenorphine’s unique partial mu agonist pharmacology and extended receptor occupation time lend to a comparatively less severe withdrawal syndrome (Tompkins, Smith, Mintzer, Campbell, & Strain, 2013; Westermeyer & McCance-Katz, 2012). A less severe withdrawal syndrome could potentially reduce relapse propensity, a hypothesis supported by the observation that longer OMT tapering procedures result in better outcomes (Dunn, Sigmon, Strain, Heil, & Higgins, 2011; Nosyk et al., 2012; Sigmon et al., 2013). Although, this is not always the case (Ling et al., 2009). In light of this less severe withdrawal syndrome, the approval of buprenorphine was heralded by enthusiasm for improved outcomes following detoxification (National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998) apropos of the high relapse rates known to accompany discontinuation of methadone OMT (Amato et al., 2013). Unfortunately, buprenorphine detoxification has not lead to increased rates of abstinence following withdrawal (Dunn et al., 2011; Horspool, Seivewright, Armitage, & Mathers, 2008), and as such, the practice of BMT discontinuation may be perpetuated by the provider expectation that abstinence is likely to follow (Newman, 2009). Furthermore, some providers may feel that abiding by program rules is necessary for BMT success to the extent that BMT is discontinued for rule infractions; however, patients often find benefit in remaining in programs despite failure to achieve program-imposed criteria (Mitchell et al., 2011).
In addition to factors associated with BMT treatment providers, patient preference is also a major factor in the discontinuation of BMT. Although patient satisfaction with buprenorphine treatment is high (Barry et al., 2007; Ling, Hillhouse, Ang, Jenkins, & Fahey, 2013), many patients ask to discontinue BMT after several months of treatment (Kleber, 2007), a preference that could be driven in part by perceived low probability of relapse (Bailey, Herman, & Stein, 2013). Hence, it is essential that both physician and patient harbor realistic, evidence-based expectations of outcomes following discontinuation of BMT; however, current systematic reviews of BMT discontinuation have focused on its use in detoxification protocols (Dunn et al., 2011; Horspool et al., 2008) and no current reports could be found that link perspectives of patients or providers with outcomes. Here we bridge patient and provider perspectives of BMT with rates of abstinence following discontinuation of BMT by systematically reviewing patient and provider perspectives that may lead to BMT discontinuation after a period of stable BMT and the outcomes that follow.
2. Methods
2.1. Search strategy and inclusion criteria
Potential studies were identified using Boolean search strings within the Pubmed database. All searches were limited to articles available in English. Studies that included patient perspectives on buprenorphine were identified using the following string: buprenorphine AND patients AND (preference* OR perspective* OR attitude* OR satisfaction OR reason*) where * denotes a wildcard. This search was most recently conducted on September 24, 2014. Studies of patient perspectives on buprenorphine were included if they quantitatively assessed patients’ subjective evaluations of buprenorphine as a treatment for opioid dependence. Further, only studies that linked these subjective evaluations with decisions to continue or discontinue BMT were included. Citations within identified articles were screened for identification of additional references.
Studies that included provider perspectives on buprenorphine were identified using the following string: buprenorphine AND (physician* OR provider* OR counselor* OR psychiatrist*) AND (preference* OR perspective* OR attitude* OR satisfaction OR reason*). This search was most recently conducted on September 27, 2014. Studies of provider perspectives on buprenorphine were included if they quantitatively assessed providers’ subjective evaluations of buprenorphine as a treatment for opioid dependence. Further, only studies that linked these subjective evaluations with decisions to continue or discontinue BMT were included. Citations within identified articles were screened for identification of additional references.
Studies of cessation of buprenorphine maintenance were identified using the following search string: buprenorphine AND (detoxification OR taper OR discontinue OR cessation OR withdrawal). Search results were then limited to clinical trials. References of identified articles were also searched for additional reports that met review criteria. This search was most recently conducted on September 24, 2014. Articles were included if participants were opioid-dependent and maintained on a buprenorphine-containing medication for at least 14 days before starting medication taper. Notably, 14 days does not reflect what most providers/investigators would consider to be a maintenance period. We chose this time period for 2 reasons: 1) It is the typical time frame required to titrate a patient to a stable maintenance dose (Chiang & Hawks, 2003); thus, shorter time periods would not allow us to consider the pre-taper maintenance dose. 2) Our goal was to determine typical outcomes after cessation of buprenorphine maintenance therapy and relate these outcomes to pre-taper variables. However, very few studies have maintained patients on buprenorphine and studied outcomes following cessation. Thus, to include enough studies to consider pre-taper associations with outcomes, we required a very broad quantitative definition of maintenance period. In addition to the requirement of a minimum 14 day maintenance period, only studies that reported urine drug screens for opioids at least 1 month following completion of the taper were included, ensuring final outcome measures occurred outside of the withdrawal epoch.
2.2. Analysis
Study outcomes were analyzed as intention-to-treat with the initial patient sample size defined as the number of patients retained at the start of the taper. Primary outcome was defined as the proportion of participants retained in the study at the start of the tapering procedure who tested negative for opioids via urinalysis at least 1 month after buprenorphine taper cessation. One study reported a urinalysis-based outcome, and study authors indicated that it approximated a simple urinalysis outcome (Weiss et al., 2011). Hence, we included reported outcomes of Weiss et al. (2011) as if they were simple urinalysis outcomes; a recent buprenorphine-focused review made a similar approximation for this study (Thomas et al., 2014). All missing urinalysis data were assumed to be opioid-positive. Pre-taper parameters reported to predict opioid urinalysis 1 month or more after taper cessation within included studies were compared across studies. Statistical analyses were performed using IBM SPSS Statistics (Version 19). Weighted-least-squares regression with weights determined by study sample size was used to relate potential predictors to outcome. Due to the low number of studies included, multivariate comparisons were not performed.
3. Results
3.1. Patient perspectives of buprenorphine
The search for studies that quantitatively assessed patient perspectives associated with BMT discontinuation most recently (September 24, 2014) returned 203 possible studies. After screening all article titles and abstracts for relevancy, 11 articles were screened in their entirety. One of these articles was excluded, because although it quantified patients’ reasons for ceasing maintenance therapy, 90% of these patients were maintained on methadone and no comparison was performed to determine if the distribution of reasons was similar for both buprenorphine and methadone (Awgu, Magura, & Rosenblum, 2010). One article was excluded on the grounds that it did not quantify reasons patients discontinued treatment (Guichard, Lert, Brodeur, & Richard, 2007). Seven of these articles were excluded on the basis that they did not include patients’ reasons for discontinuing BMT. Two studies of patient perspectives on buprenorphine met all criteria (Gryczynski et al., 2013; Winstock, Lintzeris, & Lea, 2011). Screening citations within these 2 articles did not result in identification of additional relevant references. These studies are summarized here in chronological order with emphasis on reasons patients decided to discontinue BMT as well as the limitations present in each study.
Winstock and colleagues (2011) surveyed 145 patients from public clinics in Sydney, Australia who were maintained on either buprenorphine (n = 56) or methadone (n = 89). The purpose of the survey was to determine variables associated with patients’ level of interest (i.e., low vs. high) in cessation of OMT. High interest in treatment cessation was found to be significantly associated with shorter duration of current treatment, discussing treatment cessation with more categories of people, and low concern of relapse following cessation. Patients were then asked what concerns they had about treatment cessation. The most frequent patient concerns were fear of withdrawal discomfort (68%), pain (50%), relapse (48%), and events associated with relapse, such as, “life becoming a mess” (34%), return to crime (30%), and loss of contact with clinic (17%).
There are several major limitations present in the study by Winstock and colleagues (2011). Although not an inherent limitation, for the purposes of this buprenorphine-focused review, interpretation of results is limited by pooling of data from patients maintained on both methadone and buprenorphine. However, treatment medication was not associated with level of interest in treatment cessation or frequency of concerns about cessation; thus, these results are likely relevant to both buprenorphine as well as methadone. Another limitation is that outcomes were not reported, i.e., proportion of patients who discontinued BMT. Patient interest in ceasing BMT may not predict actual duration of BMT (Gryczynski et al., 2013); thus, the implication of patients’ desire to discontinue BMT remains unknown.
More recently, Gryczynski et al. (2013) reported buprenorphine perspectives of participants from a parent randomized clinical trial (Mitchell et al., 2013) comparing levels of counseling intensity in conjunction with BMT at 2 outpatient substance abuse treatment clinics in Baltimore, Maryland. Participants were opioid-dependent African-Americans (n = 297) newly initiated on BMT. No differences in illicit opioid use or any other outcome measures were reported between counseling intensities. At initiation of BMT participants were asked how long they intended to stay in BMT. Data were coded as number of weeks patients intended to remain in treatment with all responses beyond 26 weeks collapsed to a single epoch, because patients who remained in BMT after 26 weeks were transferred to an outside buprenorphine provider. Nearly half (42.1%) of participants discontinued buprenorphine treatment before 26 weeks, and only 4% of these discontinuations were due to successful completion of treatment. No association was found between participants’ initial intended duration of BMT and actual duration, and on average, participants expected to remain in treatment longer than they actually remained. This discordance between participants’ intentions and actual durations of BMT was explained in part by participants’ reasons for BMT cessation. Nearly half (44.6%) of participants who discontinued treatment were discharged involuntarily, mostly due to conflicts with program staff (24%) or missing too many appointments (17%). In contrast, few patients discontinued BMT due to reasons fundamental to buprenorphine medication; only 4% of patients who discontinued BMT did so because they did not like the study medication (Suboxone®), 4% because they wanted to keep using illicit drugs, and 1% because they felt addiction recovery was not possible while taking medication. Other reasons could be classified as logistical conflicts, such as, program conflicts with work/school (17%), left to receive treatment from another provider (14%), incarceration (7%), financial hardship (4%), moved out of town (3%), or lack of transportation (3%).
There are several notable limitations the study by Gryczynski and colleagues (2013). Foremost, the setting within a clinical trial limits generalization to clinical practice. For example, the study was terminated at 26 weeks, but patients often are maintained on buprenorphine for much longer time periods. If the study were extended, a correlation between expected treatment duration and actual treatment duration might have become apparent. On the other hand, the clinical trial framework was also a major strength on this study, as it was the only perspectives study included in this review that included a measure of patient outcomes. The generalizability of this study is also limited given its racially homogenous population in a single city in the United States. Moreover, the setting was unique in that patients attended formally drug-free programs that recently began prescribing buprenorphine, a caveat that could explain the high rates of involuntary treatment discontinuation.
3.2. Provider perspectives of buprenorphine
The search for studies that quantitatively assessed provider perspectives related to retention in BMT for opioid dependence most recently (September 27, 2014) returned 97 articles. After screening all article titles and abstracts for relevancy, 19 articles were screened in their entirety. Sixteen of these articles were excluded on the basis that they did not include providers’ reasons for discontinuing BMT or provider perspectives associated with BMT discontinuation. Three studies of provider perspectives on buprenorphine met all criteria (Feroni et al., 2005; Quaglio et al., 2010; Suzuki, Connery, Ellison, & Renner, 2014). Screening citations within these 3 articles did not result in identification of additional relevant references. These studies are summarized here in chronological order with emphasis on provider characteristics associated with discontinuation or continuation of BMT and limitations present within studies.
Feroni et al. (2005) surveyed the attitudes and practices of a sample of 345 primary care physicians prescribing BMT in South-Eastern France. Investigators’ primary goal was to determine physician attitudes and practices associated with multiple prescribers per patient, i.e. “doctor shopping.” Physicians were randomly selected and stratified according to gender, age, and number of medical consultations per year. The primary finding of the study was that physicians who endorsed a “stringent attitude” were more likely to have patients with multiple buprenorphine prescribers. A “stringent attitude” was defined as prescribing buprenorphine for only 7 days at a time, with daily delivery by the pharmacist, and daily dose taking in the pharmacy. Notably, the survey tool utilized contained 3 items related to BMT cessation. Nearly two-thirds (64%) of physicians indicated that they would discontinue BMT if they discovered a patient had several other buprenorphine prescribers, 49% of physicians indicated they would discontinue BMT if they discovered a patient had been injecting buprenorphine, and 22% of physicians indicated they had discontinued BMT in the past because of a difficult relationship with a patient.
The major strength of Feroni and colleagues’ (2005) report is that it directly asked physicians why they might discontinue BMT and if they had discontinued BMT in the past. The study’s major limitation is of generalizability, given that it surveyed physicians from a single geographic region from a single country.
Quaglio and colleagues (2010) surveyed 305 randomly selected Italian physicians working in drug addiction centers with at least 6 months experience prescribing buprenorphine for opioid use disorders, and 185 physicians completed the survey. Approximately half of sampled physicians were male, most had been prescribing buprenorphine for more than 2 years, and the specialties of internal medicine, pharmacology/toxicology, and psychiatry were approximately evenly represented within the sample. The sample mostly supported the use of buprenorphine with 90% of physicians either completely (69%) or partially (21%) favoring use of buprenorphine for treating a patient with heroin use disorder the first time they contacted the office. Notably, 98% of physicians indicated that buprenorphine was a very (90%) or a somewhat (8%) useful treatment for long-term substitution (i.e., >6 months). Conversely, fewer (84%) of physicians indicated that buprenorphine is useful for short-term substitution (i.e., <3 months) with 49% of physicians indicating buprenorphine is very useful for short term substitution and 35% indicating it is somewhat useful. Given that the vast majority of sampled physicians viewed buprenorphine as a useful long-term treatment for opioid use disorders, these physicians’ perceived advantages and disadvantages of BMT might represent the typical views of physicians who consider buprenorphine to be a useful long-term treatment for opioid dependence. In the sample of physicians, the most frequently cited advantages of BMT in an open-ended response were as follows: easy to use for unsupervised/take-home medication (30%), smaller risk of overdose (22%), and buprenorphine in tablets is less stigmatized than methadone (17%). The most frequently cited disadvantages were as follows: diversion (31%), difficult to start tapering because of lack of tablets at a lower dosage (28%), and slow sublingual absorption (13%).
Quaglio and colleagues’ (2010) report is mostly limited by generalizability. The study took place exclusively in Italy and may not represent physician perspectives in other nations. Furthermore, given that the vast majority of surveyed physicians indicated buprenorphine was useful for long-term maintenance, it is unclear how perspectives of buprenorphine advantages and disadvantages would differ in a sample of providers who favored short-term use of buprenorphine. Finally, physician perspectives were not assessed against the duration of BMT of typical patients within their practice; hence, the implications of these findings are unclear.
Recently, Suzuki and colleagues (2014) compared BMT perspectives of physicians who had received BMT training to those who had not received BMT training. Authors contacted 359 psychiatrists via email who graduated from psychiatry residency programs in the United States between 2008 and 2011 to complete a survey of BMT training and perspectives, and 93 psychiatrists who graduated within this timeframe completed the survey. About half (56%) of physicians reported completing a minimum of 1 buprenorphine course during their training. Although 38.5% of physicians with BMT training had prescribed buprenorphine, none of the physicians who had not received BMT training had prescribed buprenorphine. Compared to participants who did not receive BMT training in residency, those who did receive training were more likely to be male (50.0% vs. 26.8%), to report confidence in treating opioid dependence (84.6% vs. 46.3%), to believe opioid dependence is treatable (98.1% vs. 75.6%), and to believe that buprenorphine is effective in treating opioid dependence (98.1% vs. 75.6%). Moreover, physicians who received BMT training were less likely to indicate that treatment cessation via detoxification should be attempted before maintenance therapy (32.7% vs. 51.2%). Thus, in this sample BMT training was associated with a physician preference for more extended use of buprenorphine through maintenance therapy.
Suzuki and colleagues’ (2014) report is limited primarily because none of the physicians who did not receive training had prescribed buprenorphine. Thus, it is unclear whether differences in physician perspectives were primarily due to training or experience. Similarly to many studies of perspectives, this study is also limited by the uncertain association between the physician perspective that detoxification should be attempted before a trial of BMT and actual duration of BMT provided by these physicians. Finally, inclusion of only physicians trained in the United States limits international applicability.
3.3. Outcomes following BMT discontinuation
The search for studies that quantitatively assessed outcomes following discontinuation of BMT most recently (September 24, 2014) returned 212 articles. After screening all article titles and abstracts for relevancy, 13 articles were screened in their entirety. One of the 13 articles met study design criteria but did not delineate drug screen results between maintenance and post-taper phases of the study (Otiashvili et al., 2013). Another study met all criteria but relied on self-reported opioid use instead of urine drug screening in the follow-up period (Kornør, Waal, & Ali, 2006). Five studies were excluded on the basis that they did not include a follow-up period of 1 month or longer. One study was excluded on the basis that it did not maintain patients for at least 14 days before taper (Rosenthal et al., 2013). Five studies of buprenorphine outcomes met all criteria (Breen et al., 2003; Ling et al., 2009; Sigmon et al., 2013; Weiss et al., 2011; Woody et al., 2008). Two randomized trials compared BMT taper durations (Ling et al., 2009; Sigmon et al., 2013); one randomized trial compared standard medical management alone or standard medical management with adjunctive individual opioid dependence counseling (Weiss et al., 2011); one randomized trial compared short-term buprenorphine detoxification with more extended BMT (Woody et al., 2008); and 1 semi-randomized trial compared methadone-to-buprenorphine transition protocols (Breen et al., 2003). Screening citations within these 5 articles did not result in identification of additional relevant references. These studies are summarized in Table 1. Given the heterogeneity in study design, summaries and study limitations are presented in chronological order.
Table 1.
Summary of reviewed studies
| Study | Sample size at taper start | Prior heroin use | Maintenance period | Mean dose | Abstinent maintenance | Taper duration | Follow-up time | Naltrexone | Abstinent post-taper |
|---|---|---|---|---|---|---|---|---|---|
| Sigmon et al. 2013 | 70 | ~50% | 2 weeks | 11.5mg | 82% | 1 week | 9 weeks | 50mg (p.o.) daily | 5/24 (21%) |
| 2 weeks | 8 weeks | 50mg (p.o.) daily | 4/24 (17%) | ||||||
| 4 weeks | 6 weeks | 50mg (p.o.) daily | 11/22 (50%) | ||||||
|
| |||||||||
| Weiss et al. 2011 | 323 (Phase 2) | 26% | 12 weeks | 20.8mg | 177/323 (54%) | 4 weeks | 8 weeks | None | 31/323 (9.6%) |
|
| |||||||||
| Ling et al. 2009 | 516 | 83% | 4 weeks | 20.3mg | 191/516 (37%) | 1 week | 4 weeks | None | 45/255 (18%) |
| 4 weeks | 4 weeks | None | 46/261 (18%) | ||||||
|
| |||||||||
| Woody et al. 2008 | 55 | 76% | 8 weeks | 15.1mg | 54% | 4 weeks | 6 months | None | 34% |
|
| |||||||||
| Breen et al. 2003 | 50 | 100% | 2 weeks (>6 months methadone) | 8.6mg | Not reported | 11 weeks | 4 weeks | Optional (p.o.) 5 participants received | 22/50 (44%) |
The less severe withdrawal syndrome engendered by buprenorphine cessation compared to that of full mu agonists was noted early in the development of buprenorphine as an opioid addiction treatment (Bickel et al., 1988; Jasinski, Pevnick, & Griffith, 1978). From these early studies Breen et al. (2003) hypothesized that buprenorphine might be used to decrease relapse rates following methadone cessation by first transferring participants to BMT and then tapering buprenorphine dose to 0 mg. The purpose of this study was to compare drug transfer protocols in a semi-randomized design. Thirty-eight participants maintained on methadone for at least 6 months at doses between 30 and 40 mg were randomly selected to either transfer to buprenorphine (Subutex®) after their methadone dose was tapered low enough to make them “uncomfortable” or to transfer after their dose was reduced to 30 mg. Another 17 participants maintained on less than 30 mg methadone were transferred to buprenorphine at their current methadone dose. Buprenorphine dose was titrated to “patient response” and up to 24 mg per day. Mean maintenance dose was 8.61 mg (the lowest of all studies reviewed) and lasted 2 weeks after a 5-day induction protocol. Fifty of the original 55 participants remained in the study at the onset of buprenorphine taper. Buprenorphine dose was then reduced at a maximum rate of 2 mg/week with the average taper lasting 11.1 weeks. Participants were given the option of naltrexone (p.o.) 5 days following buprenorphine cessation, and 5 participants were transitioned to naltrexone treatment. One month following buprenorphine cessation, 22 of 50 (44%) participants were abstinent from heroin and not in methadone treatment as indicated by urinalysis and self-report. This was the highest rate of abstinence observed in the reviewed studies 1 month or more after buprenorphine cessation. No predictors of outcome were reported.
The study by Breen et al. (2003) has several strengths, including a flexible buprenorphine-dosing regimen during the maintenance phase and a flexible tapering duration, both of which are more likely to resemble clinical practice. The major limitations of this study include no blinding of investigators or participants, lack of a control group of patients who were not discontinued from buprenorphine, no placebo used during the buprenorphine taper, and no breakdown was included of results by transfer protocol for abstinence rates at 1-month follow-up. Furthermore, the maintenance period of this study was 2 weeks, the minimum to meet criteria of this review but not as long as a typical clinical maintenance period; however, patients were maintained on methadone for several months before the switch to buprenorphine and taper initiation.
Woody et al. (2008) compared short-term (14 day) buprenorphine-assisted detoxification to 8 weeks of BMT with a 4-week terminal taper in a non-blinded, randomized trial in opioid-dependent youth. Only participants who received 8 weeks of BMT are included in the analyses, as the short-term detox group received less than 14 days of BMT, the minimal duration for inclusion in this review. Seventy-four participants were randomized to receive buprenorphine-naloxone (Suboxone®) for 8 weeks at 6 sites in the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN). Although protocol allowed for opioid-dependent participants ages 14 to 21 years, 83% of participants were 18 or older (mean age = 19.1). During the 8 weeks of BMT, participants received a mean dose of 15.1 mg/day buprenorphine (Subramaniam et al., 2011). As evidenced by urinalysis, 54% of participants were abstinent at the end of the 8-week BMT phase. After buprenorphine dose was tapered over 4 weeks, abstinence fell to 38% and remained relatively stable at the 6-month follow-up (34%). Although a secondary analysis reported several variables predictive of outcomes at the end of the taper (early treatment response, advanced illness, and use of augmenting treatments) (Subramaniam et al., 2011), predictors of outcomes at 6-month follow-up have not yet been reported.
The study by Woody et al. (2008) contains several strengths, including a longer 2-month maintenance period and a flexible dosing regiment during the maintenance period, both of which are representative of clinical practice. The major limitations of this study include no blinding of investigators or participants, lack of a control group of patients who were not discontinued from buprenorphine, and no placebo used during the buprenorphine taper. Finally, although it was the intent of the study, participants were much younger than the general population of patients with opioid use disorders.
To expand upon the findings of a smaller study (N = 8) that found a higher abstinence rate following a longer tapering procedure (Amass, Bickel, Higgins, & Hughes, 1994), Ling et al. (2009) compared abstinence rates following tapering durations of 1 vs. 4 weeks in an open-label, multisite CTN study among 748 opioid-dependent, treatment-seeking participants. Initially, participants were inducted onto buprenorphine-naloxone (Suboxone®), and 516 participants who remained in the study after 4 weeks of BMT were randomized to either a 7- (n = 255) or 28-day (n = 261) taper. Mean maintenance dose was 20.3 mg, and 191 of 516 (37%) participants were urinalysis negative for opioids at the end of stabilization (Hillhouse, Canamar, & Ling, 2013). Contrary to the authors’ hypothesis, significantly more participants were opioid-negative at the end of the 7-day taper (44%) compared to at the end of 28-day taper (30%). However, at 1-month follow-up rates of opioid-negative urinalysis were similar between groups with 91 of 516 (18%) participants testing negative. A secondary analysis revealed that primary heroin abusers were less likely than primary prescription opioid abusers to produce opioid-free urine at the end of the taper and 3 months after the end of the taper, but not at 1-month follow-up (Nielsen, Hillhouse, Thomas, Hasson, & Ling, 2013).
This study by Ling et al. (2009) contains several strengths, including a multi-center design, large sample size, and inclusion of patients with both primary heroin and prescription opioid use disorders, all of which help increase the generalizability of results. Similar to the other studies in this review, a flexible dosing protocol representative of clinical practice was utilized during the maintenance period. The major limitations of this study include no blinding of investigators or participants, lack of a control group of patients who were not discontinued from buprenorphine, and no placebo used during the buprenorphine taper.
Weiss et al. (2011; 2010) were the first to prospectively evaluate buprenorphine treatment outcomes in participants dependent on prescription opioids in a multi-center, randomized trial within the CTN. Participants (N = 653) at 10 separate cites all received buprenorphine-naloxone (Suboxone®) at a variable dose between 8 and 32 mg “adjusted for opioid use, withdrawal symptoms, adverse effects, and craving but not for pain.” Participants were randomized to receive one of two possible counseling intensities, either standard medical management alone or with additional opioid dependence counseling. No differences were observed between the counseling intensities, and the results reported here are collapsed across counseling intensity. During the first phase of the study, participants were inducted onto buprenorphine, maintained for 2 weeks, tapered over 2 weeks, and observed for an 8-week follow-up period. Participants were defined as successful in phase 1 if they reported opioid use on 4 or fewer days in a month, provided 2 consecutive opioid-negative urine samples with a maximum of 1 missing sample, and received no supplementary substance use disorder treatment, with the exception of self-help. Forty-three of 653 participants (6.6%) were successful in phase 1. Unsuccessful participants who remained in the study then entered the second phase. During phase 2, participants (n = 360) were maintained on a mean buprenorphine dose of 20.8 mg (personal communication) for 12 weeks, and 323 had their dose tapered over 4 weeks and were then observed for an 8-week follow-up period. Success in phase 2 was defined as urinalysis–verified self-reports of opioid abstinence during the final week as well as 2 of the previous 3 weeks. At the end of the buprenorphine maintenance period (week 12) 177 of 323 (54.8%) participants were successful; yet, only 31 of 323 (9.6%) participants were successful at the end of the follow-up period, 8 weeks after buprenorphine discontinuation (week 24). No variables predictive of outcomes at follow-up have yet been reported.
This study by Weiss et al. (2011) contains several strengths, including a multi-center design, a large sample size, and a 3-month maintenance period, all of which help increase the generalizability of results. Similar to the other studies in this review, a flexible dosing protocol representative of clinical practice was utilized during the maintenance period. The major limitations of this study include no blinding of investigators or participants, lack of a control group who were not discontinued from buprenorphine, and no placebo used during the buprenorphine taper. Further, the population was limited to primary prescription opioid abusers and may not recapitulate the outcomes in the general population of those with opioid use disorders.
Sigmon et al. (2013) compared outcomes following buprenorphine tapering protocols of variable duration in prescription opioid abusers in an outpatient research clinic in Burlington, Vermont. Participants (N = 70) were inducted onto buprenorphine (Suboxone®) and maintained for 2 weeks on a mean dose of 11.5 mg (2 – 20 mg) before being randomized to 1 of 3 possible tapering durations: 1, 2, or 4 weeks. Participants who did not resume opioid use were then started on naltrexone (p.o.) and observed over a minimum 6-week follow-up period. This study of buprenorphine taper duration is unique in that double-dummy medications and double-blinded administration ensured that neither staff nor participants were aware of taper duration or the point at which naltrexone treatment was initiated. There was an 82% rate of opioid-negative urine samples among participants at the end of the maintenance period, and similar to previously discussed studies, abstinence rates fell precipitously as buprenorphine dose was tapered. After taper to 0 mg of buprenorphine participants were started on naltrexone (p.o) if they produced a minimum of 1 opioid-negative urine sample and reported no opioid use in the past 24 hrs. Naltrexone was dosed daily and titrated from 12.5 mg on day 1 to 50mg on day 4. The 50 mg dose was continued until 5 weeks after the start of the buprenorphine taper and then increased to thrice weekly dosing of 100, 100, and 150 mg. Abstinence rates were relatively stable during the follow-up period and significantly higher in the 4-week taper group with 50% of participants retained, abstinent, and receiving naltrexone, compared with 17% and 21% for the 2-week and 1-week groups, respectively. Overall, 29% of participants were abstinent at follow-up. With the exception of taper duration, the only parameter predictive of abstinence at follow-up was stabilization dose. Buprenorphine doses of 8 mg or more were associated with decreased odds of abstinence at follow-up (odds-ratio = 0.26).
This study by Sigmon et al. (2013) contains several strengths, including blinding of both investigators and participants to assigned treatment groups, and a placebo control was used during the buprenorphine taper. Similar to the other studies in this review, a flexible dosing protocol representative of clinical practice was utilized during the maintenance period. The major limitations of this study include lack of a control group of patients who were not discontinued from buprenorphine, and the population was limited to primary prescription opioid abusers at a single clinic and may not recapitulate the outcomes in the general population of those with opioid use disorders. Furthermore, the maintenance period of this study was 2 weeks, the minimum to meet criteria of this review but not as long as a typical clinical maintenance period.
3.3. Predictors of abstinence following BMT cessation
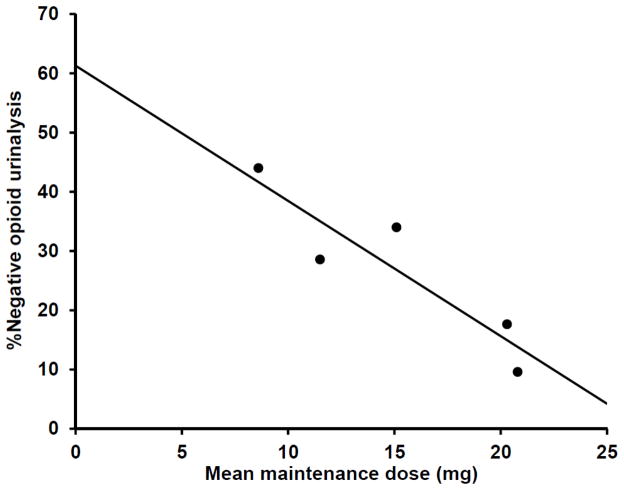
Abstinence rates 1 month or more following buprenorphine maintenance cessation ranged from 10% to 50% across the 5 reviewed studies. Collapsed across the 5 studies, 183 of 1014 (18%) of participants were abstinent. Three potential predictors of outcome (i.e., abstinence 1 month after buprenorphine maintenance cessation) were observed among the reviewed studies, including prior heroin use (Ling et al., 2009), taper duration (Sigmon et al., 2013), and buprenorphine maintenance dose (Sigmon et al., 2013). Possible associations between these parameters and outcome across reviewed studies were explored with weighted-least-squares regression with weights determined by study sample size. Prior heroin use (β = 0.61, p = 0.28) and taper duration (β = 0.44, p = 0.28.) were not significantly associated with outcome across the reviewed studies. In contrast, mean maintenance dose was found to be associated with outcome with lower maintenance doses predicting higher abstinence rates at follow-up (β = −0.90, p = 0.04, Fig. 1).
Fig. 1.
Mean buprenorphine maintenance dose predicts abstinence at follow up. Each data point represents 1 of the 5 reviewed studies and indicates the mean pre-taper buprenorphine maintenance dose and the mean outcome as the proportion of opioid-negative urinalyses after a minimum 1-month follow up. The association between maintenance dose and this outcome measure was found to be significant (β = −0.90, p = 0.04).
4. Discussion
Patient and provider perspectives associated with BMT discontinuation and the outcomes that followed were systematically reviewed. Our systematic searches identified few quantitative studies that have addressed patient or provider perspectives associated with discontinuation of BMT or the outcomes that follow BMT discontinuation. Furthermore, the heterogeneity among all reviewed studies was marked. Nevertheless, a few notable trends emerged, and these trends – elaborated in the next paragraphs – may have clinical importance and form the basis of useful hypotheses for future research.
Gryczynski and colleagues’ study of patient perspectives was unique in that it cataloged patients’ a priori planned duration of BMT (2013). Notably, these expectations were found to be unassociated with actual BMT duration during the first 6 months of treatment. This disconnect between expectation and outcome likely occurred because most patients who discontinued BMT did so involuntarily or due to logistical challenges of treatment, but not for reasons fundamental to buprenorphine medication (Gryczynski et al., 2013), an unsurprising finding given the high rates of patient satisfaction with buprenorphine treatment (Barry et al., 2007; Egan et al., 2011; Ling et al., 2013; Mitchell et al., 2013). In light of the high rate of relapse that occurs after BMT cessation reviewed here and the high rate of relapse when buprenorphine is used as a detoxification medication (Dunn et al., 2011; Horspool et al., 2008), major improvement in intention-to-treat outcomes might be realized by removal of strict barriers to continued enrollment in BMT. For example, the Drug Addiction Treatment Act of 2000 requires that patients in the United States receiving BMT also receive “appropriate counseling;” however, counseling of any type has not yet been shown to improve outcomes beyond BMT alone (Amato, Minozzi, Davoli, & Vecchi, 2011; Downey, Helmus, & Schuster, 2000; Fiellin et al., 2013; Fiellin et al., 2006; Ling et al., 2013) and, if too restrictive, may form a barrier to continuing BMT enrollment (Gryczynski et al., 2013). Although treatment providers may submit that abiding by program rules is necessary for treatment success, patients often hold divergent perspectives of treatment progress and find benefit in remaining in programs despite failure to achieve program-imposed criteria (Mitchell et al., 2011).
Continued enrollment in long-term BMT is predicated on patients having adapted to the logistical and provider-related issues that cause patients to discontinue treatment soon after initiation (Gryczynski et al., 2013). Hence, the perspectives involved in discontinuation of BMT after years of treatment are not expected to necessarily parallel those associated with early discontinuation, i.e. during the first 6 months of treatment. For example, patients with low interest in BMT cessation were found to be in BMT for a significantly longer duration than those with high interest in BMT cessation (Winstock et al., 2011). Further, in long-term BMT patients, high interest in cessation was found to be associated with low concern of relapse following cessation, and conversely patients’ most frequent concerns about BMT cessation were related to relapse and withdrawal (Winstock et al., 2011). This correlation between perceived relapse risk and desire for pharmacotherapy is also present in patients exiting short-term detoxification (Bailey et al., 2013). Although perceived high risk of relapse has not been linked to BMT adherence, if a causal relation is found, patient education of relapse risk following BMT discontinuation may be a particularly potent method of increasing adherence to long-term BMT.
The overall quality of evidence for patient perspectives associated with BMT discontinuation was found to be relatively poor. Only 2 studies quantitatively assessed patient perspectives associated with retention in BMT, and both had marked limitations. Notably, one study of patient perspectives was limited by combining perspectives across patients maintained on methadone with those maintained on buprenorphine, and another by the constraints imposed by its parent clinical trial, e.g. retention criteria and time course. Ideally, future studies of patient perspectives associated with discontinuation would include merits from both studies reviewed here. For example, a national survey that queried patients both before initiation of BMT and longitudinally tracked the evolution of their perspectives that lead to BMT discontinuation could inform the restructuring of current BMT programs to maximize retention. In light of our early analysis here, we predict that successful retention will be associated with high-perceived risk of relapse and low perceived conflict with program rules.
The association between conflict with program rules and discontinuation of BMT was also observed in our review of provider perspectives associated with BMT discontinuation. Feroni and colleagues (2005) found that nearly two-thirds of sampled French primary care physicians prescribing BMT indicated that they would discontinue BMT if they discovered a patient had several other buprenorphine prescribers, and half of these physicians indicated that they would discontinue BMT if they discovered a patient had been injecting buprenorphine. Although it is difficult to estimate actual outcomes for these providers’ patients, 22% of physicians in this sample indicated they had discontinued BMT in the past because of a difficult relationship with a patient – indicating that a significant number of patients had been discontinued secondary to conflicts with their provider. Considering that this result is without comparison to other physician samples, it raises the question of whether a group of physicians who were particularly supportive of long-term BMT might be more likely to continue BMT in the face of conflict with program mandates.
This question can be answered partially by Quaglio and colleagues (2010). These investigators found that the vast majority of Italian physicians prescribing buprenorphine in their sample supported long-term BMT, and this sampled group of physicians was less likely to view buprenorphine as a suitable short-term treatment. Thus, this sample represents providers who are less predisposed to discontinuing treatment. However, in this sample the most common cited disadvantage of buprenorphine was diversion, a behavior in conflict with program rules, indicating that patient adherence to program rules is a leading concern of providers, even in a group of physicians who strongly support BMT as a long-term treatment.
A tendency for providers to discontinue BMT might be related to whether physicians receive BMT training during residency. Suzuki and colleagues (2014) compared BMT perspectives of physicians in the United States who had received BMT training to those who had not received BMT training. Physicians who received BMT training were more likely to have prescribed buprenorphine, to believe opioid dependence is treatable, and to believe that buprenorphine is effective in treating opioid dependence. Further, physicians who received BMT training were less likely to indicate that treatment cessation via detoxification should be attempted before maintenance. Thus, BMT training might engender physician preference for more extended use of buprenorphine through maintenance therapy and a more favorable view of buprenorphine and opioid use disorder treatability. It is then plausible that increased BMT education during residency could lead to providers being less likely to discontinue BMT in the face of patient conflicts with program rules or other obstacles.
Similarly to the studies reviewed on patient perspectives associated with BMT cessation, few studies were identified that provided some quantitative insight into reasons providers discontinue BMT, and all had marked limitations. For example, the primary focus of the 3 reviewed studies of physician perspectives was not to explore reasons providers discontinue BMT; indeed, the measures discussed in this review were secondary and by no means exhaustive analyses of providers’ reasons for BMT discontinuation. These studies do, however, provide important initial insight into the provider perspectives that drive discontinuation of BMT. Ideally, future studies will focus on this topic specifically and track the patient outcomes associated with provider perspectives. Such investigations could inform the restructuring of current BMT programs to maximize retention. In light of our early analysis here, we predict that successful retention will be associated with BMT education during residency, relaxed BMT program rules, and methods that reduce diversion without preventing treatment, e.g. buprenorphine implants (Ling et al., 2010; Rosenthal et al., 2013).
Similarly to our review of perspectives associated with BMT discontinuation, our review of outcomes that followed BMT discontinuation is based on limited reports. Namely, we opted to include studies with a relatively short period of BMT (i.e., 14 days), and none of the reviewed studies included an appropriate control group. Studies with a 14-day BMT duration were included in order to expand the analysis to maximally capture both the outcomes that follow BMT as well as any possible predictors of outcome that may inform future work. However, BMT maintenance period could be particularly important given that longer durations of BMT have been linked with better outcomes (Dunn et al., 2011). Here, however, we found no association between BMT maintenance period duration and outcomes, possibly because other factors more strongly linked to outcomes also differed between studies, e.g. a high mean buprenorphine maintenance dose was reported in the study with the longest maintenance period (Weiss et al., 2011). Study designs were also not optimal. Ideally, patients would all be maintained on buprenorphine for a duration similar to typical clinical practice, i.e. months to years, and then patients would be randomized to either remain on buprenorphine or taper off using a double-blind, placebo-controlled design. Although, given the relatively certain poor outcomes that are likely to follow buprenorphine taper, it would be unethical to subject participants intending to remain on buprenorphine to a tapering procedure (Newman, 2009).
Regardless of the reasons patients discontinue BMT we found that most patients relapsed to illicit opioid use within 1 month following BMT cessation. Albeit reviewed studies were heterogeneous in both design and participant characteristics, mean relapse rate consistently surpassed 50%. Notably, high relapse rates were observed in young adults (Woody et al., 2008) as well as primary prescription opioid abusers (Sigmon et al., 2013; Weiss et al., 2010), populations often considered to be have better prognoses (Dreifuss et al., 2013; Moore et al., 2007; Sigmon, 2006). Given the association between perceived risk of relapse and interest in remaining in (Winstock et al., 2011) or initiating (Bailey et al., 2013) agonist medications, it will be important to remind all opioid-dependent, buprenorphine-maintained patients of the high probability of relapse before they consent to terminating treatment.
Nevertheless, a high mean rate of relapse does not preclude the existence of a small subgroup of patients who are able to remain abstinent following BMT cessation. In pursuit of this subgroup, candidate pre-buprenorphine-taper parameters predictive of abstinence 1 month following buprenorphine cessation were identified within reviewed studies. Taper duration (Sigmon et al., 2013), previous heroin use (Ling et al., 2009), and mean buprenorphine maintenance dose (Sigmon et al., 2013) were identified as potential predictors of outcome across studies; yet, only mean buprenorphine maintenance dose was found to be significantly associated with outcome with higher maintenance doses predicting higher rates of relapse. The association between buprenorphine maintenance dose and outcomes 1-month following discontinuation was also present in a naturalistic study of 6-months BMT followed by a 3-month taper (Kornør et al., 2006). This study met most criteria but was excluded due to reliance on self-reported opioid use rather than urinalysis. Similarly, higher maintenance doses of methadone have been found to be associated with more severe withdrawal symptoms (Glasper, Gossop, de Wet, Reed, & Bearn, 2008) as well as worse outcomes following methadone cessation (Ekhtiari, Dezfouli, Zamanian, Ghodousi, & Mokri, 2013). This association between pre-taper maintenance dose and outcome raises the possibility that a maintenance phase that gradually decreases dose over time, and results in a lower maintenance dose prior to initiating final taper to 0mg, may enhance abstinence rates at follow-up, a prediction that warrants future testing. The association between maintenance dose and outcome does not, however, indicate that lower buprenorphine maintenance doses should be enforced; higher buprenorphine doses are associated with both decreased rates of illicit opioid use during maintenance as well as increased rates of treatment retention (Hser et al., 2013; Mattick et al., 2008).
Buprenorphine maintenance dose may correspond particularly well with addiction severity within the context of clinical studies that employ flexible-dosing protocols – the protocol used by all of the buprenorphine discontinuation studies reviewed here. In this context study medication is monetarily free and titrated according to the patient response, a contrast to the many restrictions to high buprenorphine dose encountered in practice. Buprenorphine dose in the context of clinical studies with flexible-dosing protocols approximates unrestricted buprenorphine intake, i.e. the dose patients would take per day given minimal restrictions. For buprenorphine induction, medication is generally titrated until the patient is without opioid withdrawal or cravings. Those with more severe opioid addiction would in theory require a higher maintenance dose of buprenorphine. Indeed, a secondary analysis (Hillhouse et al., 2011) of Ling et al (2009) showed that within a flexible-dosing protocol patients titrated to various levels of buprenorphine (8, 16, and 24 mg per day). Notably, groups defined by these final maintenance doses differed in their drug use characteristics, with patients in the 24 mg group having more mean days of heroin use in the past 30 days, more injection drug use, and greater baseline craving and withdrawal severity before buprenorphine induction.
It is also notable that the 2 studies with the best outcomes included transition to maintenance with the opioid antagonist naltrexone after successful completion of buprenorphine taper (Breen et al., 2003; Sigmon et al., 2013), although transition was optional in one of these studies (Breen et al., 2003). Naltrexone has been shown to reduce opioid craving and opioid use (Larney et al., 2013; Syed & Keating, 2013), and may have aided these patients in extending abstinence from the end of the taper. However, orally dosed naltrexone was used in the reviewed studies, and although depot naltrexone (Syed & Keating, 2013) and naltrexone implants (Larney et al., 2013) have been shown to significantly reduce opioid use in meta-analyses, orally administered naltrexone has not (Minozzi et al., 2011), primarily due to poor treatment retention. In the latter review, naltrexone ingestion was highly variable in the 2 studies reviewed, ranging from 9% to 50% across different treatment arms. Therefore, the role of oral naltrexone in improving abstinence rates after buprenorphine detoxification is not known and warrants further study.
In conclusion, relapse rates were found to be high following BMT discontinuation, and fear of relapse was found to be a major reason patients remain in BMT (Bailey et al., 2013; Winstock et al., 2011). Most patients will express an interest in BMT cessation (Winstock et al., 2011) and many patients will discontinue BMT within 6 months (Gryczynski et al., 2013). Given the association between patient fear of relapse and interest in remaining on BMT, retention rates in BMT might be augmented by emphasizing the high risk of relapse to patients who indicate an interest in BMT cessation. If BMT cessation is necessary, a long taper period (Dunn et al., 2011; Sigmon et al., 2013) of at least 1 month that transitions into naltrexone treatment (Kornør, Waal, & Sandvik, 2007; Sigmon et al., 2013) may help minimize relapse risk. Although, the efficacy of naltrexone may depend on using the implantable (Larney et al., 2013) or depot (Syed & Keating, 2013) forms, as oral naltrexone has not been shown to reduce illicit opioid use (Minozzi et al., 2011). However, most patients will relapse as buprenorphine taper occurs, before transition to naltrexone can take place; hence, BMT cessation should be avoided if possible to achieve the greatest rates of opioid abstinence.
Highlights.
We reviewed outcomes 1 month following discontinuation of buprenorphine therapy.
Relapse rates ranged from 50% to 90%.
We reviewed patient and provider perspectives of buprenorphine treatment.
Fear of relapse and withdrawal were associated with treatment retention.
Patients were frequently forced to cease buprenorphine treatment by program policy.
Acknowledgments
Financial support for this study was provided by National Institutes of Health grants F30 DA035065 (PI: Brandon S. Bentzley), T32 GM008716 (PI: Perry V. Halushka), K12 HD055885 (PI: Kelly S. Barth) and R25 DA020537 (PI: Sudie E. Back). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institutes of Health had no role in the study design or the decision to publish the paper.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brandon S. Bentzley, Email: BSBentzley@gmail.com.
Kelly S. Barth, Email: stephen@musc.edu.
Sudie E. Back, Email: backs@musc.edu.
Sarah W. Book, Email: booksw@musc.edu.
References
- Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. Journal of Addictive Diseases. 1994;13(3):33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews (Online) 2013;2:CD003409. doi: 10.1002/14651858.CD003409.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews (Online) 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. The American Journal of Drug and Alcohol Abuse. 2004;30(1):129–153. doi: 10.1081/ADA-120029870. [DOI] [PubMed] [Google Scholar]
- Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine or methadone maintenance. Journal of Psychoactive Drugs. 2010;42(3):339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GL, Herman DS, Stein MD. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. Journal of Substance Abuse Treatment. 2013;45(3):302–305. doi: 10.1016/j.jsat.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Moore BA, Pantalon MV, Chawarski MC, Sullivan LE, O’Connor PG, et al. Patient satisfaction with primary care office-based buprenorphine/naloxone treatment. Journal of General Internal Medicine. 2007;22(2):242–245. doi: 10.1007/s11606-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, Pemberton DA. The effect of methadone prescribing in a clinic setting on the criminal activity of drug users. Scottish Medical Journal. 1996;41(6):173–175. doi: 10.1177/003693309604100606. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clinical Pharmacology and Therapeutics. 1988;43(1):72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Breen CL, Harris SJ, Lintzeris N, Mattick RP, Hawken L, Bell J, et al. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug and Alcohol Dependence. 2003;71(1):49–55. doi: 10.1016/S0376-8716(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug and Alcohol Dependence. 2003;70(2 Suppl):S39–47. doi: 10.1016/S0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug and Alcohol Dependence. 2008;94(1–3):151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction (Abingdon, England) 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction (Abingdon, England) 2005;100(6):820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Experimental and Clinical Psychopharmacology. 2000;8(2):176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug and Alcohol Dependence. 2013;131(1–2):112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug and Alcohol Dependence. 2011;119(1–2):1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JE, Netherland J, Gass J, Finkelstein R, Weiss L, BHIVES Collaborative. Patient perspectives on buprenorphine/naloxone treatment in the context of HIV care. Journal of Acquired Immune Deficiency Syndromes. 2011;56(Suppl 1):S46–53. doi: 10.1097/QAI.0b013e3182097561. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Dezfouli A, Zamanian B, Ghodousi A, Mokri A. Treatment outcome predictors in flexible dose-duration methadone detoxification program. Archives of Iranian Medicine. 2013;16(10):599–601. [PubMed] [Google Scholar]
- Feroni I, Peretti-Watel P, Paraponaris A, Masut A, Ronfle E, Mabriez JC, Obadia Y. French general practitioners’ attitudes and prescription patterns toward buprenorphine maintenance treatment: does doctor shopping reflect buprenorphine misuse? Journal of Addictive Diseases. 2005;24(3):7–22. doi: 10.1300/J069v24n03_02. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O’Connor PG, Schottenfeld RS. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. The American Journal of Medicine. 2013;126(1):74, e11–7. doi: 10.1016/j.amjmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, Schottenfeld RS. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. The New England Journal of Medicine. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Giacomuzzi SM, Riemer Y, Ertl M, Kemmler G, Rössler H, Hinterhuber H, Kurz M. Buprenorphine versus methadone maintenance treatment in an ambulant setting: a health-related quality of life assessment. Addiction (Abingdon, England) 2003;98(5):693–702. doi: 10.1046/j.1360-0443.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- Glasper A, Gossop M, de Wet C, Reed L, Bearn J. Influence of the dose on the severity of opiate withdrawal symptoms during methadone detoxification. Pharmacology. 2008;81(2):92–96. doi: 10.1159/000109982. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database of Systematic Reviews (Online) 2011;(8):CD004145. doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: Patients’ reasons for cessation of care. Journal of Substance Abuse Treatment. 2013 doi: 10.1016/j.jsat.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A, Lert F, Brodeur JM, Richard L. Buprenorphine substitution treatment in France: drug users’ views of the doctor-user relationship. Social Science & Medicine (1982) 2007;64(12):2578–2593. doi: 10.1016/j.socscimed.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Ling W. Predictors of outcome after short-term stabilization with buprenorphine. Journal of Substance Abuse Treatment. 2013;44(3):336–342. doi: 10.1016/j.jsat.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Doraimani G, Thomas C, Hasson A, Ling W. Participant characteristics and buprenorphine dose. The American Journal of Drug and Alcohol Abuse. 2011;37(5):453–459. doi: 10.3109/00952990.2011.596974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horspool MJ, Seivewright N, Armitage CJ, Mathers N. Post-treatment outcomes of buprenorphine detoxification in community settings: a systematic review. European Addiction Research. 2008;14(4):179–185. doi: 10.1159/000141641. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction (Abingdon, England) 2013 doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, O’Keeffe C. From morphine clinics to buprenorphine: regulating opioid agonist treatment of addiction in the United States. Drug and Alcohol Dependence. 2003;70(2 Suppl):S3–11. doi: 10.1016/S0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Archives of General Psychiatry. 1978;35(4):501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues in Clinical Neuroscience. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. Jama. 2008;300(19):2303–2305. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- Kornør H, Waal H, Ali RL. Abstinence-orientated buprenorphine replacement therapy for young adults in out-patient counselling. Drug and Alcohol Review. 2006;25(2):123–130. doi: 10.1080/09595230500537209. [DOI] [PubMed] [Google Scholar]
- Kornør H, Waal H, Sandvik L. Time-limited buprenorphine replacement therapy for opioid dependence: 2-year follow-up outcomes in relation to programme completion and current agonist therapy status. Drug and Alcohol Review. 2007;26(2):135–141. doi: 10.1080/09595230601146603. [DOI] [PubMed] [Google Scholar]
- Larney S, Gowing L, Mattick RP, Farrell M, Hall W, Degenhardt L. A systematic review and meta-analysis of naltrexone implants for the treatment of opioid dependence. Drug and Alcohol Review. 2013 doi: 10.1111/dar.12095. [DOI] [PubMed] [Google Scholar]
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey GL, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. Jama. 2010;304(14):1576–1583. doi: 10.1001/jama.2010.1427. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction (Abingdon, England) 2013;108(10):1788–1798. doi: 10.1111/add.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction (Abingdon, England) 2009;104(2):256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews (Online) 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews (Online) 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews (Online) 2011;(4):CD001333. doi: 10.1002/14651858.CD001333.pub4. [DOI] [PubMed] [Google Scholar]
- Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, Jaffe JH. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug and Alcohol Dependence. 2013;128(3):222–229. doi: 10.1016/j.drugalcdep.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Morioka R, Reisinger HS, Peterson JA, Kelly SM, Agar MH, et al. Redefining retention: recovery from the patient’s perspective. Journal of Psychoactive Drugs. 2011;43(2):99–107. doi: 10.1080/02791072.2011.587392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. Journal of General Internal Medicine. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. Jama. 1998;280(22):1936–1943. [PubMed] [Google Scholar]
- Newman RG. Comparing buprenorphine “tapers--”to what end? Addiction (Abingdon, England) 2009;104(8):1428–9. doi: 10.1111/j.1360-0443.2009.02612.x. author reply 1429–30. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. Journal of Addiction Medicine. 2013;7(1):33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, et al. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Affairs (Project Hope) 2013;32(8):1462–1469. doi: 10.1377/hlthaff.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Guh DP, Sun H, Oviedo-Joekes E, Brissette S, Marsh DC, et al. Health related quality of life trajectories of patients in opioid substitution treatment. Drug and Alcohol Dependence. 2011;118(2–3):259–264. doi: 10.1016/j.drugalcdep.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Sun H, Evans E, Marsh DC, Anglin MD, Hser YI, Anis AH. Defining dosing pattern characteristics of successful tapers following methadone maintenance treatment: results from a population-based retrospective cohort study. Addiction (Abingdon, England) 2012;107(9):1621–1629. doi: 10.1111/j.1360-0443.2012.03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior--outcomes of a randomized trial. Drug and Alcohol Dependence. 2013;133(2):376–382. doi: 10.1016/j.drugalcdep.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. The American Journal of Drug and Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Pattaro C, Gerra G, Mezzelani P, Montanari L, Jarlais DCD, Lugoboni F. Buprenorphine in maintenance treatment: experience among Italian physicians in drug addiction centers. American Journal on Addictions. 2010;19(3):222–230. doi: 10.1111/j.1521-0391.2010.00040.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal RN, Ling W, Casadonte P, Vocci F, Bailey GL, Kampman K, et al. Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction (Abingdon, England) 2013 doi: 10.1111/add.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Medication-Assisted Treatment for Opioid Addiction: 2010 State Profiles. Substance Abuse and Mental Health Services Administration; 2011. pp. 1–163. [PubMed] [Google Scholar]
- Sigmon SC. Characterizing the emerging population of prescription opioid abusers. American Journal on Addictions. 2006;15(3):208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, Heil SH, et al. A Randomized, Double-blind Evaluation of Buprenorphine Taper Duration in Primary Prescription Opioid Abusers. JAMA Psychiatry (Chicago, Ill) 2013 doi: 10.1001/jamapsychiatry.2013.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam GA, Warden D, Minhajuddin A, Fishman MJ, Stitzer ML, Adinoff B, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(11):1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Connery HS, Ellison TV, Renner JA. Preliminary survey of office-based opioid treatment practices and attitudes among psychiatrists never receiving buprenorphine training to those who received training during residency. American Journal on Addictions. 2014 doi: 10.1111/j.1521-0391.2014.12143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YY, Keating GM. Extended-release intramuscular naltrexone (VIVITROL®): a review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs. 2013;27(10):851–861. doi: 10.1007/s40263-013-0110-x. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatric Services (Washington, DC) 2014;65(2):158–170. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- Tkacz J, Volpicelli J, Un H, Ruetsch C. Relationship Between Buprenorphine Adherence and Health Service Utilization and Costs Among Opioid Dependent Patients. Journal of Substance Abuse Treatment. 2013 doi: 10.1016/j.jsat.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Smith MT, Mintzer MZ, Campbell CM, Strain EC. A double-blind, within subject comparison of spontaneous opioid withdrawal from buprenorphine versus morphine. The Journal of Pharmacology and Experimental Therapeutics. 2013 doi: 10.1124/jpet.113.209478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. The Journal of Pharmacology and Experimental Therapeutics. 1995;274(1):361–372. [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clinical Pharmacology and Therapeutics. 1994;55(5):569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemporary Clinical Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, McCance-Katz EF. Course and treatment of buprenorphine/naloxone withdrawal: an analysis of case reports. American Journal on Addictions. 2012;21(5):401–403. doi: 10.1111/j.1521-0391.2012.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Jagsch R, Ebner N, Thau K, Fischer G. Quality of life in patients receiving opioid maintenance therapy. A comparative study of slow-release morphine versus methadone treatment. European Addiction Research. 2008;14(2):99–105. doi: 10.1159/000113724. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lintzeris N, Lea T. “Should I stay or should I go?” Coming off methadone and buprenorphine treatment. The International Journal on Drug Policy. 2011;22(1):77–81. doi: 10.1016/j.drugpo.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. Jama. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]