Abstract
Objective
To assess the functioning of mesolimbic and striatal areas involved in reward-based spatial learning in unmedicated adults with Obsessive-Compulsive Disorder (OCD).
Methods
We compared fMRI BOLD response in 33 unmedicated adults with OCD to 33 healthy, age-matched control participants during a reward-based learning task that required learning to use extra-maze cues to navigate a virtual 8-arm radial maze to find hidden rewards. We compared groups in their patterns of brain activation associated with reward-based spatial learning versus a control condition in which rewards were unexpected because they were allotted pseudo-randomly to experimentally prevent learning.
Results
Both groups learned to navigate the maze to find hidden rewards, but group differences in neural activity during navigation and reward processing were detected in mesolimbic and striatal areas. During navigation, OCD participants, unlike healthy participants, activated left posterior hippocampus. Unlike healthy participants, OCD participants did not activate left ventral putamen and amygdala when anticipating rewards or left hippocampus, amygdala, and ventral putamen when receiving unexpected rewards (control condition). Signal in these regions decreased relative to baseline during unexpected reward receipt in OCD participants and the degree of activation was inversely associated with doubt/checking symptoms.
Conclusion
OCD participants displayed abnormal recruitment of mesolimbic and ventral striatal circuitry during reward-based spatial learning. Whereas healthy participants activate this circuitry in response to the violation of reward expectations, unmedicated OCD participants do not and instead overrely on posterior hippocampus during learning. Thus, dopaminergic innervation of reward circuitry may be altered and future study of anterior/posterior hippocampal dysfunction in OCD is warranted.
Keywords: Obsessive-Compulsive Disorder, Reward, Learning, fMRI, Virtual Reality, Hippocampus
Introduction
Functional abnormalities in fronto-striatal circuits underlie inhibitory control deficits and cognitive inflexibility in OCD(1), while dysfunction in mesolimbic regions (e.g., hippocampus and amygdala) underlies fear expression in these patients(2). Together with ventral fronto-striatal regions (e.g. orbitofrontal cortex and ventral striatum), these mesolimbic regions comprise a reward processing system(3) that allows us to anticipate, respond to, and learn from reward outcomes in quotidian life. Whereas ventral/anterior hippocampus is intrinsically connected to ventral striatum(4), processes reward-related information, and is preferentially involved in anxiety(5), dorsal/posterior hippocampus preferentially processes spatial information(6). Using an ecologically valid task of reward-based spatial learning adapted from animal research, we sought to identify functional impairments in mesolimbic and ventral striatal circuitry that may contribute to OCD behaviors.
OCD patients perform poorly on tasks that require adjusting responses based on changing reward feedback(7), consistent with findings of aberrant processing of reward in OFC and ventral striatum during reversal-learning(8) and reward anticipation(9). Visuospatial impairment has also been described(10, 11), but the neural correlates of spatial learning have not been assessed in OCD. Together with anatomical findings of reduced gray matter in corticolimbic areas(12) involved in reward expectancy(13), and smaller amygdala and hippocampal volumes in refractory OCD patients compared to controls(14), these data suggest that OCD participants have functional and structural abnormalities in the brain regions that support reward-based spatial learning.
Spatial learning is often assessed in rodents by having them navigate an 8-arm radial maze(15). We adapted this paradigm to a virtual-reality environment for use with fMRI(16). Both the animal and human tasks require learning to use extra-maze cues to navigate and find hidden rewards. Healthy individuals activate temporoparietal areas when searching the maze, as occurs with other spatial navigation tasks(17–20). Importantly, our task includes a control condition in which the use of spatial cues to find hidden rewards (i.e. spatial learning) is experimentally disabled, allowing us to assess the neural correlates of reward processing in the absence of spatial learning and disentangle the neural correlates of reward processing and learning. Healthy individuals activate hippocampus and amygdala when receiving unpredicted rewards in the control compared to the learning condition, a finding we speculated could be attributed to enhanced dopaminergic firing from ventral tegmental area to ventral striatum and these mesolimbic areas in response to unpredicted rewards(21).
In this study, we used our translational fMRI task to assess the neural correlates of reward-based spatial learning in unmedicated individuals with OCD who were free of comorbid illnesses. Twenty-one of these individuals were treatment naïve and 12 were off psychotropic medications for at least 12 weeks. Given findings of functional deficits in reward processing circuits(9) and compensatory hippocampal engagement during other learning tasks(22) in OCD, together with the differential roles of posterior and anterior hippocampus in processing spatial and reward information, respectively(6), we made the following hypotheses. First, we hypothesized that whereas both groups would engage tempoparietal areas while navigating the maze, OCD participants would over-engage posterior hippocampus during spatial learning. Second, we suspected that OCD participants would not engage anterior hippocampus and ventral striatum to the same extent as healthy participants during reward anticipation or in response to reward receipt, especially when rewards were unpredicted in the control compared to the learning condition. We also explored associations of mesolimbic and temporoparietal activations with OCD severity and symptom dimensions.
METHODS
Participants
Unmedicated adults with OCD (n=33) and healthy (n=33) participants, group-matched by age, sex, and ethno-racial groups, were recruited through flyers, internet advertisements, and word-of-mouth. The Institutional Review Board of the New York State Psychiatric Institute approved this study. Participants provided written informed consent.
Details of the exclusion criteria, clinical assessments, MRI pulse sequence, and behavioral analyses are described in the online data supplement.
Reward-Based Spatial Learning Paradigm
Our reward-based spatial learning paradigm has been described(16, 23). The virtual reality environment consisted of an 8-arm radial maze surrounded by a naturalistic landscape (e.g., mountains, trees and flowers) that constituted the extra-maze cues that could be used for spatial navigation (Figure 1). Prior to scanning, participants practiced navigating a similar maze on a desktop computer.
Figure 1. The Virtual Reality Environment.
(a) Schematic of the virtual maze depicting the four events modeled: ‘searching,’ ‘reward anticipation,’ and the two types of reward feedback, ‘reward’ and ‘no-reward.’ (b) Some of the naturalistic spatial cues in the Virtual Reality maze. (c) Participants’ view of the VR maze. (d) Baited area at the end of an arm, with $ indicating successful receipt of reward.
Stimuli during scanning were presented through non-magnetic goggles. Participants used an MRI-compatible joystick (Current Designs Inc.) to navigate the maze. Before scanning, participants were informed that they will be in the center of a maze with eight identical arms extending outwards, and that hidden rewards ($) would be available at the end of the arms. They were instructed to navigate the maze to collect the rewards and that they could keep any money they found, but that they would lose money if they revisited an arm. They were told that they would complete several sessions of the task, but not that the sessions differed from one another. They therefore believed that they would perform the same task multiple times.
The paradigm included an active learning and a control condition. In the learning condition, all 8 arms were baited with rewards. As participants navigated the maze, they had to learn the spatial layout of the extra-maze cues to avoid revisiting arms. After each arm visit (trial), participants reappeared at the center of the maze with their viewing perspective randomly reoriented to prevent use of strategies such as chaining (systematically selecting neighboring arms). After collecting all 8 rewards, the learning condition terminated.
Next, participants were presented with a screen indicating a new session was beginning. In this control condition, identical extra-maze cues used in the learning condition were randomized among locations after each trial to destroy any possibility of using the spatial layout of the cues (spatial learning). To control for the reward/punishment frequency with the learning condition, participants were rewarded at the same frequency but without regard to their actual performance. This control condition thus shared all salient features with the learning condition, including lower-order stimulus features and higher-order task features. This condition terminated following the number of trials that a given participant needed to obtain all 8 rewards in the learning condition. If a participant required 18 trials to find all 8 rewards in the learning condition (i.e. 8 correct and 10 error trials), they would be given 10 unbaited trials randomly in the control condition. Thus, contrasting neural activity in the learning condition (during spatial learning) and the control condition (where spatial learning is impossible) reveals the neural correlates of reward-based spatial learning.
Participants underwent two runs of each condition. The learning condition always preceded the control condition to establish the number of trials and reward frequency for the control condition. Thus, the paradigm contained 32 rewarded navigation events (8 rewards × 2 conditions × 2 runs), but the number of unrewarded events varied for each participant. Following completion of the paradigm, participants were provided the same amount of money regardless of performance.
Image Acquisition and Processing
A GE Signa 3 Tesla LX scanner (Milwaukee, WI) and a standard quadrature GE head coil were used for image acquisition. Axial functional images were positioned parallel to the anterior commissure-posterior commissure line using a T1-weighted sagittal localizing scan. Functional images were obtained using a T2*-sensitive gradient-recalled, single-shot, echo-planar pulse sequence having a TR=2800msec, TE=25msec, 90° flip angle, single excitation per image, 24×24cm FOV, a 64×64 matrix, 43 slices 3mm thick, no gap, and covering the entire brain. The number of EPI volumes collected was determined by the performance of each participant in the learning condition, with a maximum of 322 volumes/run.
As described(16), image preprocessing procedures were run in batch mode using MATLAB 7.9 (Mathworks, Natick, MA) and implemented in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and FSL (FMRIB Software Library; www.fmrib.ox.ac.u). Preprocessing consisted of slice-time correction, using a windowed Fourier interpolation to minimize dependence on the reference slice, motion-correction, and realignment to the middle image of the middle scanning run(24). Images with estimates for peak motion exceeding 3mm (one voxel) translation were repaired with ArtRepair(25). Runs with more than 15% of such images were discarded for poor quality (26). Motion-corrected functional images of each participant were co-registered to the corresponding 3D spoiled gradient recall anatomical image, which was spatially normalized to MNI space (avg152T1) with a voxel size of 2×2×2 mm3. Normalization parameters warped the functional images into the same MNI space as the SPGR image. Normalized images were spatially smoothed using a Gaussian-kernel filter with a full width at half maximum (FWHM) of 8 mm. Spatially smoothed fMRI time series were temporally high-pass filtered with a cutoff frequency of 1/128 Hz via a discrete cosine transform to remove low-frequency noise (e.g., scanner drift).
Image Analysis
Extraction of subject-level signal differences across the learning and control conditions of the spatial learning task was conducted using general linear models in SPM8. Four regressors corresponding to specific events that occurred during each trial of each condition were defined (Figure 1A). ‘Searching’ was defined from the start of a trial until an arm was selected and committed to (and 10% of its length was traversed). Reward ‘anticipation’ began after the first 10% of an arm was traversed and extended until reaching its baited area. The two types of reward feedback possible at an arm’s terminus were defined as ‘reward,’ when a monetary reward was won, and ‘no-reward,’ when no monetary reward was won. These regressors were convolved with a canonical hemodynamic response function, with the durations of the regressors for each participant modeled according to the durations of these events during performance in the learning condition. For these regressors, a t-contrast vector was applied to the parameters (beta_j) estimated for each voxel j producing 4 contrast images for each participant representing each regressor/event (searching, anticipation, reward, no reward) compared across the 2 conditions (learning, control).
A random-effects “omnibus” analysis (F test in SPM8) was used to test the significance of interactions between group (OCD, HC), condition (learning, control), and event (searching, anticipation, reward, no reward) across the whole brain, covarying for sex. To correct for multiple comparisons, we applied a cluster extent threshold with an a priori significance threshold of P = 0.01. The cluster extent threshold was obtained with Monte Carlo simulations (10,000 iterations) implemented in custom software written in Matlab. Group composite activation maps generated for each contrast were used to examine the interactions resulting from the omnibus test; voxels identified using a p-value threshold <0.01 together with a cluster extent threshold of 25 are reported. Subject-level fMRI signal differences across the learning and control conditions and an implicit baseline (consisting of the unmodeled components of the task) were extracted to derive parameter estimates for individual participants at specific peaks of the statistical map for that contrast. These post-hoc tests determined group differences in activation associated with the learning and control conditions for each event.
RESULTS
Participants
Thirty-three OCD and 33 healthy participants were scanned. The groups were matched on demographic characteristics (Table 1). OCD participants were free of psychotropic medications (21 treatment-naïve; 12 free of any psychotropic medications for (mean±SD) 109± 127 weeks, Table S1) and of current comorbid Axis I disorders; 9 had a lifetime history of a depressive disorder. OCD symptoms were distributed across the five symptom dimensions(27). The two groups did not differ significantly in measures of head motion within the scanner (supplemental material).
Table 1.
Demographic and Clinical Characteristics of Participants
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | OCD (n = 33) | Healthy Control (n = 33) | Analysis | ||||
| Mean | SD | Mean | SD | t | df | p | |
| Age, years | 29.4 | 8.19 | 29.42 | 7.98 | −0.12 | 64 | 0.99 |
| WASI IQ Score (Full-4) | 110.45 | 13.1 | 111.26 | 13.2 | −0.23 | 54 | 0.81 |
| Duration of Illness, yrs. | 14.80 | 8.60 | … | … | |||
| Age of OCD Onset, yrs | 15.05 | 7.33 | … | … | |||
| HAM-D scores | 4.83 | 3.50 | |||||
| Y-BOCS Total | 25.65 | 3.69 | |||||
| Obsessions | 12.28 | 1.98 | |||||
| Compulsions | 13. 37 | 2.12 | |||||
| N | % | N | % | ||||
| Sex | |||||||
| Male | 19 | 57.60 | 19 | 57.60 | |||
| Female | 14 | 42.40 | 14 | 42.40 | |||
| Handedness | |||||||
| Right | 28 | 84.84 | 30 | 90.90 | |||
| Left | 5 | 15.62 | 3 | 10.00 | |||
| Ethnicity | |||||||
| Asian | 1 | 3.00 | 1 | 3.00 | |||
| African American | 5 | 15.20 | 8 | 24.20 | |||
| Caucasian | 25 | 75.75 | 21 | 63.60 | |||
| Hispanic | … | … | … | … | |||
| Other | 2 | 6.10 | 3 | 9.10 | |||
Abbreviations: OCD, Obsessive-compulsive disorder; WASI, Weschler Abbreviated Scale of Intelligence; Y-BOCS, Yale-Brown Obsessive Compulsive Scale; HAM-D Hamilton Depression Scale.
Behavioral Performance
Both groups demonstrated significant improvement in performance speed and took fewer trials in the learning condition from Run 1 to Run 2 (main effects of Run, ps ≤ 0.01, Table 2 and S2). However, OCD participants took more trials to obtain all 8 rewards in Run 1, contributing to a significant group-by-run interaction. In addition, performance speed in the learning condition correlated positively with OCD severity ratings on the doubt/checking dimension (p=0.03). Performance differences across conditions (analogous to the BOLD contrast between the learning and control conditions) revealed a significant main effect of group for the time taken to complete both conditions in Run 1 (p=0.03), deriving from the slower performance speed of OCD participants (Table S3).
Table 2.
Group Differences in Reward-Based Spatial Learning
| Comparison | HC | OCD | Main Effectb Run F(p) | |
|---|---|---|---|---|
| Performance Speed (SD) | Run1 | 125.9 (100) | 184.2 (132) | 15.15 (<0.01) |
| Run2 | 100.5 (59) | 85.7 (39.2) | ||
| Test Stat Run 1 v 2 (p)a | 1.35 (0.19) | 4.35 (<0.01) | ||
| Main Effect Groupb F(p) | 0.44 (0.51) | Group × Runb 3.61 (0.06) | ||
| Number of Trials (SD) | Run1 | 14.9 (6.4) | 20.7 (8.5) | 5.82 (0.019) |
| Run2 | 14.8 (10.2) | 14.2 (7.9) | ||
| Test Stat Run 1 v 2 (p)a | 0.16 (0.87) | 3.25 (<0.01) | ||
| Main Effect Groupb F(p) | 1.61 (0.21) | Group × Runb 4.79 (0.03) | ||
Boldface denotes statistically significant findings.
Omnibus Test of Neural Activity
The omnibus analysis revealed a significant three-way interaction (group-by-condition-by-event) in a large left hemisphere cluster ([coordinates: −27, −7, −11], 454 voxels [756 mm3], F=8.74, P<0.05, corrected), encompassing anterior and posterior hippocampus, amygdala, and ventral putamen (Fig. 2). Group composite maps were then used to examine group-by-condition interactions within these regions separately for each event.
Figure 2. Whole-brain analysis indicating 3-way interactions (diagnosis-by-condition-by-event).
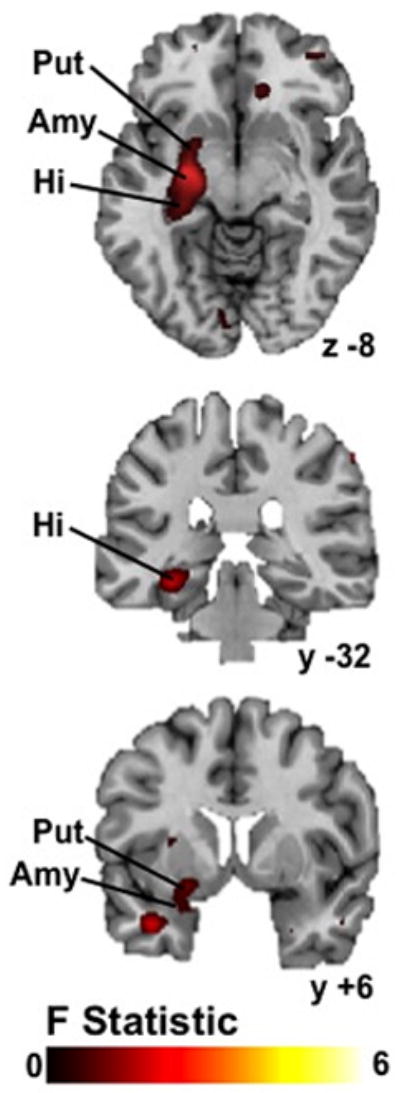
Interactions were detected in a left hemisphere cluster comprising ventral putamen, amygdala, and hippocampus (maximum peak −27, −7, −11; 454 voxels (756 mm3); F=8.74, P<0.05, corrected). Abbreviations: Put, putamen; Amy, amygdala, Hi, hippocampus.
Neural Activity During Spatial Navigation
A significant group-by-condition interaction in left posterior hippocampus derived from its activation in the OCD but not the healthy participants when navigating the maze and searching for rewards in the learning compared to control conditions (Fig. 3a&b). Both groups activated temporal and parietal regions, including bilateral superior temporal gyrus and lateral inferoparietal cortex (Fig 3a).
Figure 3. Neural Activity During Spatial Navigation.
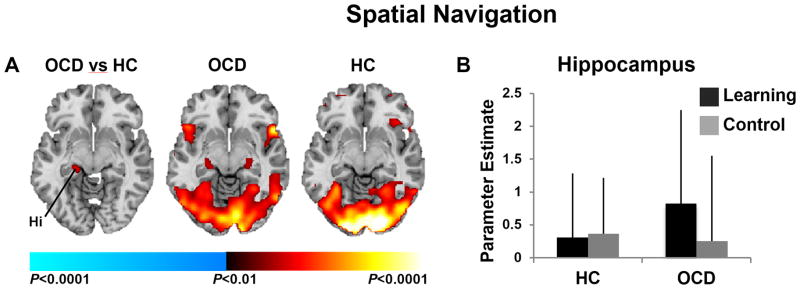
(A) Left image shows group differences in brain activations associated with searching the maze in the learning versus the control condition detected in left posterior hippocampus. Center and right images show group average brain activations for the OCD and healthy participants with increases in signal during searching in the learning vs. control condition in red, and increases during searching in the control vs. active condition in blue. These maps are thresholded at our a priori significance threshold (P = 0.01, cluster filter of 28). (B) Parameter estimates at the labeled left hippocampal cluster (−33, −32, −13) in both conditions and for both groups. Abbreviations: OCD, Obsessive-Compulsive Disorder; HC, healthy control; Hi, hippocampus.
Neural Activity During Reward Anticipation
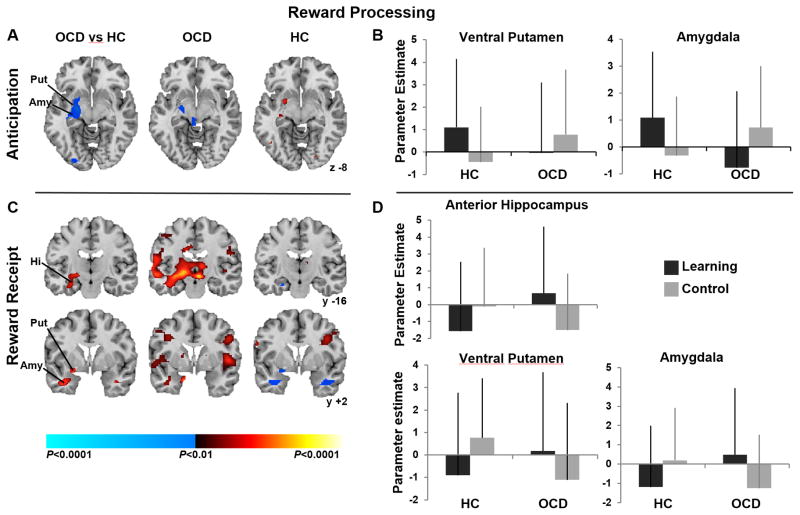
Significant group-by-condition interactions in left amygdala and ventral putamen (Fig. 4a) derived from activation during reward anticipation in the learning condition and deactivation in the control condition in healthy participants, in contrast to activation in the control condition and deactivation (left amygdala) in the learning condition in OCD participants (4b).
Figure 4. Neural Activity During Reward Processing.
(A) Left image shows group differences in activations associated with reward anticipation in the learning versus control conditions detected in left putamen and amygdala. Center and right columns (A & C) show group average brain activations for the OCD and healthy participants with increases in signal associated with reward anticipation (A) and receipt (C) in the learning vs. control condition in red, and increases in the control vs. active condition in blue. (B) Parameter estimates at the labeled putaminal (−21, 6, −10) and amygdalar (−27, −7, −11) clusters in both conditions for both groups. (C) Group differences (left column) in activations associated with reward receipt in the learning versus control conditions are shown in left anterior and posterior hippocampus, putamen and amygdala with parameter estimates (D) at the labeled hippocampal (−33, −32, −13), putaminal (−21, 6, −10), and amygdalar (−27, 7, −11) clusters in both conditions and groups. These maps are thresholded at P = 0.025 with a cluster filter of 30. Abbreviations: OCD, Obsessive-Compulsive Disorder; HC, healthy control; Hi, hippocampus, Put, putamen, Amy, amygdala.
Neural Activity During Reward Receipt
Significant group-by-condition interactions in left anterior hippocampus, amygdala, and ventral putamen were detected in response to receiving expected (learning condition) and unexpected (control condition) rewards (4c). In healthy participants, activation of these regions in response to receiving unexpected rewards was accompanied by deactivation in response to receiving expected rewards. In contrast, in OCD participants, activation of these same regions in response to receiving expected rewards was accompanied by their deactivation in response to receiving unexpected rewards in the control condition (Fig. 4d).
Performance Correlates
Performance speed in the OCD participants correlated positively with their activation of left posterior hippocampus during navigation in the learning condition (p=0.01) and inversely with their activation of left anterior hippocampus, amygdala and putamen during reward receipt in that condition (p’s ≤ 0.01).
Symptom Severity Correlates
OCD severity ratings on the doubt/checking dimension correlated positively with activation of left posterior hippocampus during navigation (p=0.01) and inversely with activation of left anterior hippocampus and ventral putamen during reward receipt in the control condition (p’s ≤ 0.01). Thus, the OCD participants with the most severe doubt/checking symptoms activated left hippocampus the most during navigation and both left hippocampus and ventral putamen the least during the receipt of unexpected rewards.
Discussion
We used a translational paradigm to investigate the neural correlates of reward-based spatial learning in unmedicated individuals with OCD. Participants had to use extra-maze cues to navigate the maze and find rewards in the learning condition, but randomization of the scene prevented use of the cues to learn the reward locations; thus, spatial learning was experimentally prevented in the control condition. Both OCD and healthy participants demonstrated spatial learning, taking less time and fewer trials to find all 8 rewards on the second compared to the first scan run. Group differences in neural activity associated with searching the maze, anticipating, and receiving rewards were detected in a left hemisphere cluster encompassing hippocampus, amygdala and ventral putamen. Although both groups engaged temporoparietal areas typically engaged by healthy individuals during spatial navigation(17–20), only OCD participants engaged left posterior hippocampus. Also, healthy participants activated left anterior hippocampus, amygdala and ventral putamen when receiving unexpected rewards in the control condition, consistent with our prior findings with this task in another sample of healthy individuals(16). In contrast, in OCD participants, signal in these mesolimbic regions decreased relative to baseline in response to receiving unexpected rewards; activation was instead detected in response to receiving expected rewards in the learning condition. Finally, only healthy participants activated left ventral putamen and amygdala when anticipating rewards in the learning condition. These findings suggest abnormal functioning of mesolimbic and ventral striatal circuitry in OCD during reward-based spatial learning.
Healthy participants did not activate posterior hippocampus when searching the maze, a finding we previously interpreted as evidence that (posterior) hippocampus works with other medial temporal regions to create a map of the environment(28). In contrast, OCD participants activated left posterior hippocampus when searching and receiving rewards in the learning condition, suggesting that they required additional neural processing resources to learn/remember the spatial layout of the cues, consistent with their needing more trials (attempts) than healthy participants to obtain all 8 rewards in Run 1 and with the role of left hippocampus in episodic memory(29). OCD participants took more time to find all rewards in Run 1 and their performance speed correlated positively with activation of left posterior hippocampus during navigation. Perhaps their greater engagement of this region contributed to their greater improvement (than healthy participants) in performance (speed and number of trials) from Run 1 to Run 2. Greater reliance on hippocampus is consistent with findings of compensatory hippocampal engagement in OCD participants during performance of other learning tasks(22). Both performance speed and activation of left posterior hippocampus during navigation was positively associated with doubt/checking symptoms, suggesting that the OCD participants who endorsed more of these symptoms required the most time and greatest reliance on posterior hippocampus to find all rewards.
Unlike healthy participants, unmedicated OCD participants did not activate ventral striatum in response to receiving unexpected rewards in the control condition. Lesion, neurophysiological, and fMRI studies typically implicate ventral striatum, specifically nucleus accumbens, in processing reward prediction errors(30). FMRI data from healthy individuals suggest that ventral striatal activation increases with positive prediction errors (i.e. when reinforcement is greater than expected(31, 32)). Our findings suggest that the receipt of unexpected rewards is the prediction error signal that activates ventral striatum on this task in healthy participants. In OCD participants, however, the receipt of unexpected rewards was associated with decreased BOLD signal relative to baseline in ventral putamen, an effect typically associated with omitted rewards in healthy individuals(32, 33). Abnormal ventral striatal function when processing rewards is consistent with findings from studies using a monetary incentive delay task of reward processing in OCD patients(9, 34). Our finding of attenuated ventral striatal activation during reward anticipation in OCD participants is also consistent with those previous data(9).
Together, these findings suggest ventral striatal dysfunction in reward signaling in OCD pathophysiology, perhaps contributing, in part, to the inflexible control over behaviors. Blunted reward signaling, for example, might decrease the rewarding relief that should normally result from a behavior, thereby contributing to difficulty controlling the urge to repeat it. These findings can also be interpreted in terms of the dopaminergic system since dopamine is associated with reward-based learning(21). Neurophysiological findings suggest that ventral striatal dopaminergic neurons fire in response to unpredicted rewards(35). If ventral striatal activation reflects the normal phasic activity of dopaminergic neurons in response to reward unpredictability, then our fMRI findings suggest abnormal phasic activity of striatal dopaminergic neurons in OCD, consistent with PET data(36).
In healthy participants, activation of left ventral putamen along with anterior hippocampus and amygdala was detected in response to receiving unexpected rewards. Ventral striatum is intrinsically connected to anterior hippocampus(4), which has a preferential role over posterior hippocampus in processing reward information(6), and to amygdala, which is also involved in reward prediction error signaling(37). In OCD participants, signal in these regions decreased relative to baseline in response to receiving unexpected rewards, suggesting that the processing of reward prediction errors is abnormal in OCD. Given the role of anterior hippocampus in anxiety(5), however, such failure to activate may also represent greater baseline activity within these connected regions in persons with OCD, particularly in right hippocampus since our findings of group differences were localized to the left hemisphere. Thus, baseline psychophysiological measures of anxiety should be incorporated into future studies.
In OCD participants, activation of left ventral putamen and anterior hippocampus during reward receipt in the control condition was inversely associated with severity ratings on the doubt/checking dimension, suggesting the least activation in those who endorsed the most doubt/checking symptoms. Mesolimbic dysfunction specific to this symptom dimension may, in part, be due to reduced grey matter volume in mesolimbic areas in OCD patients with prominent checking compulsions(12, 38). Electrophysiological(39) and fMRI(40) data indicate that anterior hippocampus encodes uncertainty, consistent with our findings of anterior hippocampal activation in response to unexpected reward in healthy individuals. Thus, the processing of uncertainty within these regions is likely altered in OCD, consistent with evidence that OCD patients – especially those with checking compulsions -- are highly intolerant of uncertainty(41).
This study is limited by the modest sample size and spatial resolution of fMRI that does not allow differentiation of detailed hippocampal subregions that may contribute differently to reward-based learning. We also cannot exclude the possibility that group differences in brain activations were due, in part, to group differences in visuospatial processing or affective responses to receiving/not receiving rewards. Finally, searching- and reward-related activations might be less distinct than we suggest, given the timing of the task and slowness of the hemodynamic response function.
In conclusion, this is the first study to assess the neural correlates of reward-based spatial learning using a translational fMRI paradigm in unmedicated participants with OCD. Our data point to mesolimbic and ventral striatal dysfunction associated with reward-based spatial learning in OCD, confirm findings of hippocampal compensation(22) and suggest that the neural processing of unpredictable rewards is abnormal in OCD. Future studies will determine whether these functional abnormalities precede its clinical expression (and could be biomarkers of risk) or whether these abnormalities follow the clinical expression of OCD (and could be targets for treatment).
Supplementary Material
Table 3.
Group differences in neural activity associated with spatial navigation and reward processing in the learning versus the control condition.
| Location | |||||||
|---|---|---|---|---|---|---|---|
| MNI Coordinates | Significance Testing | ||||||
| Activated Regions | Side | # of voxels | x | y | z | Statistic | p |
| Omnibus Test | |||||||
| Left hemisphere cluster | L | 474 | −27 | −7 | −11 | F=8.74 | <0.05, corrected |
| Spatial Navigation (Searching) | |||||||
| Posterior Hippocampus | L | 53 | −33 | −32 | −13 | T=3.15 | < 0.001 |
| Reward Anticipation | |||||||
| Ventral Putamen | L | 429 | −21 | −6 | −10 | T=3.05 | < 0.01 |
| Amygdala | L | −27 | −7 | −11 | T=3.47 | < 0.01 | |
| Reward Receipt | |||||||
| Anterior Hippocampus | L | 248 | −31 | −16 | −20 | T=3.04 | <0.01 |
| Ventral Putamen | L | −21 | −6 | −10 | T=3.43 | <0.01 | |
| Amygdala | L | 63 | −27 | −7 | −11 | T=2.43 | <0.05 |
Acknowledgments
This work was supported in part by NIMH grants R21MH093889-09 (RM & HBS), K01-MH077652 (RM), K24 MH091555 (HBS), and K02 MH74677 (BSP) as well as by the New York State Office of Mental Hygiene.
Footnotes
Financial Disclosures
In the last 36 months, Dr. Simpson has received research funds for clinical trials from Janssen Pharmaceuticals (2006–2012) and Transcept Pharmaceuticals (2011–2013), consulted for Quintiles, Inc (on therapeutic needs for OCD, 2012), and receives royalties from Cambridge University Press and UpToDate, Inc. All other authors report no biomedical financial interests. All authors report no potential conflicts of interest.
References
- 1.Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered Activation in Fronto-Striatal Circuits During Sequential Processing of Conflict in Unmedicated Adults with Obsessive-Compulsive Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23(3):187–92. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Behrendt RP. Conscious experience and episodic memory: hippocampus at the crossroads. Front Psychol. 2013;4:304. doi: 10.3389/fpsyg.2013.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168(7):718–26. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(11):1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 9.Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, et al. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry. 2011;69(9):867–74. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Abramovitch A, Dar R, Hermesh H, Schweiger A. Comparative neuropsychology of adult obsessive-compulsive disorder and attention deficit/hyperactivity disorder: implications for a novel executive overload model of OCD. J Neuropsychol. 2012;6(2):161–91. doi: 10.1111/j.1748-6653.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 11.Morein-Zamir S, Craig KJ, Ersche KD, Abbott S, Muller U, Fineberg NA, et al. Impaired visuospatial associative memory and attention in obsessive compulsive disorder but no evidence for differential dopaminergic modulation. Psychopharmacology (Berl) 2010;212(3):357–67. doi: 10.1007/s00213-010-1963-z. [DOI] [PubMed] [Google Scholar]
- 12.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(7):720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 13.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 14.Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, et al. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1283–6. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9(5):1465–72. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh R, Hao X, Xu D, Wang Z, Duan Y, Lui J, et al. A Virtual Reality-Based FMRI Study of Reward-Based Spatial Learning. Neuropsychologia. 2010;48(10):2912–21. doi: 10.1016/j.neuropsychologia.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. European Journal of Neuroscience. 2007;25(3):890–9. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- 18.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37(5):877–88. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 19.Spiers HJ, Maguire EA. A navigational guidance system in the human brain. Hippocampus. 2007;17(8):618–26. doi: 10.1002/hipo.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6(6):823–9. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5(6):483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 22.Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):330–6. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Brooks SJ, O’Daly O, Uher R, Friederich HC, Giampietro V, Brammer M, et al. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One. 2012;7(3):e34000. doi: 10.1371/journal.pone.0034000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jazzard P, Matthews PM, Smith SM. Functional MRI—An introduction to methods. New York: Oxford University Press; 2002. [Google Scholar]
- 25.Mazaika P, Hoeft F, Glover GH, Reiss AL. Hum Brain Mapp. San Francisco, CA: 2009. Methods and Software for fMRI Analysis for Clinical Subjects. [Google Scholar]
- 26.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 27.Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, 3rd, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160(1):83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp PE. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus. 1999;9(4):432–43. doi: 10.1002/(SICI)1098-1063(1999)9:4<432::AID-HIPO9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061–8. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Science. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 31.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 32.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 33.Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23(3):777–86. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Jung WH, Kang DH, Han JY, Jang JH, Gu BM, Choi JS, et al. Aberrant ventral striatal responses during incentive processing in unmedicated patients with obsessive-compulsive disorder. Acta Psychiatr Scand. 2011;123(5):376–86. doi: 10.1111/j.1600-0447.2010.01659.x. [DOI] [PubMed] [Google Scholar]
- 35.Schultz W. Behavioral dopamine signals. Trends in Neuroscience. 2007;30(5):203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.McAnarney ER, Zarcone J, Singh P, Michels J, Welsh S, Litteer T, et al. Restrictive anorexia nervosa and set-shifting in adolescents: a biobehavioral interface. J Adolesc Health. 2011;49(1):99–101. doi: 10.1016/j.jadohealth.2010.11.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132(Pt 4):853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 39.Vanni-Mercier G, Mauguiere F, Isnard J, Dreher JC. The hippocampus codes the uncertainty of cue-outcome associations: an intracranial electrophysiological study in humans. Journal of Neuroscience. 2009;29(16):5287–94. doi: 10.1523/JNEUROSCI.5298-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carleton RN, Fetzner MG, Hackl JL, McEvoy P. Intolerance of Uncertainty as a Contributor to Fear and Avoidance Symptoms of Panic Attacks. Cogn Behav Ther. 2013 doi: 10.1080/16506073.2013.792100. [DOI] [PubMed] [Google Scholar]
- 41.Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJ. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 2012;26(3):468–79. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.