Abstract
We compared outcomes of adult patients receiving T-cell depleted (TCD) hematopoietic stem cell transplantation (HCT) without additional GVHD prophylaxis at Memorial Sloan Kettering Cancer Center (MSKCC, N=52) with patients receiving conventional grafts at MD Anderson Cancer Center (MDACC, N=115) for acute lymphoblastic leukemia in CR1 or CR2. Patients received myeloablative conditioning. Thirty-nine patients received ATG at MSKCC and 29 at MDACC. Cumulative incidence of grades 2-4 acute (p=0.001, 17.3% vs. 42.6% at 100 days) and chronic GVHD (p=0.006, 13.5% vs. 33.4% at 3 years) were significantly lower in the TCD group. The NRM at day 100, 1 and 3 years was 15.4%, 25.0% and 35.9% in the TCD group and 9.6%, 23.6% and 28.6% in the unmodified group (p=0.368). There was no difference in relapse (p=0.107, 21.3% vs. 35.5% at 3 years), OS (p=0.854, 42.6% vs. 43.0% at 3 years), or RFS (p=0.653, 42.8% vs. 35.9% at 3 years). In an adjusted model, age >50, cytogenetics and CR status were associated with inferior RFS (HR=2.16, p=0.003, HR=1.77, p =0.022, HR=2.47, p<0.001), while graft type was not significant (HR=0.90, p=0.635). OS and RFS rates are similar in patients undergoing TCD or conventional HCT, but TCD effectively reduces the rate of GVHD.
Keywords: Hematopoietic stem cell transplantation/methods, acute lymphoblastic leukemia, T cell depletion, graft versus host disease
Introduction
Allogeneic hematopoietic stem cell transplant (HCT) has been shown to confer a survival advantage to high-risk patients with acute lymphoblastic leukemia (ALL) in first complete remission (CR1) and in patients in second CR (CR2), with overall survival at 5 years of 40-50%.1-5 Graft-versus-host disease (GVHD) remains one of the main causes of post-transplant morbidity and mortality. Manipulation of allografts with T-cell depletion (TCD) has established efficacy in patients with acute myelogenous leukemia (AML), myelodysplastic syndrome and non-Hodgkin lymphoma, and is associated with a significantly decreased incidence of GVHD6-10. A recent comparison of conventional and TCD grafts in patients with AML demonstrated similar outcomes but significantly less GVHD in patients receiving TCD grafts11. The use of TCD HCT was also recently reported in patients with ALL (n=56), and demonstrated favorable overall survival (OS) and relapse-free survival (RFS) with low rates of GVHD4. However, there are no published reports comparing TCD versus conventional transplantation in ALL.
In this study we compared the outcomes of patients receiving TCD-HCT at Memorial Sloan Kettering Cancer Center (MSKCC) with patients receiving conventional grafts at MD Anderson Cancer Center (MDACC) for ALL in CR1 or CR2, and found that overall and relapse-free survival were similar, but acute and chronic GVHD incidences were significantly lower in the TCD group.
Patients and Methods
Patients
Patients older than 18 undergoing HCT for ALL in CR1 or CR2 between 2000 and 2010 were identified through the institutional BMT registries at MDACC and MSKCC after approval by each institution's institutional review board. Patient demographics, disease characteristics, treatment, GVHD, and outcome data were obtained from departmental databases at each institution. Complete remission was defined as ≤ 5% blasts in the bone marrow. Cytogenetic risk was assigned according to standard criteria12. Donor-recipient human leukocyte antigen (HLA) matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, Cw, -DQB1, and -DRB1 loci, in both institutions. Patients were considered to be mismatched if they did not match at 10/10 alleles.
Transplant procedure and supportive care
Patients at MSKCC received TCD grafts (TCD group, n = 52) after myeloablative (MA) cytoreduction consisting of hyperfractionated total body irradiation (HFTBI), followed by thiotepa and high dose cyclophosphamide (n = 16) or thiotepa and fludarabine (n = 31), as described previously6, 7, 9. One patient was treated with busulfan, melphalan and fludarabine, as previously described,8 and 4 with clofarabine, thiotepa and melphalan, as part of an ongoing clinical trial (clinicaltrials.gov NCT01119066). Peripheral blood stem cell (PBSC) grafts (n = 46) underwent CD34+ cell selection using CliniMACS cell selection system (Miltenyi Biotech, Gladbach, Germany, n=7), or by positive selection of CD34+ cells using the ISOLEX 300i Magnetic Cell Separator (Baxter, Deerfield, Illinois) and subsequent sheep RBC rosette depletion (n=39)7. T cells were removed from bone marrow (BM) grafts (n = 6) by sequential soybean lectin agglutination and sheep RBC rosette depletion6, 8. Thirteen recipients of an HLA-matched related donor graft who were treated with HFTBI, thiotepa, and fludarabine (n = 10), or HFTBI, thiotepa, and cyclophosphamide (n = 3) did not receive any rejection prophylaxis. Thirty-nine patients received anti-thymocyte globulin (ATG) for graft rejection prophylaxis. Eight recipients of matched related identical donors received ATG either because they received chemotherapy only conditioning (n=4) or at the discretion of the practicing providers for recipients of TBI based conditioning (n=4). No patients received GVHD prophylaxis post-transplant.
Patients at MDACC received unmodified grafts (unmodified group, n = 115) after myeloablative conditioning regimens as follows: 62 patients received TBI-based conditioning with either cyclophosphamide (n = 44) or etoposide (n = 18), 51 intravenous busulfan-based conditioning with either melphalan (n = 31) or clofarabine (n = 20), and 2 received carmustine, etoposide, cytarabine, melphalan and alemtuzumab. Recipients of matched unrelated grafts received a total dose of 4mg/kg ATG (n = 29). Tacrolimus and mini-methotrexate (5mg/m2 on days 1,6 and 11 post transplant) were used for GVHD prophylaxis in all patients. Forty patients received BM grafts, and 75 PBSC.
Patients were managed at both institutions according to each institution's standard guidelines. GVHD was diagnosed clinically, confirmed pathologically when possible and graded according to standard criteria13. GVHD diagnosed after day 100 post-transplant was classified as chronic GVHD14. Only patients who engrafted were evaluable for GVHD assessment. The cause of death was determined using a standard algorithm15.
Data collection and statistical methods
Data was updated as of December 2012. Patient characteristics were compared between the TCD and unmodified graft groups using the Wilcoxon rank sum test for continuous covariates, and chi-square test or Fisher's exact test for categorical covariates where appropriate. Overall survival (OS) and relapse-free survival (RFS) were defined as the time from HCT until death from any cause and disease relapse or death, respectively. Non-relapse mortality (NRM) was defined as death in a patient without leukemia relapse. Univariate probabilities and 95% confidence intervals of OS and RFS were estimated using Kaplan-Meier methodology, and survival distributions were compared across patient and treatment characteristics using a logrank test. The cumulative incidence of NRM, leukemia relapse, and GVHD was estimated based on the cumulative incidence method of competing risks. Leukemia relapse, death in the absence of leukemia relapse, and relapse or death in the absence of GVHD were considered competing risks for NRM, leukemia relapse, and GVHD, respectively. Gray's test was used to compare the incidence of NRM, leukemia relapse, and GVHD between patients receiving unmodified versus TCD grafts. The incidence of relapse for the two graft types was additionally compared separately for CR1 and CR2 patients. Cox proportional hazards regression was used to evaluate the univariate associations between patient and transplant characteristics with respect to OS and RFS. Patient and transplant characteristics included, type of graft (TCD, unmodified), age (older than 50 years), gender, immunophenotype (B-cell, T-cell, other), cytogenetic risk, CR status at transplantation (CR1, CR2), donor type (HLA-matched related, HLA-matched unrelated, HLA-mismatched), and stem cell source (bone marrow, peripheral blood). A multivariate Cox regression model was implemented to further investigate the association between risk of relapse or death and graft type, CR status, age, and cytogenetics. Statistical analysis was performed using R 3.0.0.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Donor type differed between the unmodified and TCD groups, with a higher percentage of patients in the unmodified group receiving a transplant from a matched related donor (MRD) and only one patient in this group receiving a transplant from a mismatched donor (p<0.001). Additionally, a higher percentage of patients received grafts from peripheral blood in the TCD cohort compared to the unmodified group (88.5% vs. 65.2%, p=0.003). Since in-vivo T-cell depletion with alemtuzumab or ATG could affect outcomes in the unmodified graft group, we compared the use of in-vivo T-cell depletion within this group and found no significant difference with respect to OS and RFS (data not shown). As a result, all patients in the unmodified group were pooled for subsequent analysis except for GVHD.
Table 1. Patient Characteristics.
| Characteristics | TCD (N=52) | Unmodified (N=115) | p-value | ||
|---|---|---|---|---|---|
| Followup - survivors (months)* | 38.9 (20.5-97.4) | 47.9 (6.0,126.1) | - | ||
|
| |||||
| Age (years)* | 40.4 (18.0-67.2) | 34.0 (18.0,64.0) | 0.176 | ||
|
| |||||
| >50 Years | 13 (25.0) | 18 (15.7) | 0.221 | ||
|
| |||||
| Sex = Female | 18 (34.6) | 48 (41.7) | 0.483 | ||
|
| |||||
| Time to CR1 (days)* | 38.5 (7,180) | 30 (14,210) | 0.047 | ||
|
| |||||
| Time from CR1 (days) to HCT* (CR1 patients only) | 109.0 (15.0,288.0) | 101.0 (16.0,570.0) | 0.787 | ||
|
| |||||
| Immunophenotype | 0.099 | ||||
| T-Cell | |||||
| WBC ≤ 50,000 | 3 (5.8) | 10 (8.7) | |||
| WBC > 50,000 | 3 (5.8) | 1 (0.9) | |||
| missing | 1 (1.9) | 1 (0.9) | |||
| B-Cell | 0.102 | ||||
| WBC ≤ 30,000 | 25 (48.1) | 53 (46.1) | |||
| WBC > 30,000 | 6 (11.5) | 32 (27.8) | |||
| missing | 4 (7.7) | 18 (15.7) | |||
| Other | 9 (17.3) | - | |||
| missing | 1 (1.9) | - | |||
|
| |||||
| Cytogenetic Risk | 0.747 | ||||
| Good | 1 (2.0) | 5 (4.9) | |||
| Standard | 29 (58.0) | 61 (59.8) | |||
| Poor | 20 (40.0) | 36 (35.3) | |||
| missing | 2 | 13 | |||
|
| |||||
| Donor Type | <0.001 | ||||
| Matched Related | 21 (40.4) | 72 (62.6) | |||
| Matched Unrelated | 15 (28.8) | 42 (36.5) | |||
| Mismatch | 16 (30.8) | 1 (0.9) | |||
|
| |||||
| Stem Cell Source | 0.003 | ||||
| Bone Marrow | 6 (11.5) | 40 (34.8) | |||
| Peripheral Blood | 46 (88.5) | 75 (65.2) | |||
|
| |||||
| TKI1 | 0.849 | ||||
| yes | 5 (22.7) | 13 (28.3) | |||
| no | 17 (77.3) | 33 (71.7) | |||
|
| |||||
| CR Status | |||||
| 1st | 36 (69.2) | 60 (52.2) | 0.058 | ||
| 2nd | 16 (30.8) | 55 (47.8) | |||
|
| |||||
| ATG | < 0.001 | ||||
| yes | 39 (75.0) | 29 (25.2) | |||
| no | 13 (25.0) | 86 (74.8) | |||
|
| |||||
| TBI | < 0.001 | ||||
| yes | 47 (90.4) | 62 (53.9) | |||
| no | 5 (9.6) | 53 (46.1) | |||
Median(range)
Counts (percents) based on total po ssible individuals rec eiving TKI
Numeric variables tested via Wilcoxon Signed-Ranked Test
Categorical variables tested via Pearson's Chi-squared or Fisher's Exact Tests (excluding missing values) where appropriate.
Overall survival, relapse free survival, and relapse incidence
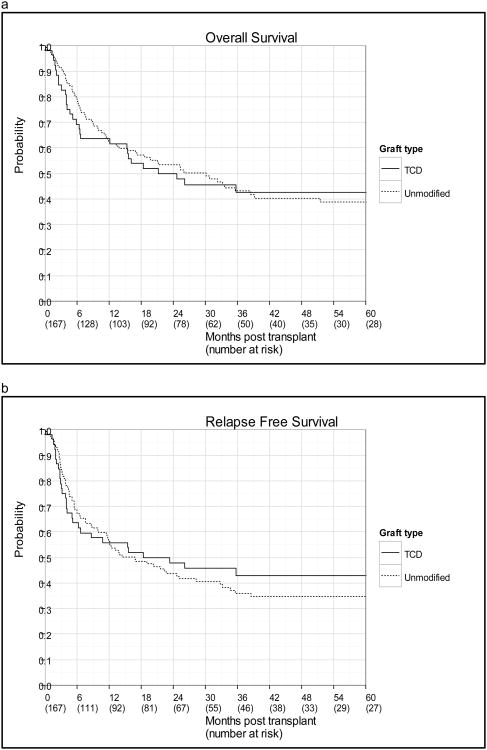
As of December 2012, the median-follow up among surviving patients was 38.9 months (range: 20.5-97.4 months) in the TCD group and 47.9 months (6.0-126.1 months) in the unmodified group. No statistically significant difference was observed between the TCD and unmodified groups with respect to OS, and RFS (Figure 1, Table 2). The 3 year survival probabilities in the TCD and unmodified groups were 42.6% vs. 43.0% (p=0.854) for OS and 42.8% vs. 35.9% for RFS (p=0.653). The 3-year cumulative incidence of relapse was 21.3% in the TCD group and 35.5% in the unmodified group (p=0.107). Separated by CR status, the incidence of relapse remained lower for the TCD cohort; however, neither comparison reached statistical significance (CR1: p=0.261, 3-yr estimates 16.7% vs. 29.0%; CR2: p= 0.431, 3-yr estimates 32.3% vs. 42.5%).
Figure 1.
(a) OS and (b) relapse-free survival by graft type.
Table 2. Outcomes in T-Cell Depleted and Unmodified Grafts.
| Outcome | TCD (95% CI) : N=52 | Unmodified (95% CI) : N=115 | p-value |
|---|---|---|---|
| Relapse-free survival | 0.653 | ||
| 1 year | 55.8 (41.3-68.0) | 56.3 (46.7-64.8) | |
| 3 year | 42.8 (28.8-56.1) | 35.9 (26.8-45.0) | |
|
| |||
| Overall survival | 0.854 | ||
| 1 year | 63.5 (48.9-74.9) | 62.4 (52.8-70.5) | |
| 3 year | 42.6 (28.6-56.0) | 43.0 (33.3-52.4) | |
|
| |||
| Relapse incidence | 0.107 | ||
| 1 year | 19.2 (9.8,31.0) | 20.1 (13.3-27.9) | |
| 3 year | 21.3 (11.3,33.4) | 35.5 (26.5-44.6) | |
|
| |||
| Non-relapse mortality | 0.368 | ||
| 100 day | 15.4 (7.1-26.5) | 9.6 (5.1-15.8) | |
| 1 year | 25.0 (14.2-37.4) | 23.6 (16.3-31.8) | |
| 3 year | 35.9 (22.6-49.4) | 28.6 (20.5-37.3) | |
|
| |||
| Acute GVHD (grade 2-4) | 0.001 | ||
| 100 day | 17.3 (8.5-28.8) | 42.6 (33.4-51.5) | |
|
| |||
| Acute GVHD (grade 3-4) | 0.164 | ||
| 100 day | 7.7 (2.4,17.0) | 15.7 (9.7,22.9) | |
|
| |||
| Chronic GVHD | 0.006 | ||
| 3 year | 13.5 (5.8-24.3) | 33.4 (24.9-42.2) | |
Non-relapse mortality
The NRM at day 100, 1 year and 3 years was 15.4%, 25.0% and 35.9% in the TCD group and 9.6%, 23.6% and 28.6% in the unmodified group (p=0.368). Causes of death in the TCD group included relapsed leukemia (n = 11, 37.9%), GVHD (n = 5, 17.2%), infection (n = 7, 24.1%), organ toxicity (n = 5, 17.2%), and graft failure (n =1, 3.4%). In the unmodified group, causes of death included relapse (n = 34, 51.5%), GVHD (n = 21, 31.8%), infection (n = 8, 12.1%), and organ toxicity (n = 3, 4.5%). Death in the first 100 days post-transplant occurred in 9 (17.3%) patients in the TCD group (4 from infection, 1 acute GVHD, 1 non-engraftment, 2 organ failure, 1 relapse), and 11 (9.6%) patients in the unmodified group (4 from infection, 6 from acute GVHD, 1 from organ toxicity). Patients in the unmodified group had a lower incidence of death due to infection than the patients in the TCD group (p=0.046).
Graft-versus-host-disease
The rate of grade 2-4 acute GVHD was significantly lower in the TCD group than in the unmodified group (p=0.001, 100 day estimates 17.3% vs. 42.6%). No significant difference was noted in the rate of grade 3-4 acute GVHD, which was low in both cohorts (p=0.164, 100 day estimates 7.7% vs. 15.7% in the TCD and conventional groups, respectively). The rate of chronic GVHD was significantly lower in the TCD group compared to the unmodified group (p=0.006, 3 year estimates 13.5% vs. 33.4%). In the unmodified group, 39 patients developed chronic GVHD, including 26 with extensive cGVHD, while 7 patients in the TCD group developed chronic GVHD, including 3 with extensive cGVHD. Due to potential differences in aGVHD incidence among patients receiving ATG, a subset analysis was conducted looking at the grade 2-4 aGVHD incidence among patients whom received ATG. A significant difference remained between TCD and unmodified grafts with TCD grafts exhibiting a significantly lower incidence of aGVHD 2-4 (23.1% vs. 55.2% at 100 days, p=0.005). Among patients receiving ATG, the TCD group exhibited a lower incidence of chronic GVHD compared to the unmodified group (p=0.100, 3 year estimates 17.9% vs. 32.3%).
Use of Tyrosine Kinase Inhibitors (TKIs)
TKIs were administered to a subset of patients with Philadelphia chromosome positive (Ph+) ALL at both centers. At MDACC 13 patients out of 46 Ph+ patients (28.3%) received TKI therapy (all with imatinib) and at MSKCC 5 out of 22 patients (22.7%) received TKI therapy (3 with imatinib, 2 with dasatinib). There was no significant difference between Ph+ patients that received TKI and those who did not receive a TKI with respect to OS, RFS, aGVHD or cGVHD.
Prognostic Factors
In the univariate analyses shown in Table 3, age > 50 and CR status increased both the risk of death (HR=2.08, p=0.003 and HR=1.55, p=0.034) and the risk of relapse or death (HR=1.95, p=0.005 and HR=1.55, p=0.029). No other factor achieved statistical significance at the 0.05 level. In a multivariate model shown in table 4, age > 50 (HR=2.41, p=0.001, HR=2.16, p=0.003), and CR2 status (HR=2.26, p=0.002, HR=2.47, p<0.001), were associated with an inferior OS and RFS, respectively, poor risk cytogenetics was also associated with inferior RFS (HR=1.77, p=0.022). There was no association between graft type and OS or RFS (HR 1.039, p=0.869 and HR=0.90, p=0.635 respectively).
Table 3. Univariate Analyses of risk factors associated with relapse-free survival (RFS) and overall survival (OS).
| RFS, HR (95% CI) | p-value | OS, HR (95% CI) | p-value | |
|---|---|---|---|---|
| Age | 0.005 | 0.003 | ||
| ≤ 50 years | reference | Reference | ||
| > 50 years | 1.946 (1.228,3.085) | 2.084 (1.290,3.366) | ||
|
| ||||
| Gender | 0.747 | 0.775 | ||
| Male | reference | Reference | ||
| Female | 0.937 (0.628,1.396) | 0.942 (0.623,1.423) | ||
|
| ||||
| Immunophenotype | 0.179 | 0.280 | ||
| T-Cell | reference | Reference | ||
| B-Cell | 1.349 (0.701,2.596) | 1.212 (0.628,2.339) | ||
| Other | 0.505 (0.139,1.834) | 0.495 (0.136,1.800) | ||
|
| ||||
| Cytogenetic Risk | 0.191 | 0.568 | ||
| Good or Standard | reference | Reference | ||
| Poor | 1.325 (0.869,2.022) | 1.134 (0.737,1.745) | ||
|
| ||||
| Status at HSCT | 0.029 | 0.034 | ||
| CR1 | reference | Reference | ||
| CR2 | 1.547 (1.047,2.287) | 1.546 (1.033,2.313) | ||
|
| ||||
| Donor type | 0.768 | 0.630 | ||
| Matched related | reference | Reference | ||
| Matched unrelated | 0.899 (0.585,1.381) | 0.967 (0.622,1.503) | ||
| Mismatch | 1.144 (0.600,2.183) | 1.339 (0.698,2.567) | ||
|
| ||||
| Stem Cell Source | 0.381 | 0.387 | ||
| Bone marrow | reference | Reference | ||
| Peripheral blood | 1.224 (0.778,1.925) | 1.228 (0.771,1.956) | ||
|
| ||||
| Graft type | 0.654 | 0.853 | ||
| Unmodified | reference | Reference | ||
| TCD | 0.906 (0.589,1.395) | 1.042 (0.673,1.613) | ||
|
| ||||
| TBI | ||||
| no | reference | 0.281 | reference | 0.725 |
| yes | 0.800 (0.533,1.201) | 0.926 (0.603,1.423) | ||
Table 4. Multivariate analysis of risk factors associated with relapse-free survival (RFS) and overall survival (OS).
| RFS, HR (95% CI) | p-value | OS, HR (95% CI) | p-value | |
|---|---|---|---|---|
| Age | 0.003 | 0.001 | ||
| ≤ 50 years | reference | reference | ||
| > 50 years | 2.158 (1.296,3.595) | 2.414 (1.409,4.137) | ||
|
| ||||
| Cytogenetic Risk | 0.022 | 0.178 | ||
| Good/standard | reference | reference | ||
| Poor | 1.771 (1.085,2.892) | 1.409 (0.856,2.318) | ||
|
| ||||
| Graft Type | 0.635 | 0.869 | ||
| Unmodified | reference | reference | ||
| TCD | 0.897 (0.573,1.405) | 1.039 (0.658,1.643) | ||
|
| ||||
| CR status | < 0.001 | 0.002 | ||
| 1 | reference | reference | ||
| 2 | 2.468 (1.514,4.023) | 2.257 (1.359,3.748) | ||
Discussion
Our results represent the first published comparison of TCD versus conventional graft transplantation solely in ALL. A randomized, prospective study done by the Unrelated Donor Marrow Transplantation Trial compared patients with a variety of hematologic malignancies, TCD only achieved a 1 Log reduction and necessitated post-transplant cyclosporine16. Thus, our study is the largest, albeit retrospective, comparison of outcomes of different graft sources in ALL. Overall survival was similar between the two groups. However the cumulative incidences of relapse, grades 2-4 GVHD and cGVHD were lower in the TCD group.
Improvements in supportive care, the use of reduced intensity conditioning and more accurate HLA typing contribute to decreased treatment-related mortality with allogeneic transplantation. T-cell depletion potentially represents an important advance towards further reducing toxicity from transplantation, mainly in the form of decreased GVHD. Few reports exist comparing outcomes for these two transplantation strategies11, 17. We recently reported outcomes of patients receiving TCD versus conventional grafts for AML and showed similar findings, primarily a reduced incidence of GVHD without adversely affecting relapse or survival11.
There are few publications with TCD transplantation in ALL. Patel et al18 reported on long-term outcomes of 48 patients receiving in-vivo TCD grafts with alemtuzumab for patients with poor risk Ph- ALL in CR1. Outcomes were favorable, with OS at 5 years of 61%, aGVHD of 27% and cGVHD at 5 years of 22%. The largest report to date of TCD transplantation in ALL (n = 56) was recently published by Goldberg et al4, with a 2 year OS of 39% and incidence of GVHD of 20% at 1 year, without any grade 4 GVHD reported. In addition, a significant proportion of patients in this series had high-risk cytogenetic features. These reports, along with our current study, add to the growing body of literature suggesting that TCD is a safe and effective transplantation strategy.
The current study does have some limitations. The retrospective nature of the study is an inherent limitation. The patients in each treatment group were not homogeneous and included patients on clinical trials, which may be associated with selection bias. Furthermore, a variety of conditioning regimens were used. Although data is missing for the induction and consolidation therapy patients received, there is likely variation in this regard as well. In addition, post-transplant maintenance with TKIs were variably used in both centers for Ph+ patients, although not significantly different between the two centers. Finally, CR was defined by the absence of blasts. Normalization of blood counts and minimal residual disease (MRD) were not taken into account, and thus our comparison of relapse rates between the two transplant approaches may be limited.
Our study also challenges the applicability of findings from recent studies showing that PBSCT is associated with higher rates of GVHD when compared to bone marrow after ablative conditioning19. In our study, patients at MSKCC almost exclusively received PBSC (88.5%), compared to MDACC where 34.8% of patients received BM. However, the rates of GVHD at MSKCC were significantly lower. It is likely that the protective benefit of TCD with respect to the occurrence of GVHD outweighs the benefit for BM as a graft source. A recent CIBMTR study evaluated the outcomes of patients with ALL receiving cord blood (CB), PBSC and BM grafts20. Similar rates of aGVHD were seen in patients receiving PBSC and BM grafts, with lower rates in the CB group, and similar rates of cGVHD regardless of graft source. In addition to demonstrating comparable outcomes in the CB group compared to the PBSC and BM groups, this study suggests that all three graft sources are acceptable for patients with ALL.
In addition to more patients receiving PBSCT, patients in the TCD group were significantly more likely to receive a mismatched graft (30.8% vs. 0.9%). Despite this, GVHD rates were significantly lower in the TCD group. This result is in line with prior reports; in the study by Bayraktar11 et al 23% of TCD recipients vs. 8% of unconventional graft recipients received mismatched grafts and rates of GVHD were significantly lower in the TCD group. This suggests that less stringent HLA matching may be acceptable in TCD recipients.
In our study, patients receiving TCD grafts experienced lower relapse and incidence of GVHD, with similar OS between both groups. This suggests that other factors, such as infection, may be responsible for the added mortality in the TCD group. Although there was no statistically significant difference in the incidence of death due to infection, the numbers may be too small to conclusively determine this. Another possible explanation for similar OS seen in both groups is that although the rates of aGVHD were significantly different in both groups, the rates of grades 3-4 aGVHD were similar in both graft groups. To further understand the difference in relapse incidence, analysis were conducted separately for CR1 and CR2, and although relapse incidence remained lower in the TCD group, the numbers in the subset were small and did not reach statistical significance.
The similar outcomes in both T-cell replete and TCD groups further supports a limited role for graft versus leukemia (GVL) effect in ALL21, 22 suggesting that other mechanisms may explain the benefits of HCT for ALL.
In summary, TCD and unmodified grafts offer similar overall and relapse free survival rates, but TCD grafts are associated with significantly lower incidence of GVHD. A future prospective randomized study is being planned to compare these different transplant approaches.
Acknowledgments
This work was supported in part by PO1 CA23766 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MDACC authors had no acknowledgements.
Footnotes
Authorship: M.-A.P. and P.K. designed and performed research, analyzed data, enrolled patients in the study, and wrote the manuscript; G.S.H. and A.H. performed research, analyzed data, and wrote the manuscript; J.D.G., E.B.P. A.A.J. R.J.O R.E.C, S.G. enrolled patients in the study and wrote the manuscript G.R., P.H. and S.M.D. analyzed data and wrote the manuscript. All authors approved the final manuscript.
Conflict of Interest: R.E.C and P.K. receive research support from Otsuka. There are no other conflicts of interest to report.
References
- 1.Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in Philadelphia-positive acute lymphoblastic leukaemia. Bone Marrow Transplant. 2008;41(5):447–453. doi: 10.1038/sj.bmt.1705904. [DOI] [PubMed] [Google Scholar]
- 2.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111(4):1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 3.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22(20):4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg JD, Linker A, Kuk D, Ratan R, Jurcic J, Barker JN, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(2):208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebriaei P, Saliba R, Rondon G, Chiattone A, Luthra R, Anderlini P, et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact of tyrosine kinase inhibitors on treatment outcomes. Biol Blood Marrow Transplant. 2012;18(4):584–592. doi: 10.1016/j.bbmt.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–1090. [PubMed] [Google Scholar]
- 7.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(4):458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perales MA, Jenq R, Goldberg JD, Wilton AS, Lee SS, Castro-Malaspina HR, et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone marrow transplantation. 2010;45(9):1408–1416. doi: 10.1038/bmt.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayraktar UD, de Lima M, Saliba RM, Maloy M, Castro-Malaspina HR, Chen J, et al. Ex Vivo T Cell Depleted versus Unmodified Allografts in Patients with Acute Myeloid Leukemia in First Complete Remission. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 14.Sullivan KM. Chronic graft-versus-host disease. Cancer treatment and research. 1990;50:79–98. doi: 10.1007/978-1-4613-1493-6_5. [DOI] [PubMed] [Google Scholar]
- 15.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366(9487):733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 17.Pasquini MC, Devine S, Mendizabal A, Baden LR, Wingard JR, Lazarus HM, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel B, Kirkland KE, Szydlo R, Pearce RM, Clark RE, Craddock C, et al. Favorable outcomes with alemtuzumab-conditioned unrelated donor stem cell transplantation in adults with high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in first complete remission. Haematologica. 2009;94(10):1399–1406. doi: 10.3324/haematol.2009.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagler A, Labopin M, Shimoni A, Niederwieser D, Mufti GJ, Zander AR, et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(9):1422–1429. doi: 10.1016/j.bbmt.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2013 doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 22.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]