Abstract
Cross-fostering studies suggest cocaine-induced deficits in maternal behavior could be associated with altered behavior of offspring following prenatal cocaine-exposure. Neonatal vocalizations are an important offspring cue facilitating early interactions between dam and rodent pup offspring and have been shown to be altered following prenatal cocaine-exposure. It is unclear how variations in acoustic parameters of USVs impact maternal behavior and the mechanism(s) underlying these processes. The present study examined differences in cocaine-exposed and control rodent dam maternal preference of cocaine-exposed or untreated pups in a dual choice apparatus. Relationship of preference-like behavior with pup USVs and dam oxytocin expression was explored. Gestational cocaine-exposure interfered with preference-like behavior of dams on postpartum day 1 with cocaine-exposure associated with decreased time spent on the cocaine-exposed pup side compared to the control pup side, and decreases in preference-like behavior associated in part with decreased number of USVs being emitted by cocaine-exposed pups. On postpartum day 5, decreased oxytocin expression in the medial preoptic area was associated with altered preference-like behavior in cocaine-exposed dams, including frequency and latency to touch/sniff pups. Results indicate cocaine’s effects on the mother-infant relationship is likely synergistic, in that cocaine influences mother and offspring both independently and concertedly and that variations within pup vocalizations and the oxytocin system may be potential mechanism(s) underlying this synergistic relationship during the postpartum period.
Keywords: maternal behavior, vocalization, cocaine, oxytocin, MPOA
1. Introduction
Parental substance abuse, cocaine in particular, is associated with child neglect and abuse [1;2]. Parallel to studies on human cocaine abuse, animal models indicate gestational cocaine-exposure alters maternal behavior with the greatest deficits occurring during the early postpartum period (postpartum days (PPDs) 1–5) [3–7]. Cross-fostering studies suggest that cocaine-induced deficits in maternal behavior could be in part a consequence of altered behavior of offspring following prenatal cocaine-exposure (PCE) [3]. Yet there is limited data examining the impact of PCE changes in offspring behavior on the maternal environment.
Cocaine-using mothers have previously been shown to differentially respond to changes in the acoustic signal of cries [8], with studies also showing PCE can lead to variations in the acoustic properties of neonatal cries [9–11], suggested to contribute to altered maternal-infant interactions. Parallel to human infant cries, neonatal vocalizations are an important offspring cue facilitating early interactions between dam and rodent pup offspring [12–14], with sustained high-rate ultrasonic vocalizations (USVs) emitted by pups being the most effective for eliciting retrieval from dams [15–18]. Studies also suggest that frequency modulation [19;20] and amplitude of USVs [21] may be important acoustic cues for maternal approach and response.
Most studies examining USV differences following prenatal drug exposure have focused solely on the rate of USV emissions, warranting the need for extending USV analysis to other acoustic measures, and even fewer studies have explored how variations in acoustic characteristics of vocalizations might affect the maternal environment. Prenatal cocaine is associated with altered fundamental frequency and rate of vocalizations during the early postnatal period in rodent offspring [22–24]. Since PCE is associated with increased maternal neglect [3], understanding how variations in pup USVs impact the maternal environment could help to further elucidate the mechanism(s) underlying maternal neglect. To our knowledge, no study has determined the impact of individual pup vocalizations on early postpartum maternal behavior following PCE.
The present study examined differences in maternal preference-like behavior of rodent dams (cocaine-exposed and controls) by observing the amount of time spent with a neonatal pup when given the choice between a cocaine-exposed or an untreated male pup. It also determined if dam preference-like behavior was correlated with variations in pup USVs on each respective test day to elucidate to what extent a dam’s preference-like behavior is influenced by variations in USV characteristics such as call rate, fundamental frequency, and amplitude. Additionally, dam preference-like behavior and relationship with oxytocin (OT) expression in the medial preoptic area (MPOA) was explored, as OT has been shown to play an essential role in onset and maintenance of early maternal behavior in both human [25;26] and rodent models [27–29]. Additionally, OT dynamics has been shown to be altered in rat dams gestationally-exposed to cocaine [30–32], including decreased expression in the MPOA [33;34], a region consistently shown to be critical for maternal behavior [35–39]. However, the mechanisms underlying these relationships are still unclear with studies suggesting cocaine can directly alter OT dynamics [33;40;41] and recent studies suggesting maternal-infant interactions may regulate OT dynamics across the postpartum period [3;42–44].
2. Methods
2.1 Animals
Following a one-week habituation period, virgin female (200–240 grams) Sprague-Dawley rats (Charles River, Raleigh, NC) were placed with males on a breeding rack until a sperm plug was found, which was designated as gestation day (GD) zero. Subjects were randomly assigned to one of three treatment or control groups or one of two pup-provider groups and singly housed and maintained on a reversed 12:12 reverse light cycle (lights off at 0900 hours) for seven days. They were then transferred to a room with a regular light cycle (lights on at 0700 hours) for the remainder of the experiment, a procedure that generally results in the majority of dams delivering their litters during daylight hours [45]. All procedures were conducted under federal and institutional animal care and use committee guidelines for humane treatment of laboratory subjects.
2.2. Treatment
Treatment groups included: chronic cocaine (CC), and two control groups: chronic saline (CS) and untreated (UN) dams. CC and CS dams received subcutaneous injections on alternating flanks of 15 mg/kg cocaine HCL (dose calculated as the free base, Sigma Chemical Company, St. Louis, MO) dissolved in 0.9% normal saline (total volume 2 ml/kg), or the same volume of normal saline (0.9%), respectively. Injections were delivered twice daily (at approximately 0800 and 1600 hours) throughout gestation beginning on GD 1 and continuing until the day before delivery (GD 20) with the CS dams serving as controls for injection stress and early food consumption rates. UN dams were weighed and handled daily, but received no drug treatment. CC and UN dams had free access to water and food (rat chow), while CS-treated dams were yoke-fed over the first week to match maximum consumption rates of CC dams to control for the anorectic effects of cocaine, as previously described [4]. A second group of dams were bred simultaneously and served as pup-providers for the test dams. Pup-providers either received no treatment (UN dam), other than weighing and handling, or they were treated with cocaine as described above. The pup-providers reared their natural culled litters (ten pups, six males, four females) for behavioral testing. Each test dam had two pup-providers (one UN and one CC) assigned to her for postpartum testing so each dam would be tested with unfamiliar pups. No pup from a pup-provider litter was used twice during preference testing.
2.3 Maternal Preference Testing
2.3.1. Preference Apparatus
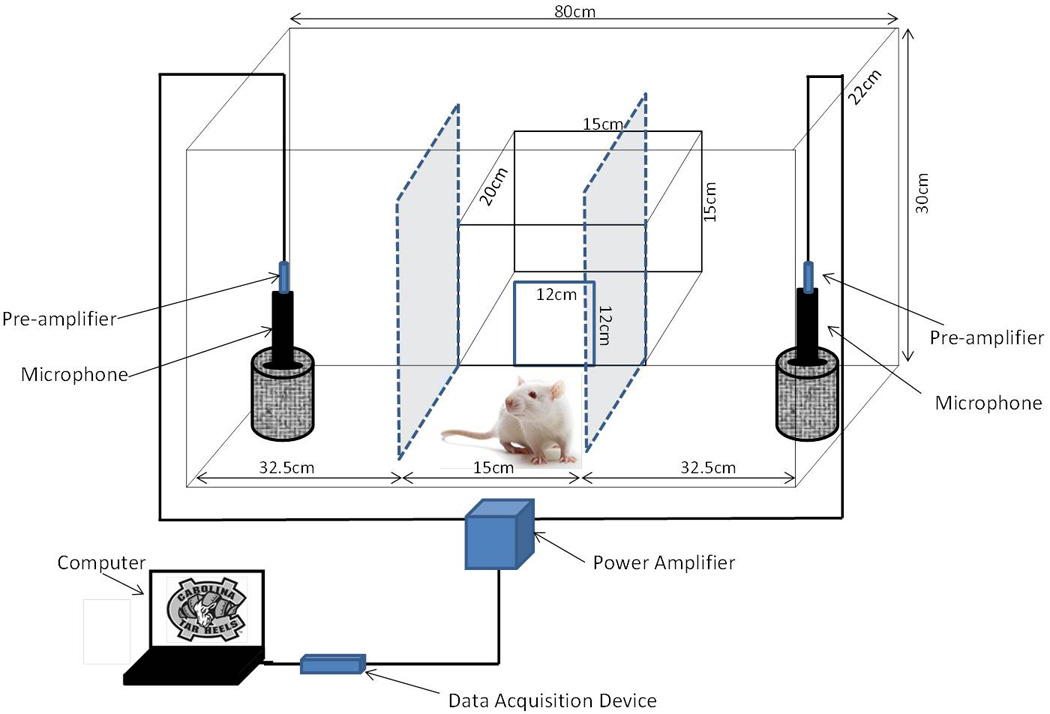
The maternal preference apparatus (See Figure 1) was modified from a social test apparatus [46;47], and consisted of a rectangular Plexiglas box (80 cm × 22 cm × 30 cm). On either side of the apparatus were partial dividing walls that were 32.5 cm from both left and right ends of the box, thus dividing the box into a left, right, and center (no choice) chamber. Attached to the center of the box was a start box (20 cm × 15 cm × 15 cm) where the dam was placed immediately prior to the start of her test session. The start box had a guillotine door separating it from the center chamber, which was raised to permit entry into the center chamber of the testing box and promptly closed after dam entry into the apparatus. In each side chamber there was a mesh cage attached to the wall for placement of the stimulus pup. The mesh cage wires prevented the dam from touching the pup. Above each mesh cage, microphones were secured for recording rodent pup ultrasonic vocalizations during preference testing. USVs were recorded with model CM16/CMPA40-5V microphones (Avisoft Bioacoustics; Berlin, Germany) connected to a desktop computer through a National Instruments instrumentation recorder (PCI-6132), as described previously [22], sampled at a rate of 1 MS/s (1 million samples per second) at 14 bit. Microphones were calibrated prior to each use as previously described [48]. National Instruments software (LabView, 2009) began the acquisition of USVs at the beginning and terminated at the end of the test session.
Figure 1. Diagram of Maternal Preference Apparatus.
2.3.2. Preference Testing Procedure
On GDs 16 and 17, all dams were shuttled from the animal housing to the testing room to habituate to travel and testing room. On GD 19, each test dam (not pup-providers) was habituated to the preference apparatus for 15 minutes. On the day of delivery, after all pups were born, cleaned, and had a visible milk band (designated as PPD 1), a triad consisting of a test dam with her litter and two pup-provider litters (one CC and one UN within 24 hours of the test dam) were brought to the test room. A 10 minute rest period was allowed following travel. After the rest period, pups were removed from the PPD 1 test dam’s home cage, and test dam was moved in her home cage to a separate room where behavioral testing would take place, isolated from her pups for 20 minutes. Pups from the test dam litter were placed on a heating pad together and were culled during this isolation period. At this same time, a CC and UN male pup from a CC and UN pup-provider litter respectively was removed from the pup-provider litter and isolated on a heat pad in two separate secondary containers. Pups from pup-provider litters were matched by age and weight to control for differences in heat loss and stages of brain development. During the first ten minutes of isolation, the test dam was placed in the start box and allowed ten minutes of exploration of the box which was videotaped and subsequently analyzed for baseline measures. The dam was then returned to her isolated cage for the remaining 10 minutes of the 20 minute isolation period. At the end of the dam’s 20 minute isolation period, one isolated CC and UN male pup (from pup-providers) were placed and secured on opposite sides of the testing apparatus in the wire mesh cage. The side of pup placement varied between test dams and across test days. Dams were placed in the start box, the guillotine door was opened, and then closed behind the dam once she left. All dams left the start box within 5 minutes. Preference-like behavior was videotaped for ten minutes and stimulus pup vocalizations recorded for later analysis and correlation with preference. After preference testing, the test dam was returned to her home cage along with her culled natural litter. Both the CC and UN male pups from pup-provider litters were marked with a sharpie, with the pup used on PPD 1 differentially designated so as not to be reused in future testing, and returned to the pup-provider litter. Test dam and litter weight (before and after culling) were recorded, along with the weight of the two stimulus pups from pup-provider litters prior to testing. Additionally, the temperature of each pup was recorded before testing using a laser thermometer held a half inch from the rump of the pup for four seconds. The testing triad, including all dam’s with natural litters, were returned to the animal colony room until the next testing day. Natural litters remained with dams when testing was not occurring.
On PPDs 3 and 5 the same test dams and pup-provider litters were shuttled back to the testing room for preference testing as described above, the only exception being that dams did not undergo baseline testing during their 20 min isolation period. Procedures for CC and UN pup-providers were the same and placement side for pup treatment group was alternated on across test days. Dam preference-like behavior and pup vocalizations were recorded as before for later analysis.
2.3.3. Maternal Preference-like Behavioral Scoring
Video recordings of maternal preference testing trials (baseline, PPDs 1, 3, and 5) were scored by two independent observers (blind to stimulus pup side) for frequency, duration and latency of behaviors displayed by dams. All behavioral scoring was reconciled to inter and intra-reliability within 95–100% concurrence for frequency and latency and 80% or better for duration of behaviors. The following behaviors were scored and were the focus of preference analysis: in center (begins when dam leaves the start chamber and enters the center chamber, i.e. all four feet cross the threshold), in chamber left or right (scored differentially when dam enters (up to one rear leg but not including tail) one of the chamber sides where the pup cage is (chamber left or right was later defined as CC or UN pup side), touch/sniff (T/S) pup stimulus cage in left or right chamber (scored when dam touches or sniffs either pup cage on the left or right side, respectively). First choice of stimulus alley by each dam was scored as well.
2.4. Rodent Pup Ultrasonic Vocalization Analysis
The acoustic characteristics of rat pup USVs were determined from sound spectrographic analyses using previously established methods in analyses of rodent and human infant distress vocalizations [22;49]. Analysis of acoustic characteristics was conducted with the aid of Avisoft-SASLab Pro by experienced researchers blind to treatment group membership and experimental conditions. The software program provides a wide range of digitally-based acoustic analyses and visual displays, including (1) sound spectrograms of user-selected segments of the vocal signal, (2) frequency by amplitude power spectra resulting from Fast Fourier Transforms (FFT) of the acoustic signal at user-selected points in the spectrogram, (3) displays of frequency and amplitude at cursor placement in all acoustic analysis procedures, including power spectra and (4) time intervals between user-juxtaposed cursors. Software routines also allow summation, means and summary statistics of measures of fundamental frequency (basic pitch in Hz), amplitude (dB) and durations of voiced components (sec) across user-selected points of time. Temporal measures including the number of USVs emitted, mean duration, and mean interval between USVs were collected along with spectral measures including the mean frequency and amplitude at the a) maximum amplitude, b) minimum frequency, and c) the maximum frequency within a USV. All USVs emitted during the first minute of testing (the first minute the dam was in the apparatus) were analyzed, as pilot data showed the most robust differences in USVs during the first minute of testing followed by a steady decline in rate of USVs over the remaining testing period in both CC and UN pups. Standard deviation of the fundamental frequency and amplitude of USVs were calculated as previously described [49].
2.5. Tissue Fixation and Sectioning
Within two hours following PPD 5 behavior testing, dams were anesthetized with pentobarbital (60 mg/kg, 1 ml/kg, i.p.) and transcardially perfused with 0.1M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were placed in 4% paraformaldehyde in PBS for twenty-four hours at 4°C before being rinsed in PBS. Whole brains were sectioned serially at 50µm on a Leica SM200R sliding frozen microtome. Serial free-floating sections were stored in cryoprotectant at −20°C until immunohistochemistry was performed.
2.6. OT Immunohistochemistry
Using a random sampling method half of the dams in the CC and UN groups were selected for OT staining. The other half was used for other immunohistochemistry staining (unpublished). For OT staining, sections containing the MPOA were selected and 3,3’-Diaminobenzidine (DAB) visualized immunohistochemistry was performed using standardized methods previously published [50]. The MPOA was identified according to a rat brain atlas [51]. All analyzed sections were stained with cresyl violet after immunohistochemical processing and prior to ethanol dehydration and cover slipping to facilitate anatomical land mark identification. For a section to be considered for cell counts for either treatment or control groups it had to contain all of the following anatomical references: the corpus callosum, fornix, anterior commissure and optic chiasm ensuring all sections analyzed were with −0.5 and −1.20 mm to the bregma suture. Briefly, free floating sections were washed in PBS, treated with 1% hydrogen peroxide, incubated in blocking solution (10% rabbit serum in 0.1% Triton-X, 0.1M PBS), and incubated for 48 hours in primary antibody (1:10,000 rabbit anti-oxytocin, Immunostar, Hudson WI). Tissue was then rinsed in PBS, incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), followed by avidin-biotin complex kit (Vector Laboratories, Burlingame, CA), and DAB chromophore attachment (Polysciences, Warrington, PA). Sections were mounted onto Fisherbrand Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) and cover slipped with permount.
2.7. Microscopy and Analysis of OT
Single-labeled DAB-positive cell bodies were visualized with a BX53 Olympus bright-field light microscope (Olympus Corporation, Shinjuku, Japan) with 20X air objectives. Anatomic landmarks, i.e. the anterior commissure, were employed to ensure consistent imaging across tissue sections. Images were acquired from the left and right hemisphere for at least four sections of tissue. Image analysis was adapted from previously described methods [52]. Briefly, DAB-positive OT cell bodies were assessed using the plug-in for nucleus detection in Image J v1.41 (National Institutes of Health, Bethesda MD). Counts generated by the nucleus detection plug-in were found to be consistent using a manual counting protocol.
2.8. Statistical Analysis
2.8.1. Gestational, Litter, and Pup Stimulus Comparisons
A one-way Analysis of Variance (ANOVA) for treatment group was used to assess gestational and postpartum weight gain of test and pup-provider dams and litter weight at birth, total number of offspring, percent of offspring being males, culled litter weight, and culled litter weight gain (PNDs 1–5), with p<0.05 being significant. To assess that stimuli used for all test dams were the same, two-way ANOVAs (for stimulus pup group by test dam group) were ran on each test day for pup weight, temperature of pup at the start of testing, and pup temperature.
2.8.2. Rodent Dam Preference-like behavior
Rodent preference-like behavior on each test day was adjusted for baseline measures, as some dams showed a clear preference for a side at the time of baseline testing. Baseline adjustments were made by subtracting the duration of time dams spent engaging in a behavior on a particular side during baseline testing from the duration of time dams spent engaging in that behavior on the respective side during testing. Duration, but not frequency and latency, was analyzed for preference-like behavior to decrease multiple comparisons and based on our a priori hypothesis that total duration of time dams spent on the CC pups side would be decreased in CC dams, indicating decreased preference-like behavior for CC pups. Rodent dam adjusted behavior was analyzed on each test day separately, with a two-way ANOVA (dam group by pup group side) for the duration of time dams spent on a respective side (not including time spent touch/sniffing) and for the duration of time dams spent on the CC or UN pup side in touch/sniff behavior. The duration of time spent in the center compartment was tested with a one-way (treatment group) ANOVA. Following a significant dam group or interaction effect (p<0.05), Tukey HSD post hoc tests were used to make between group comparisons. Fisher’s exact tests were used to compare first choice entry of dams on each day.
2.8.3. Rodent Offspring Stimulus- Vocalizations
A multiple analyses of variance (MANOVA) was used to assess exposure group (CC or UN pup) differences on selected cry variables during the first minute of preference testing (the first minute after the dam left the start box). A MANOVA allowed for control of multiple comparisons on acoustic measures of interest. Acoustic measures were grouped into rhythm of cry sound (total number, average duration and interval between USVs), spectral frequency acoustic measures (average peak frequency, average minimum frequency, average frequency at loudest portion of USV, standard deviation of frequency), and spectral amplitude acoustic measures (average amplitude at peak frequency, average amplitude at minimum frequency, average amplitude at loudest portion of USVs, and standard deviation of amplitude).
2.8.4. Maternal Preference-like Behavior Relationship to Pup Vocalizations
Pearson Correlations were used to assess whether maternal preference-like behavior was related to offspring vocalizations during testing. We examined the relationship between total time spent on a pup side (adjusted for baseline preference) and mean vocalization measures emitted by the pup on that respective side. We also assessed correlations between the duration of time dams spent engaged in T/S behavior on a pup side (adjusted for baseline preference) and mean vocalization measures emitted by the pup on that respective side. Mean vocalization measures assessed included total number of USVs, average peak frequency, standard deviation of frequency, average amplitude at the peak frequency, and standard deviation of amplitude of USVs emitted by pups on each respective test day. These measures were chosen as a priori based on recent findings suggesting CC-exposed offspring emit fewer USVs on PND 1 [23], and frequency modulation [19;20] and amplitude of USVs [21] may be important cues for maternal approach and response. To account for multiple comparisons (dam preference-like behavior correlated with five USV measures) we used the bonferroni correction method.
2.8.5. OT Expression and Relationship with Pup Preference-like Behavior on PPD5
A two-tailed t-test was used to examine the effect of treatment (CC and UN) on number of DAB-positive OT-expressing cell bodies in the MPOA. Pearson Correlations were used to assess whether decreased expression in OT in CC dams was related to their preference-like behavior on PPD 5. We examined the relationship between average number of OT-positive cells and frequency, latency, and duration of time engaged in T/S behavior (uncorrected data). In addition to duration, latency and frequency were also examined as OT in the MPOA has been suggested to play a central role in onset of maternal behavior [28;37].
3. Results
3.1. Gestational and Litter Comparisons
There were no significant treatment group differences in test dam gestational or postpartum weight gain. There was no difference among test or pup-provider dams in the number of pups in their litter, weight of birth litters, gender ratio, or weight gain of culled litters during the postpartum testing period.
3.2. Rodent Dam Preference-like Behavior
There were no differences in weight, temperature before testing, or temperature change during testing on any PPD of testing between UN and CC stimulus pups used for any test dam group. On PPD 1, a significant dam group by pup side interaction was observed in the duration of time dams spent on a pup side (F=4.210, p<0.05, See Figure 2A). Specifically, while UN and CS dams did not differ in the amount of time they spent on the UN or CC pup side we found CC dams spent more time on the UN pup side than they spent on the CC pup side (p<0.05). CC dams also spent less time on the CC pup side than CS (p<0.05) and UN (p<0.05) dams spent on the CC pup side. There were no significant group differences in the duration of time dams spent touching/sniffing pup cages or in the center compartment.
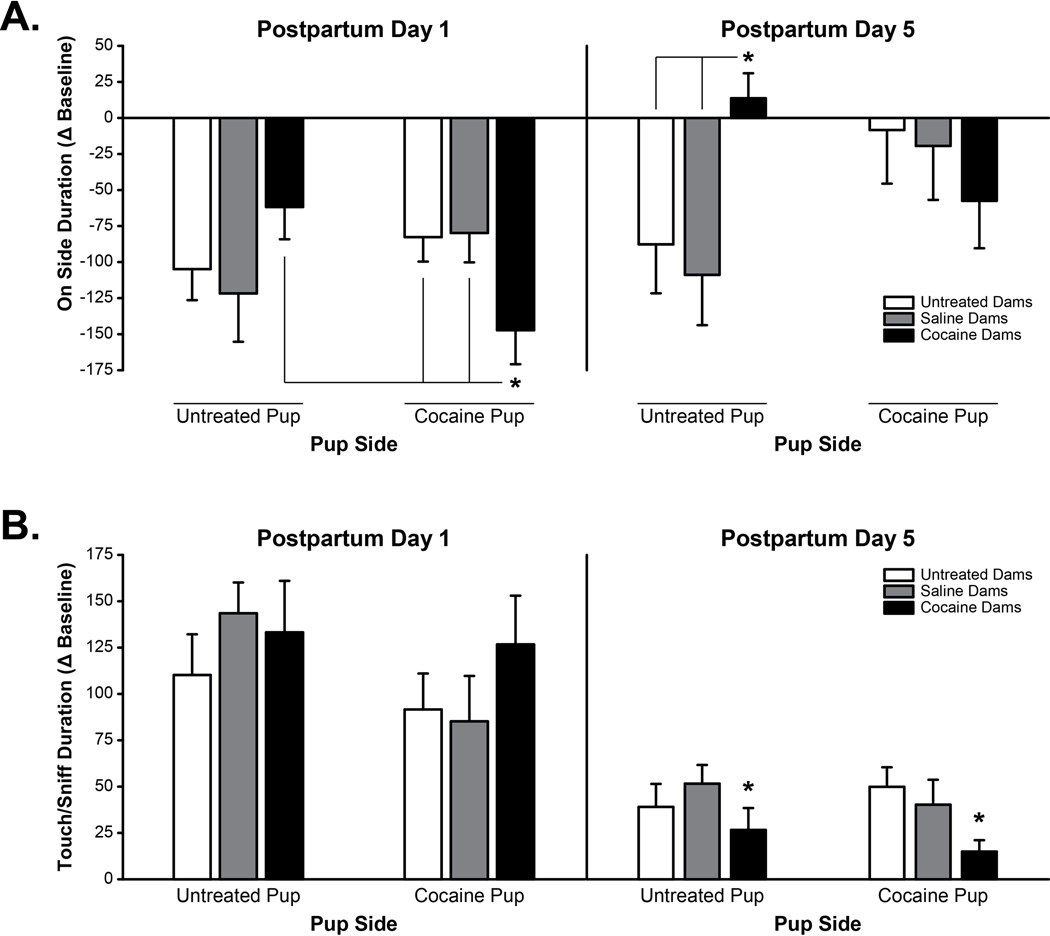
Figure 2. Maternal Preference-Like Behavior in UN, CS, and CC dams.
(A) Duration spent on each pup side during the pup preference task. On PPD 1, CC dams spent less time on the CC pup side than UN and CS dams spent on the CC pup side, and compared to amount of time CC dams spent on the UN pup side. On PPD 5, CC dams spent more time on the UN pup side compared to UN and CS dams. (B) Duration spent touching/sniffing pups during the pup preference task. On PPD 1, there was no difference in amount of time dams spent engaged in touch/sniff behavior; however, on PPD 5, CC dams engaged in touch/sniff behavior less (regardless of pup side) compared to UN and CS dams. N=13 UN dams, 12 CS dams, 12 CC dams, * p<0.05
There were no statistically significant group differences observed in any pup preference-like behaviors on PPD 3. A significant dam group by pup side interaction was however observed on PPD 5 in the duration of time dams spent on a pup associated side (F=3.615, p<0.05, See Figure 2A). Specifically, CC dams spent more time on the UN pup side than did CS (p<0.01) and UN dams (p<0.05). There was also a main dam group effect on duration of time spent engaged in T/S behavior (F=3.228, p<0.05, See Figure 2B). Specifically, CC dams touched/sniffed both UN and CC pup cages for a shorter duration than did UN and CS dams (p<0.05). There were no group differences in the duration of time dams spent in the center compartment and there were no significant differences in the first choice of alley on each postpartum day or in latency to enter the alley.
3.3. Rodent Offspring Vocalizing Behavior
Group differences in USVs were only observed on PPD 1. CC pups had significant differences in rhythm of USVs (MANOVA, Wilks’s Lambda p<0.01, See Figure 3A,B), specifically CC pups emitted fewer USVs (F=10.564, p<0.005) with an increased average interval between USVs (F=5.971, p<0.05). There was no group difference in the average duration of USVs or in spectral frequency or amplitude measures.
Figure 3. Variations in Stimulus Pup USVs and Relationship with Maternal Preference-Like behavior.
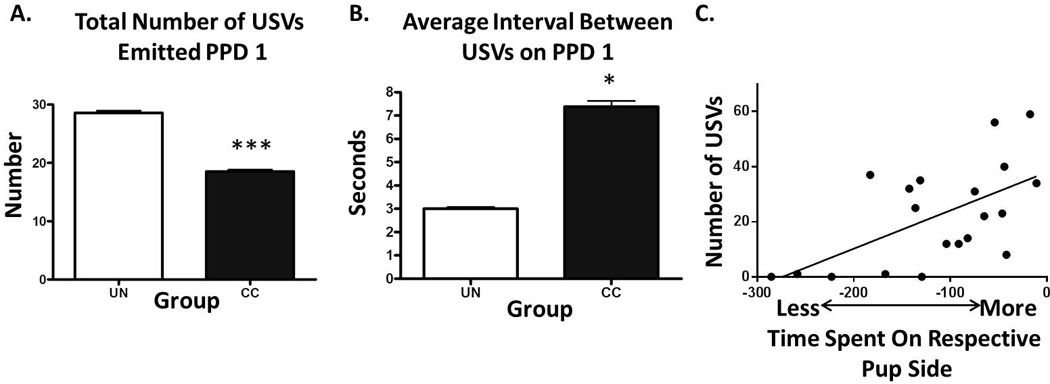
On PPD 1, CC stimulus pups (A) emitted fewer USVs with (B) a greater interval between USVs. On PPD 1, the amount of time CC dams spent on a pup side (not engaged in touch/sniff behavior) was positively associated with (C) total number of USVs pups emitted. * p<0.05, *** p<0.005
3.4. Dam Preference Correlations with Stimulus Offspring Vocalizing Behavior
On PPD 1, we found a positive correlation between time spent on a side by CC dams (but not in T/S) where stimulus pups emitted a greater number of USVs on that respective side (r=0.590, p<0.006, See Figure 3C), however CS and UN dams did not show a significant relationship on these measures. No other significant associations were observed between dam preference-like behavior and variations in pup USVs on PPD 1. There were no significant correlations on PPD 3 or 5 with USV measures assessed.
3.5. OT-expression in the MPOA and Correlations with Dam Preference-Like Behavior
CC dams had significantly fewer OT-labeled cell bodies in the MPOA compared to UN dams on PPD 5 (t(10)=3.368, p<0.01, See Figure 4A–C). Additionally, an OT and behavior relationship was highlighted by a significant relationship between number of OT-positive cell bodies and preference-like behavior in CC dams (Figure 4D). Specifically, within CC dams, greater OT expression in the MPOA was associated with greater engagement in T/S behavior [frequency of T/S on CC (r=0.84, p<0.05) and UN pup side (r=0.81, p<0.05) and latency to T/S on CC pup side (r=−0.95, p<0.005)]. No significant relationship was observed within UN dams between OT expression and preference-like behavior.
Figure 4. Average OT-expressing cells in MPOA and relationship with maternal preference-like behavior on PPD 5.
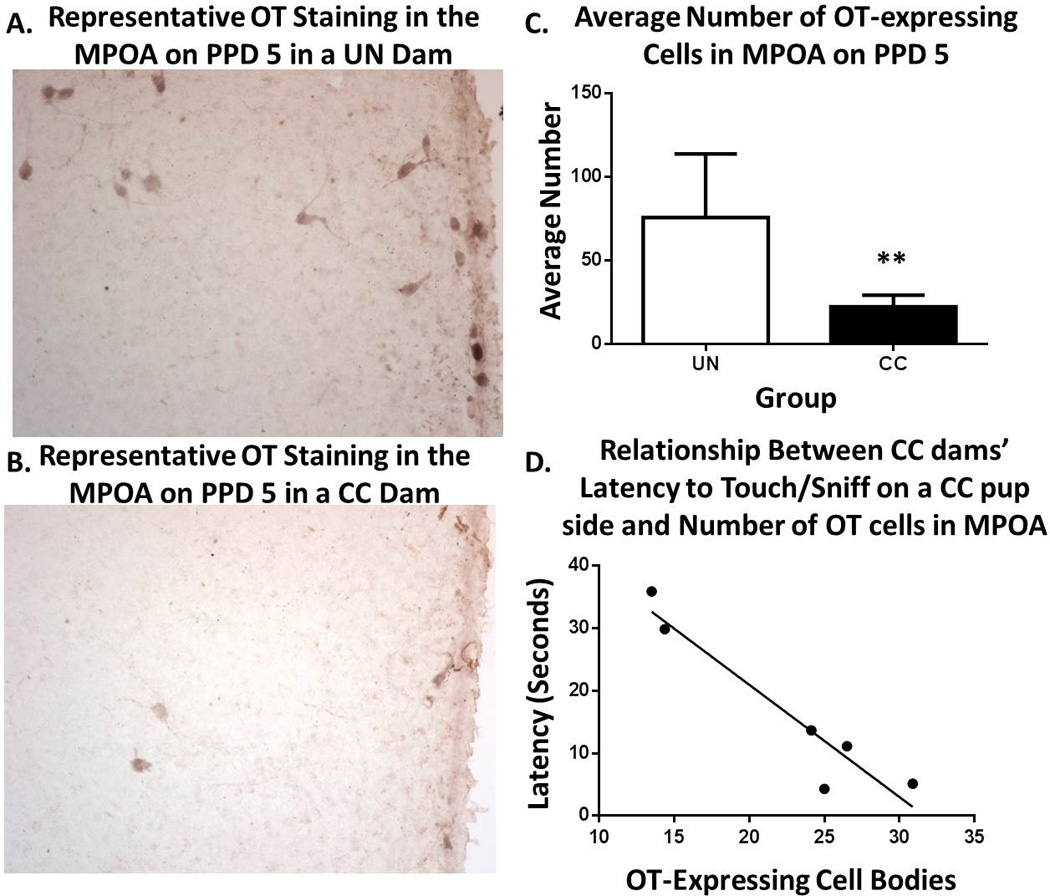
Representative images from UN (A) and CC (B) dam showing CC dams had fewer OT-expressing cells in the MPOA on PPD 5 (C) which was associated with a greater latency to engage in touch/sniff behavior on the CC pup side (D). ** p<0.01; Representative images were acquired at 40X magnification.
4. DISCUSSION
Results suggest gestational cocaine-exposure interferes with preference-like behavior of dams towards a CC-exposed or UN male pup on PPD 1, and that change in preference-like behavior following gestational cocaine-exposure appears to be associated in part with decreased number of USVs being emitted by CC-exposed pups. Cocaine administration has previously been found to interfere with preference-like behavior in a dualchoice conditioned place preference apparatus when given the choice of chambers associated with either cocaine or age-matched pups [53]. Findings reported here further support gestational cocaine interferes with pup saliency and extends this previous study showing that subtle changes in preference-like behavior are evident on PPD 1 specifically and can be observed when given a choice of CC-exposed or UN pup stimulus. Additionally, previous studies have shown that high-rates of vocalizations emitted by pups are the most effective for eliciting retrieval from dams [14–18]. Findings reported here extend these findings and suggests high-rates of vocalizations can influence preference-like behavior of rodent dams to whole pup-stimuli (not their own biological pup), when dams are unable to touch/ retrieve pups though it seems restricted to PPD 1 in particular using this paradigm.
Interestingly, only the CC dams’ preference-like behavior positively correlated with number of USVs pups emitted. This could suggest that CC dams are differentially attending to multiple environmental cues or differentially ranking cues in their environment ultimately reflecting a failure of the CC dam to react normally in the maternal environment. Attentional processes in rodents have been shown to be altered following cocaine-exposure [54;55] and withdrawal [56]. While CC and control dams attended to both CC and UN pup stimuli during preference testing and pilot data similarly showed all dams attended to pup USVs (when recorded USVs were played back during testing in the absence of whole pup stimuli), suggesting CC dams’ initial attention towards pup stimuli and pup USVs were not significantly affected, though we did not measure attention directly. It is therefore possible cocaine’s effects on attentional processes may be driving differences in preference-like behavior on PPD 1 and warrants more study. As we found only trends and no statistically significant differences on first alley choice further study with larger groups may uncover additional differences. Additionally, the CC dams may be responding to pups differentially based on the fact that all dams were separated from their litters prior to testing. In previous tests following a 30 minute separation there was clear evidence of less maternal response in CC treated dams and the experimental procedure employed here was designed to have a separation time as well. Differential response to separation could be an aspect of the overall drug effect on CC dams and should be considered in future mechanistic studies. While the dams and pups may have been in some state of withdrawal, activity measures in general do not indicate that as a particular mechanism of choice. Previous studies of dams given gestational cocaine, similar to the dams in the present study, did not indicate differences between withdrawn and non-withdrawn groups on response to pups in a maternal behavior task. That being said it is certainly possible either dam or pup withdrawal effects could influence behavior in both dam (preference-like behavior) and pups (variations in vocalizations and other cues) making it all the more interesting to look at differential group behavior towards pup cues.
It was also previously found in this animal model that CC dams show avoidance-like behavior toward CC-pup urine on PPD 1 (not observed in UN dams) but not on PPDs 3 or 5 [57]. It is therefore plausible that CC dams are attending to multiple cues in the early postpartum period that they find aversive/prefer, specifically olfactory and auditory (urine and USVs). As mentioned above, rodent dams in this study were exposed to complex, multimodal stimuli: they could not only hear stimuli, but could also see and smell stimuli. It is possible that the pups emitting fewer USVs also had the greatest differences in urine olfactory cues based on level of cocaine-exposure, as cocaine and cocaine’s metabolites have been detected in the urine of PCE pups until postnatal day 3 (pilot work). Previous studies have found that olfactory cues from offspring play an important role in maternal behavior [58], but studies suggest that accurate maternal approach/response requires both vocalization and olfactory stimuli [12;59]. In light of these studies, and previous findings that CC dams find CC-exposed urine aversive (but not UN dams), it is very likely that olfactory cues are also playing a critical role in CC dam preference-like behavior for UN pups on PPD 1. PPD 1 is a very critical time in the pups’ survival and we have reported delayed maternal response previously on this day in particular with stronger effects on this day, compared to either PPDs 3 or 5, on several pup directed behaviors. Whether dams are responding to cry suppression or to other cues from PND 1 pups are not totally clear and the elucidation of the motivation for response will be an important factor in future experiments. Future studies aimed at elucidating the differential impact of olfactory cues and USVs in this model would prove useful in understanding the mechanism(s) underlying maternal response/preference-like behavior.
We had hypothesized that all dams would spend less time on the CC pup side, based on previous work showing that all dams (regardless of treatment history) show differential maternal behavior towards CC-exposed pups [3], and that this would be associated with variations in vocalizations emitted by pups. However, both UN and CS dams did not differ in the duration of time they spent on the CC or UN pup side, and only the CC dam’s preference-like behavior was associated with variations in USVs. Differences between this study (where the dams can see, hear, smell but not touch the vocalizing pup) and the previous study (where dams can see, hear, smell, and touch vocalizing pups) could be underlying differential findings. Somatosensory input has been shown to be an important pup cue in nursing behavior [60]. Differences in somatosensory and other behaviors, such as thermoregulation, between CC and UN offspring could in part drive differences in maternal behavior following retrieval that require contact with pups, including nest building and crouching, but were not factors explored in the present study as dams were not able to touch pups.
While we found no differences in dam preference-like behavior on PPD 3, we did find a non-significant trend for more CC dams to select the UN pup side over the CC pup side as a first choice. We found on PPD 5, CC-exposed dams spent more time on the UN pup side compared to UN and CS dams and T/S pup cages less (regardless of which pup associated side they were on). Preference-like behavior on PPD 5 did not correlate with variations in vocalizations emitted by pups. This may indicate preference-like behavior differences observed on this day may not be directly associated with variations in USVs. Interestingly, we found that decreased T/S behavior was related to decreased number of OT-positive neurons in the MPOA on PPD 5. A normally functioning MPOA is necessary for pup preference-like behavior [39] and OT in the MPOA is necessary for a full complement of rodent maternal behaviors [36–38;61]. Additionally, rodent dams exhibiting cocaine induced decreases in maternal behavior [4] have also previously been shown to have decreased oxytocin levels in the MPOA [62;63]. Findings further support the importance of OT in the MPOA and OT’s role in regulating maternal proximity and response behavior. Findings could suggest that variations in OT dynamics may drive subtle differences in preference-like behavior since the most robust correlate with OT expression was latency to T/S on a CC pup side (OT was not associated with latency to T/S on the UN pup side). While only speculative, this could provide a possible potential mechanism partially underlying the complex and cyclic nature of maternal-infant behavioral deficits following PCE, in which an infant with altered behavior following PCE fails to elicit normal maternal care, and deficits in these interactions prime the maternal brain (and potentially OT dynamics), further altering maternal behavior that is context dependent (based on self-experience with offspring as we only observed an OT relationship with latency to T/S towards CC pups), and consequently infant behavioral outcome. Human and rodent maternal behavior has previously been shown to be experience-dependent [64;65]. Additionally, it was recently reported that sensory experience in neonatal mice regulates OT synthesis and secretion [66] and recent studies suggest maternal-infant interactions may regulate OT dynamics across the postpartum period [3;43;44]. The mechanisms underlying experience-dependent changes in OT dynamics in the maternal brain are unclear. As this was a small sample size, future studies using a larger number of subjects and looking at other brain regions of interest would prove useful. We did not have the resources to examine CS dam brains but given the behavioral data showing no differences between control groups we feel UN dam brain OT analyses may be generalizable to both control groups. Future work should also examine the temporal relationship of these findings, i.e. if the early maternal environment, e.g. differences in olfactory, USV, and somatosensory cues of a dam’s own litter, is in part driving differences in OT expression on PPD 5, and thus specific decreases in preference-like behavior towards cocaine-exposed offspring. It’s possible that OT dynamics also played a role in initial differences in preference-like behavior observed in CC dams on PPD 1, however more research is warranted on the underlying mechanisms of initial dam preference-like behavior, as the present study only assessed OT on PPD 5. Future work on OT dynamics, including synthesis and receptor binding, temporal relationship of variations within this system and relationship with infant cues, and impact on maternal preference-like behavior are needed and could provide critical insight into the mechanisms that drive maternal deficits following gestational cocaine-exposure.
We did not observe differences in vocalizations between CC and UN stimulus pups on PPDs 3 or 5 in this study. Therefore, minimal variation in USVs emitted by pups could underlie non-significant associations between stimulus pup vocalizations and dam preference-like behavior on PPDs 3 and 5. Previous studies have found greater differences in acoustic measures of USVs following PCE than what was found in this study [22–24]. Most studies have examined USVs in an isolation paradigm, with the pup isolated from littermates and dams and USVs elicited by a painful stimulus or thermal challenge. It is possible that a greater thermal challenge could have resulted in greater variations in USVs and subsequent maternal preference-like behavior. However, findings could also suggest that in the presence of a rodent dam fewer differences are observed in USVs compared to when a pup is isolated.
A limitation of this study is that we only looked at USV differences during the first minute of preference testing. It is possible that USV differences may have been more robust if we had analyzed all USVs emitted during the 10 minute testing session and that differences would have been associated with preference-like behavior. Pilot studies showed the most robust differences in USVs during the first minute of testing, with both CC and UN pups showing a peak in USV production during this minute followed by a sharp decline in USV production over the remaining testing period. We therefore hypothesized that this initial minute may drive initial maternal preference/attention toward pup stimuli as opposed to longer care patterns of maternal care, e.g. nest building and crouching behavior observed following retrieval in other maternal testing paradigms. Short term discrete pup cues, i.e. USVs and olfactory cues, may play a larger role in initial attention, approach/response, and preference-like behavior while thermoregulation and other tactile stimuli may play a role in other behaviors associated with maternal care. We’ve previously found rodent dams quickly make a choice to retrieve pups, or not, once pups are placed in the cage [67]. Similarly we observed in the paradigm used in this study that rodent dams explored and attended to both CC and UN pup stimuli during the first minute and then dams that showed a preference between pup sides appeared to make a choice to explore one pup side more. We therefore feel we captured this initial period when USVs may have the greatest influence on maternal choice or response though further tests looking at initial arousal factors would be interesting. Future studies are needed to dissect the role of pup cues on distinct patterns of maternal behavior, i.e. maternal response and preference vs. behaviors following retrieval. Studies manipulating auditory stimuli (i.e. USV playback in the absence of pups) are now underway to elucidate the role USVs have in the early postpartum environment (alone and in combination with olfactory cues).
5. Conclusions
The overall findings of this study are generally in accord with established literature and indicates that cocaine’s effects on the mother-infant relationship is likely synergistic in that it influences both mother and offspring independently and concertedly. Though direct comparisons with previous studies of maternal response are not possible given the different paradigm, design, apparatus and stimuli, several things findings are consistent nonetheless with previous findings. CC dams do respond differently from controls in their early maternal response; PCE pups have different behavioral profiles than do control pups; and oxytocin system dynamics are different in CC dams compared to untreated controls in brain regions important to maternal response in the early postpartum period. In light of study limitations, the association between temporal variation in USVs and CC-exposed dam preference-like behavior remains striking and suggests subtle changes in offspring stimuli may be contributing to changes in the maternal environment. Also, while OT findings should be considered preliminary, they could suggest as we have suggested previously that variations within the OT system are a potential mechanism underlying this synergistic relationship. Findings also highlight the need for additional research to elucidate the mechanism(s) underlying altered maternal response behavior during early postpartum and how these differences may lead to greater neglectful behavior observed later in the postpartum period towards a larger litter. The importance of continued research in this field is highlighted by its potential application in translational value for informing mother-infant studies of cocaine abuse in humans. Perhaps as important is the need for elucidating exactly how cocaine is affecting infant biological and behavioral processes to alter cues which play an important role in eliciting maternal and social response both early and later in development. Continued research has the possibility to unlock clues into the mechanisms by which mother-infant communication fails and whether such failure is driven primarily by altered changes in the maternal sensory and perceptual systems, by altered infant cues, or interactions between the two.
Highlights.
-
■
Gestational cocaine disrupts maternal preference-like behavior
-
■
Variations in USVs of cocaine-exposed pups contribute to altered maternal preference
-
■
MPOA oxytocin expression is associated with preference behavior in later postpartum
-
■
Results support cocaine’s effects on the mother-infant relationship is synergistic
Acknowledgements
We would like to acknowledge the invaluable contributions of Dr. Sarah Williams, Dr. Joan Morrell, Ms. Abigail Jamieson-Drake, and Ms. Marlana Radcliffe for their help in the preparation of this manuscript and data collection as well as their thoughtful discussions with the authors. This project was supported though the National Institute on Drug Abuse by Award Number P01DA022446 (awarded to Josephine M. Johns) and pre-doctoral training grants F31DA030060 (awarded to Elizabeth T. Cox) and F31DA026251 (awarded to Matthew S. McMurray). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Murphy JM, Jellinek M, Quinn D, Smith G, Poitrast FG, Goshko M. Substance abuse and serious child mistreatment: prevalence, risk, and outcome in a court sample. Child Abuse Negl. 1991;15(3):197–211. doi: 10.1016/0145-2134(91)90065-l. [DOI] [PubMed] [Google Scholar]
- 2.Leventhal JM, Forsyth BW, Qi K, Johnson L, Schroeder D, Votto N. Maltreatment of children born to women who used cocaine during pregnancy: a population-based study. Pediatrics. 1997;100(2):E7. doi: 10.1542/peds.100.2.e7. [DOI] [PubMed] [Google Scholar]
- 3.Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, et al. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119(6):1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994;108(1):107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- 5.Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113(2):377–390. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- 6.Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47(4):857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 7.Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996;110(2):315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- 8.Scheutze P, Zeskind PS, Eiden RD. The perceptions of infant distress signals varying in pitch by cocaine-using mothers. Infancy. 2003;4:25–34. doi: 10.1207/S15327078IN0204_06. [DOI] [PubMed] [Google Scholar]
- 9.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 10.Lester BM, Corwin MJ, Sepkoski C, Seifer R, Peucker M, McLaughlin S, et al. Neurobehavioral syndromes in cocaine-exposed newborn infants. Child Dev. 1991;62(4):694–705. doi: 10.1111/j.1467-8624.1991.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 11.Corwin MJ, Lester BM, Sepkoski C, McLaughlin S, Kayne H, Golub HL. Effects of in utero cocaine exposure on newborn acoustical cry characteristics. Pediatrics. 1992;89(6 Pt 2):1199–1203. [PubMed] [Google Scholar]
- 12.Farrell WJ, Alberts JR. Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol. 2002;116(3):297–307. doi: 10.1037/0735-7036.116.3.297. [DOI] [PubMed] [Google Scholar]
- 13.Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5(4):371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol. 1994;108(3):298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Farrell WJ, Alberts JR. Maternal responsiveness to infant Norway rat (Rattus norvegicus) ultrasonic vocalizations during the maternal behavior cycle and after steroid and experiential induction regimens. J Comp Psychol. 2002;116(3):286–296. doi: 10.1037/0735-7036.116.3.286. [DOI] [PubMed] [Google Scholar]
- 16.Deviterne D, Desor D, Krafft B. Maternal behavior variations and adaptations, and pup development within litters of various sizes in Wistar rat. Dev Psychobiol. 1990;23(4):349–360. doi: 10.1002/dev.420230406. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerberg B, Kim JH, Davidson AN, Rosenthal AJ. Early deprivation alters the vocalization behavior of neonates directing maternal attention in a rat model of child neglect. Ann N Y Acad Sci. 2003;1008:308–313. doi: 10.1196/annals.1301.039. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007;97(1):937–941. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- 19.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122(4):357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 20.Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93(4–5):766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Wohr M, Dahlhoff M, Wolf E, Holsboer F, Schwarting RK, Wotjak CT. Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: an embryo-transfer study. Behav Genet. 2008;38(6):579–595. doi: 10.1007/s10519-008-9221-4. [DOI] [PubMed] [Google Scholar]
- 22.McMurray MS, Zeskind PS, Meiners SM, Garber KA, Tien H, Johns JM. Effect of prenatal cocaine on early postnatal thermoregulation and ultrasonic vocalization production. Front Psychol. 2013;4:882. doi: 10.3389/fpsyg.2013.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox ET, Hodge CW, Sheikh MJ, Abramowitz AC, Jones GF, Jamieson-Drake AW, et al. Delayed developmental changes in neonatal vocalizations correlates with variations in ventral medial hypothalamus and central amygdala development in the rodent infant: effects of prenatal cocaine. Behav Brain Res. 2012;235(2):166–175. doi: 10.1016/j.bbr.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn ME, Benno RH, Schanz N, Phadia E. The effects of prenatal cocaine exposure and genotype on the ultrasonic calls of infant mice. Pharmacol Biochem Behav. 2000;67(4):729–738. doi: 10.1016/s0091-3057(00)00418-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Fonagy P, Koos O, Dorsett K, Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res. 2013 doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galbally M, Lewis AJ, Ijzendoorn M, Permezel M. The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv Rev Psychiatry. 2011;19(1):1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- 27.Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18(3):267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 29.Rich ME, deCardenas EJ, Lee HJ, Caldwell HK. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS ONE. 2014;9(6):e98839. doi: 10.1371/journal.pone.0098839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams SK, Johns JM. Prenatal and gestational cocaine exposure: Effects on the oxytocin system and social behavior with implications for addiction. Pharmacol Biochem Behav. 2013 doi: 10.1016/j.pbb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nephew BC, Febo M. Effects of cocaine on maternal behavior and neurochemistry. Curr Neuropharmacol. 2012;10(1):53–63. doi: 10.2174/157015912799362760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurray MS, Cox ET, Jarrett TM, Williams SK, Walker CH, Johns JM. Impact of gestational cocaine treatment or prenatal cocaine exposure on early postpartum oxytocin mRNA levels and receptor binding in the rat. Neuropeptides. 2008;42(5–6):641–652. doi: 10.1016/j.npep.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35(2):127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- 34.Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides. 1997;31(5):439–443. doi: 10.1016/s0143-4179(97)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numan M. The role of the medial preoptic area in the regulation of maternal behavior in the rat. Ann N Y Acad Sci. 1986;474:226–233. doi: 10.1111/j.1749-6632.1986.tb28014.x. [DOI] [PubMed] [Google Scholar]
- 36.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 37.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Pereira M, Morrell JI. The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: Facilitation followed by inhibition. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira M, Morrell JI. The medial preoptic area is necessary for motivated choice of pup- over cocaine-associated environments by early postpartum rats. Neuroscience. 2010;167(2):216–231. doi: 10.1016/j.neuroscience.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23(1):27–31. doi: 10.1016/0143-4179(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 41.Johns JM, Caldwell JD, Pedersen CA. Acute cocaine treatment decreases oxytocin levels in the rat hippocampus. Neuropeptides. 1993;24(3):165–169. doi: 10.1016/0143-4179(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 42.Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25(50):11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasawa M, Okabe S, Mogi K, Kikusui T. Oxytocin and mutual communication in mother-infant bonding. Front Hum Neurosci. 2012;6:31. doi: 10.3389/fnhum.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buisman-Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, Tops M. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol Biochem Behav. 2014;119:22–38. doi: 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in Sprague-Dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32(2):131–136. [PubMed] [Google Scholar]
- 46.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 47.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMurray MS, Hubbard DK. A novel device for the calibration of sonic and ultrasonic recording transducers. J Neurosci Methods. 2013;217(1–2):39–43. doi: 10.1016/j.jneumeth.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeskind PS, McMurray MS, Garber KA, Neuspiel JM, Cox ET, Grewen KM, et al. Development of translational methods in spectral analysis of human infant crying and rat pup ultrasonic vocalizations for early neurobehavioral assessment. Front Psychiatry. 2011;2:56. doi: 10.3389/fpsyt.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besheer J, Schroeder JP, Stevenson RA, Hodge CW. Ethanol-induced alterations of c-Fos immunoreactivity in specific limbic brain regions following ethanol discrimination training. Brain Res. 2008;1232:124–131. doi: 10.1016/j.brainres.2008.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. 3rd ed. San Diego: Academic Press; 1997. [Google Scholar]
- 52.Byun J, Verardo MR, Sumengen B, Lewis GP, Manjunath BS, Fisher SK. Automated tool for the detection of cell nuclei in digital microscopic images: application to retinal images. Mol Vis. 2006;12:949–960. [PubMed] [Google Scholar]
- 53.Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology (Berl) 2007;194(3):309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr., Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26(38):9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grilly DM, Gowans GC, McCann DS, Grogan TW. Effects of cocaine and d-amphetamine on sustained and selective attention in rats. Pharmacol Biochem Behav. 1989;33(4):733–739. doi: 10.1016/0091-3057(89)90463-2. [DOI] [PubMed] [Google Scholar]
- 56.Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182(4):579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 57.Williams SK. Gestational cocaine: effects on postpartum behaviors and endocrine signaling in a rodent model University of North Carolina at Chapel Hill. 2011 [Google Scholar]
- 58.Levy F, Keller M, Poindron P. Olfactory regulation of maternal behavior in mammals. Horm Behav. 2004;46(3):284–302. doi: 10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12(1):55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- 60.Stern JM, Lonstein JS. Nursing behavior in rats is impaired in a small nestbox and with hyperthermic pups. Dev Psychobiol. 1996;29(2):101–122. doi: 10.1002/(SICI)1098-2302(199603)29:2<101::AID-DEV2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 61.Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91(1):146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- 62.Lubin DA, Cannon JB, Black MC, Brown LE, Johns JM. Effects of chronic cocaine on monoamine levels in discrete brain structures of lactating rat dams. Pharmacol Biochem Behav. 2003;74(2):449–454. doi: 10.1016/s0091-3057(02)01027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johns JM, Lubin DA, Walker CH, Joyner P, Middleton C, Hofler V, et al. Gestational treatment with cocaine and fluoxetine alters oxytocin receptor number and binding affinity in lactating rat dams. Int J Dev Neurosci. 2004;22(5–6):321–328. doi: 10.1016/j.ijdevneu.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donovan WL. Maternal learned helplessness and physiologic response to infant crying. J Pers Soc Psychol. 1981;40(5):919–926. doi: 10.1037//0022-3514.40.5.919. [DOI] [PubMed] [Google Scholar]
- 65.Mashoodh R, Sinal CJ, Perrot-Sinal TS. Predation threat exerts specific effects on rat maternal behaviour and anxiety-related behaviour of male and female offspring. Physiol Behav. 2009;96(4–5):693–702. doi: 10.1016/j.physbeh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, Yao H, et al. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17(3):391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]
- 67.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long- term withdrawal from gestational cocaine treatment on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1997;19(4):368–374. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]