Abstract
Background
Physical performance measures have been shown to predict mortality and incident cardiovascular disease (CVD) mainly in elderly populations. We evaluated whether physical performance measures are associated with vascular health indices (carotid intima-media thickness (cIMT), adventitial diameter (cAD) and carotid plaque) in a large sample of multi-ethnic, late midlife women.
Methods
Participants from the Study of Women’s Health Across the Nation free of CVD and who had carotid ultrasound assessed at the 12th annual visit were evaluated. Physical function (PF) measures at visit 12 included: average 40-foot walking speed and average time needed for sit-to-stand assessment.
Results
A total of 1103 women (53.7% White, 30.5% Black, 15.9% Chinese) aged 59.6±2.7 years at visit 12, were included. In models adjusted for study site, race, current age, menopausal status and systolic blood pressure, slower walking speed and longer time needed for sit-to-stand were significantly associated with wider cAD, thicker cIMT and a higher probability of a high level of carotid plaque burden (all P-values <0.05). Associations between walking speed and cAD, and between time needed for sit-to-stand and cAD, remained significant (P=0.04) or marginally significant (P=0.07), respectively, after additional adjustment for CVD risk factors, medications and physical activity. However, the associations between PF measures and cIMT and plaque burden were largely explained by traditional CVD risk factors.
Conclusions
The current study suggest that worse performance in simple objective PF tests may be an early indicator of vascular structural changes that precede vascular disease among women at late midlife.
Keywords: Physical performance, Vascular health, Intima-media thickness, Plaque, Adventitial diameter, Menopause
Introduction
Physical performance measures, such as gait speed, the long-distance walking test and grip strength, predict disability, mortality (1, 2) and incident cardiovascular disease (CVD) (3) in diverse elderly populations. Carotid intima-media thickness (cIMT), adventitial diameter (cAD), and carotid plaque are markers of arterial wall alteration and vascular health that precede the development of CVD. The relationships between physical performance measures and vascular health indices remain uncertain in younger population as most prior studies were conducted in the elderly, after early stage vascular remodeling (or vascular changes) has occurred. Results from these studies have shown that coronary artery calcification (4, 5), carotid plaque (6), and cIMT (5,6) were inversely related to walking speed in older populations.
No previous studies have evaluated the relationship between physical performance measures and cAD. Furthermore, whether similar associations exist between physical performance measures and vascular health indices, including cAD, among healthy younger populations is not known. Evaluating these associations among middle aged women, in particular, is of great interest given that limitations in subjectively-assessed physical performance have been observed more frequently in women compared to men (7,8) at middle age (40–55 years) (9). Additionally, substantial changes in vascular health indices including cAD and cIMT have been reported among women at midlife (10).
The main aim of this study was to examine whether walking speed and time needed for sit-to-stand assessment were associated with vascular health indices (cIMT, cAD, and carotid plaque) in a large sample of multi-ethnic, late midlife women.
Methods
Study population
The Study of Women’s Health Across the Nation (SWAN) is an ongoing multiethnic multisite longitudinal study designed to characterize biological and psychological changes over the menopausal transition. Details of the study design and procedures have been reported previously (11). In brief, each study site recruited non-Hispanic White women and women belonging to a predetermined racial or ethnic minority group (Black women in Pittsburgh, Boston, Michigan, Chicago; Japanese in Los Angeles; Hispanic in New Jersey; Chinese in the Oakland area of California).
Baseline eligibility criteria for the SWAN study included being aged 42–52 years, having an intact uterus and at least one ovary, not pregnant or lactating, not using oral contraceptives or hormone therapy, and having at least one menstrual cycle in the 3 months prior to the baseline interview. Annual clinic assessments began in 1996–1997. Study protocols were approved by the institutional review boards at each site and each participant provided written informed consent. The current study examined associations between physical performance measures and vascular health indices completed for the first time at the 12th annual visit (2009–2011) of the SWAN study.
Of the 3,302 women enrolled in the SWAN study, 1,552 completed carotid ultrasound assessment (all SWAN study sites except Los Angeles). Of those, 172 women were excluded as a result of having no data for physical performance measures (the New Jersey site of the SWAN study did not participate in either the sit-to-stand or the walking speed assessments; therefore, Hispanic women were not included) and/or carotid ultrasound measures including cIMT, cAD and carotid plaque. Additionally, 114 women were excluded as a result of having reported stroke, angina or myocardial infarction at any time point before the 12th annual visit. Women who had hysterectomy and/or bilateral oophorectomy (n=158) and those with undetermined menopausal status at visit 12 (n=5) were also excluded. Final sample size included 1103 women.
Women who were excluded from the current analysis were more likely to be Black, less educated, current smoker, have diabetes and were more likely to ever use cardiovascular medications (antihypertensive, lipid lowering and/or heart medication). In addition, excluded women had a higher BMI, lower high-density lipoprotein cholesterol (HDL-C), higher triglycerides, insulin resistance index and systolic blood pressure, thicker cIMT and wider cAD and required longer time to complete the sit-to-stand and the 40-foot walking performance tests at visit 12 compared with those who were included in the study (all P<0.05).
Carotid Ultrasound Assessments
Carotid ultrasound images were obtained at each study site by centrally trained sonographers using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency 5 to 12 Mhz linear array transducer and were data streamed to the Ultrasound Research Laboratory, University of Pittsburgh for centralized reading. The reading software used was the AMS system developed in Sweden by Dr. Thomas Gustavsson (12). Digitized images were obtained of the left and right distal common carotid artery (CCA), 1 cm proximal to the carotid bulb. cIMT measures were obtained by tracing electronically the lumen-intima interface and the media-adventitia interface across a 1-cm segment of the near and far walls of the right and left distal CCA; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The average values for these measures were recorded for all 4 segments. For analyses, the mean of the average readings at all 4 segments was used. cAD was measured directly as the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole across the same CCA segments used for cIMT measurement. Reproducibility of cIMT and cAD measures was excellent with an intraclass correlation coefficient between sonographers > 0.77 and > 0.90, and between readers > 0.89 and > 0.89, respectively.
The presence and extent of plaque were evaluated in the left and right carotid artery in each of 5 segments (distal and proximal CCA, carotid bulb, and proximal internal and external carotid arteries) and summarized as the presence or absence of any plaque (13). Plaque was defined as a distinct area protruding into the vessel lumen that was at least 50% thicker than the adjacent cIMT. For each segment, the degree of plaque was graded between 0 (no observable plaque) and 3 (plaque covering 50% or more of the vessel diameter). The grades from all 10 segments of the combined left and right carotid artery were summed to create the plaque index. The plaque index was found to be a valid and reproducible measure of carotid atherosclerosis in a number of populations, with intraclass correlations ranging from 0.86 to 0.93 (14). For the current analysis, plaque burden was used and defined as none if plaque index=0, minimal if plaque index=1 and high if plaque index was > 1.
Physical Function Performance Measures
All physical function performance measures were obtained at each study site by trained examiners. The 40-foot walking test measured the time in seconds that elapsed while participants walked 40 feet at a brisk, purposeful pace. The area for the walk was located in a non-skid, non-wet surface and adjacent to a wall that could allow support should the participant require it. Two repetitions were performed and the average of the two was used. Walking speed was calculated from average time of the 40-foot walking assessment by dividing distance in meters (12.192) by time in seconds.
The sit-to-stand test measured the time in seconds that it took for participants to rise from a normal-height wooden bench or a wooden chair (approximately 18″ off the floor) with their arms crossed over their chests to a standing position with both arms down at the sides. Five repetitions were performed and an average time was calculated.
In secondary analyses, the minimum 40-foot walking time of the two repetitions and the minimum time needed for sit-to-stand of the 5 repetitions were used as well and the results were similar to what was reported in the current manuscript (data not shown).
Study Covariates
For the current analysis, all covariates were obtained from the 12th annual visit unless otherwise specified.
Blood assays
A fasting blood specimen was obtained during the early follicular phase of the menstrual cycle (day 2–5) if participants were still menstruating. If a timed sample could not be obtained, a random fasting sample was taken within the 90-day period of the 12th annual visit. Lipids, glucose and insulin were performed on a Siemens ADVIA 2400 automated chemistry analyzer utilizing Siemens ADVIA chemistry system reagents (Siemens Healthcare Diagnostics, Deerfield IL) at the University of Michigan Pathology Laboratory, CLIA certified and accredited by the College of American Pathologists. Triglycerides, high-density lipoprotein cholesterol (HDL-C) and direct low density lipoprotein cholesterol (LDL-C) (15) were analyzed using a coupled enzymatic methods that utilized lipase, glycerol kinase, glycerol-3-phosphate oxidase (G3PO), and peroxidase (for triglycerides) and cholesterol esterase, cholesterol oxidase, and peroxidase (for HDL-C and direct LDL-C). Serum insulin was measured by a two-site sandwich immunoassay using direct chemiluminescent technology which uses constant amounts of two antibodies. Glucose was measured using a two-step enzymatic reaction that utilizes hexokinase and glucose-6-phosphate dehydrogenase (G6PDH) enzymes. The HOMA index was calculated from fasting insulin and glucose as (insulin (mU/Liter)* glucose (mmoles/Liter)/22.5) (16).
Physical measures
BMI was calculated as measured weight/measured height2. Blood pressure was averaged after two sequential measures in the right arm with the participant seated after at least 5 minutes of rest. Physical activity was assessed via a modified Baecke Scores of Habitual Physical Activity with higher scores indicating more physical activity (17).
Demographic, socioeconomic, co-morbidity and smoking variables
Race/ethnicity was self-reported at the SWAN screening interview. Age was calculated using the date of the 12th annual interview minus date of birth. The following variables were created using data from baseline through visit 12: 1) Smoking status was defined as never: never reported smoking at any previous visit, ever: reported smoking at any previous visit or current: reported smoking at visit 12; 2) ever use of medications (self-reported) including antihypertensive, lipid-lowering and heart medications at any visit (Yes/No) and ever use of hormone replacement therapy (self-reported) at any visit (Yes/No); 3) participants were classified as having diabetes by visit 12 if they self-reported diabetes or had fasting glucose levels ≥126 mg/dL, or reported any use of insulin or anti-diabetic agents on at least 70% of the visits and/or for at least 3 consecutive visits.
Menopausal status
Menopausal status was determined based on reports about frequency, regularity of menstrual bleeding as follow: 1) Premenopause: monthly bleeding with no perceived change in cycle interval, 2) Early peri-menopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months, 3) Late peri-menopause: 3 consecutive months of amenorrhea, 4) Postmenopause: 12 consecutive months of amenorrhea. Given that, the majority of the women were postmenopausal by visit 12, premenopausal, early perimenopausal and late perimenopausal women were grouped as “not postmenopausal.”
Statistical Analysis
Independent (physical performance measures), dependent variables (cIMT, cAD), and covariates were examined for distributions and outliers and transformation was applied as appropriate. Time needed for sit-to-stand, triglycerides and HOMA insulin resistance index were log transformed. First, we evaluated cIMT, cAD, and level of plaque burden as a function of physical functioning performance measures (separate models). Unadjusted linear regression was used for cIMT and cAD and unadjusted multinomial logistic regression was used for level of plaque burden since the proportional odds of assumption was violated. Next, models were adjusted for potential confounders as identified from the bivariate analyses (supplemental table 1) and/or based on biological plausibility. Effect modification by race and BMI were tested and not significant. To better understand the relationships between cAD and walking speed and time needed for the sit-to-stand test, both walking speed and time needed for sit-to-stand were additionally modeled as quartiles instead of as continuous measures. Analyses were performed with SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Study population characteristics and summary statistics of vascular health indices and physical performance measures are summarized in Table 1.
Table 1.
Characteristics of the Study Sample at the 12th Annual Visit
| Study Variables | Total N=1103 |
|---|---|
| Age, years, mean(SD) | 59.6 (2.7) |
| Race, n(%)* | |
| White | 592 (53.7) |
| Black | 336 (30.5) |
| Chinese | 175 (15.9) |
| Menopausal Status, n(%) | |
| Not postmenopausal | 32 (2.9) |
| Postmenopausal | 1071 (97.1) |
| Body mass index, Kg/m2, mean(SD) | 29.5 (7.3) |
| Systolic blood pressure, mmHg, mean(SD) | 120.6 (16.7) |
| LDL-C, mg/dL, mean(SD) | 120.4 (31.6) |
| HDL-C, mg/dL, mean(SD) | 62.7 (16.3) |
| Triglycerides, mg/dL, median (IQR) | 96.0 (73.0, 134.0) |
| HOMA insulin resistance index, median (IQR) | 2.0 (1.2, 3.6) |
| Smoking status, n(%) | |
| Never | 689 (62.5) |
| Ever | 321 (29.1) |
| Current | 93 (8.4) |
| Ever use of cardiovascular medications, n(%)† | 584 (53.0) |
| Ever use of HT, n(%) | 390 (35.4) |
| Diabetic, n(%) | 105 (9.5) |
| Physical activity score, mean(SD) | 7.7 (1.8) |
| cIMT, mm, mean(SD) | 0.79 (0.1) |
| cAD, mm, mean (SD) | 7.2 (0.6) |
| Level of plaque burden, n(%) | |
| None | 609 (55.2) |
| Minimal | 215 (19.5) |
| High | 279 (25.3) |
| Average time needed for sit-to-stand, s, median (IQR) | 1.1 (0.8, 1.5) |
| Average walking speed, m/s, mean (SD) | 1.4 (0.3) |
LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, cIMT: carotid intima-media thickness, cAD: carotid adventitial diameter
Los Angeles site did not participate in carotid ultrasound assessment and therefore data were not available for the Japanese participants of the SWAN study
Ever use of cardiovascular medications including antihypertensive, lipid-lowering and heart medications
Table 2 provides unadjusted and adjusted results for associations between walking speed and each index of vascular health. In minimally adjusted models (model1), faster walking speed was significantly associated with thinner cIMT, narrower cAD and a lower probability of a high burden of carotid plaque (all P-values <0.01). Further adjustment for BMI eliminated the significant association between cIMT and walking speed only. Additional adjustment for lipids, insulin resistance, smoking status, ever use of cardiovascular medications and hormone therapy, diabetes and level of physical activity (model 3) attenuated the association between walking speed and high burden of carotid plaque (P=0.1). On the other hand, walking speed remained significantly associated with cAD independent of all CVD risk factors included in final model (P=0.04). Walking speed was not associated with minimal burden of plaque in either bivariate (supplemental table 1) or multivariable models.
Table 2.
Multivariable Associations between Vascular Health Measures and Walking Speed (m/s)
| cIMT (mm) | cAD (mm) | Carotid plaque burden | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (SE) | P value | β (SE) | P value | OR (95% CI) None vs. minimal |
P value | OR (95% CI) None vs. high |
P value | |
|
| ||||||||
| Unadjusted | −0.080 (0.012) | <.001 | −0.497 (0.066) | <.001 | 1.140 (0.669, 1.943) | 0.6 | 0.625 (0.384, 1.018) | 0.06 |
| Model 1 | −0.045 (0.014) | 0.001 | −0.427 (0.076) | <.001 | 1.030 (0.529, 2.005) | 0.9 | 0.339 (0.182, 0.631) | 0.001 |
| Model 2 | −0.017 (0.015) | 0.3 | −0.189 (0.081) | 0.02 | 1.201 (0.583, 2.477) | 0.6 | 0.423 (0.213, 0.839) | 0.01 |
| Model 3 | −0.016 (0.015) | 0.3 | −0.176 (0.085) | 0.04 | 1.346 (0.635, 2.852) | 0.4 | 0.569 (0.277, 1.169) | 0.1 |
LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, cIMT: carotid intima-media thickness, cAD: carotid adventitial diameter
Model 1 : Adjusted for age, race, site, menopausal status, SBP.
Model 2 : Model 1 + BMI.
Model 3 : Model 2 + LDL-C, HDL-C, log transformed triglycerides, log transformed HOMA index, smoking status, ever use of cardiovascular medications (antihypertensive/lipid lowering/heart medication), ever use hormone therapy, diabetes status, and physical activity.
Adjusted and unadjusted associations between time needed for sit-to-stand and vascular health indices were provided in Table 3. In model 1, longer time needed for the sit-to-stand test was significantly associated with thicker cIMT, wider cAD and a higher probability of a high burden of carotid plaque (all P-values <0.05). Further adjustment for BMI eliminated the significant associations between time needed for the sit-to-stand test and both cIMT and high burden of carotid plaque. Additional adjustment for other covariates (model 3) attenuated the association between time needed for sit-to-stand and cAD (p=0.07). Time needed for the sit-to-stand test was not associated with minimal burden of plaque in either bivariate (supplemental table 1) or multivariable models.
Table 3.
Multivariable Associations between Vascular Health Indices and Time needed for Sit-To-Stand*
| cIMT (mm) | cAD (mm) | Carotid plaque burden | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (SE) | P value | β (SE) | P value | OR (95% CI) None vs. minimal |
P value | OR (95% CI) None vs. high |
P value | |
| Unadjusted | 0.028 (0.009) | 0.001 | 0.225 (0.048) | <.001 | 0.709 (0.478, 1.053) | 0.09 | 1.562 (1.098, 2.223) | 0.01 |
| Model 1 | 0.025 (0.010) | 0.009 | 0.204 (0.055) | 0.0002 | 0.882 (0.547, 1.424) | 0.6 | 1.613 (1.049, 2.478) | 0.03 |
| Model 2 | 0.015 (0.010) | 0.1 | 0.118 (0.054) | 0.03 | 0.836 (0.513, 1.363) | 0.5 | 1.432 (0.921, 2.225) | 0.1 |
| Model 3 | 0.015 (0.010) | 0.1 | 0.103 (0.056) | 0.07 | 0.809 (0.490, 1.335) | 0.4 | 1.182 (0.734, 1.901) | 0.5 |
LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, cIMT: carotid intima-media thickness, cAD: carotid adventitial diameter
Log transformed
Model 1: Adjusted for age, race, site, menopausal status, SBP.
Model 2: Model 1 + BMI.
Model 3: Model 2 + LDL, HDL, log transformed triglycerides, log transformed HOMA index, smoking status, ever use of medications (antihypertensive/lipid lowering/heart medication), ever use hormone therapy, diabetes status, and physical activity.
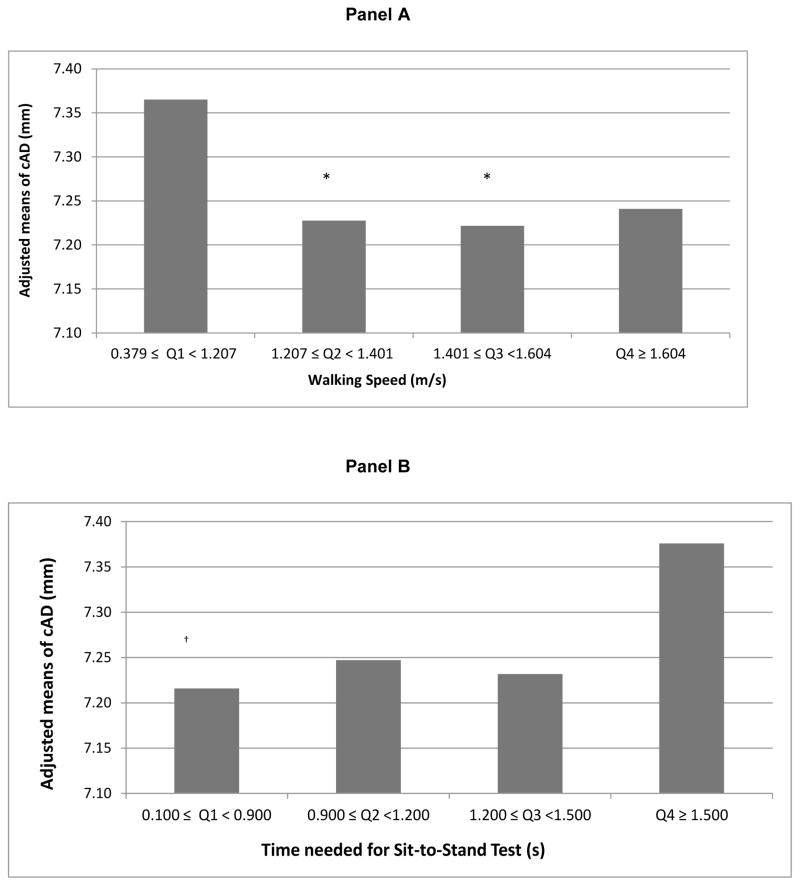
When evaluating cAD as a function of quartiles of walking speed and time needed for sit-to-stand (separate models) in final models, threshold effects seem to emerge (Figure 1, panel A and B, respectively). Women whose walking speed was less than 1.207 m/s, and those who needed 1.5 seconds or more to perform the sit-to-stand test, had significantly wider cAD (~2% wider relative to the comparison group (Figure 1)) compared to those with waking speed equal to or higher than 1.207 m/s (P<0.05 for Q1 vs. Q2 and Q1 vs. Q 3; P=0.07 for Q1 vs. Q4) and those who needed less than 0.9 seconds (P<0.05 for Q1 vs. Q4) to perform the sit-to-stand test, respectively.
Figure 1.
Adjusted Means of cAD by Quartiles of Walking Speed (A) and Time Needed for Sit-To-Stand Test (B)
* Significantly differ from 1st quartile, P <0.05.
†Significantly differ from 4th quartile, P <0.05.
Models adjusted for age, race, site, menopausal status, SBP, BMI, LDL-C, HDL-C, log transformed triglycerides, log transformed HOMA index, smoking status, ever use of cardiovascular medications (antihypertensive/lipid lowering/heart medication), ever use of hormone therapy, diabetes status, physical activity score
Discussion
Using a multi-ethnic sample of community-dwelling women, the current study showed that poor performance in simple objective measures of physical function, such as the 40-foot walking speed test is associated with vascular structural changes at late midlife. Women with a slower walking speed had a wider adventitial diameter of the carotid artery and tended to have a higher probability of a high burden of carotid plaque independent of age, study site, race, BMI, traditional CVD risk factors, use of medications and hormone therapy, level of physical activity and diabetic status. The associations between cAD, walking speed and time needed for sit-to-stand quartiles suggest possible non-linear effects. Late midlife women whose walking speed is less than 1.207 m/s or who needed at least 1.5 seconds to perform the sit-to-stand assessment significantly had wider (2% wider) cAD compared with those who walked faster or performed the sit-to-stand test in a shorter time. A threshold of 1 m/s for walking speed has been shown to predict persistent lower extremity limitation, hospitalization, and death in older adults (mean age 74.2) (18). The higher threshold for walking speed that we reported is likely due to our relatively younger study population. Additionally, it suggests that gradient vascular changes can be identified in initially healthy midlife women.
The direction of the association between cAD and walking speed among midlife women is in agreement with previous reports which were mainly conducted in elderly populations using other indices of vascular health. In a study of 387 healthy elderly participants (mean age 78.7±3.7, 35% men) in the Cardiovascular Health Study, coronary artery calcification was inversely related to gait speed in women, but not in men (4). Hamer et al. reported independent associations between faster walking speed and lower prevalence and extent of coronary artery calcification, and lower cIMT, in 530 healthy older adults (mean age 63±6, 50.3% men) from the Whitehall II cohort study (5). Further, in the Three-City study, cIMT and carotid plaque were inversely associated with objectively measured maximum walking speed (6). We extend these findings to a younger population of healthy women at late midlife and show for the first time that walking speed is significantly associated with vascular remodeling as measured by carotid artery dilation. cAD has been shown to be an informative measure of vascular health. Wider cAD is correlated with adverse cardiovascular risk factors (19), and is associated with a higher risk of coronary heart disease (20). Compensatory enlargement of the central arterial diameter due to a chronic increase in arterial pressure is associated with the breaking of elastin fibers as a result of high tensile wall stress (21). A dilated artery may itself lead to a disturbance in blood flow as the wider artery may have less ability to dilate further in response to a stimulus, which may make the artery more vulnerable to damage. Recent data suggests that an enlarged carotid artery diameter may serve as a useful clinical marker for identifying subjects at risk for heart failure (22).
In the current study we were not able to report an independent association between any of the evaluated physical performance measures and cIMT, as has been reported in previous manuscripts using data from the Whitehall II cohort study (5) and the Three-City study (6). This is most likely due to discrepancies between study populations rather than the use of different protocols to measure cIMT. Both the Whitehall II and Three-City studies were conducted in older adults (mean age 63 and 73, respectively) when changes in cIMT are obvious and can be easily detected. Subclinical measures appear to worsen, and functional status to decline, across the age range of 65–85 in both men and women (23). In addition, both the Whitehall II cohort study and the Three-City study included men and women while our study only included women at late midlife. In postmenopausal women of similar age to our study, Pettee et al did not find an independent association between cIMT and walking performance (24).
Several possible mechanisms may explain the association between low levels of physical performance and vascular health alterations. Due to the cross-sectional nature of this work, we are unable to determine whether vascular health alteration is a consequence of physical functioning limitation or vice versa. For example, a lower level of physical activity is a well-known CVD-risk factor (25) and slower walking speed could be considered as a marker of poor fitness. On the other hand, physical functioning limitations as measured by slower walking speed could be a manifestation of vascular health alterations.
Higher cerebral white matter hyperintensities, the most commonly imaged biomarker of small-vessel (microcirculation) disease, which was found to be associated with impaired physical functioning as measured by slower walking speed (26), has also been found to be independently associated with larger common carotid artery diameter in a sample of hypertensive individuals without a prior history of stroke and neuroimaging evidence of asymptomatic cerebral infarction (27). The same study failed to document any relationship between cIMT and white matter lesions, suggesting that alterations in carotid arterial diameter may be more specifically related. Unfortunately we did not evaluate white matter hyperintensities among SWAN women and therefore we were not able to test the above hypothesis.
Another possibility is that carotid arterial dilation could be a marker of cardiac geometry alteration (22), which has been found to be associated with physical performance limitation (28). Frailty, defined based on the presence of three out of 5 criteria including weight loss, low grip strength, low energy, slow gait speed and low physical activity, appeared to be associated with cardiac geometry alterations including reduced global left ventricular function and increased left ventricular mass (28). Interestingly, larger carotid artery diameter was found to be associated with worse left ventricular geometry in patients with heart failure and preserved ejection fraction (22). Finally, slow walking speed could be a marker of peripheral arterial disease. Out of 1103 participants included in the current study, only 9 women self-reported peripheral arterial disease (PAD) in the neck and/or legs/feet by visit 12. Medical records were available for only 3 women and were used to confirm PAD among 2 of them. With these limitations in adjudicating PAD events, we could not rule out the possibility of some confounding effect.
We excluded women who experienced surgical menopause as those women have been shown to report higher levels of physical functioning limitations (29). Additionally, CVD events that are related to vascular health alteration (e.g. stroke, heart attack and angina) may be a source of confounding and therefore were excluded from this analysis. It is unlikely that the current study was biased by the applied exclusion criteria. Participants who were excluded were more likely to be obese, diabetic, ever used cardiovascular medications, have lower HDL, higher triglycerides, insulin resistance index and systolic blood pressure, thicker IMT and wider AD and required longer time to complete the sit-to-stand and the 40-foot walking performance tests. It is most likely that including the excluded participants would strengthen the current findings. Still, we were able to document significant or marginal associations between both walking speed and time needed for sit-to-stand, and vascular remodeling indicator among apparently healthy midlife women.
The current study has some limitations. The cross-sectional design limits our ability to understand the temporality and the cause-effect aspects of the evaluated associations. The current study only included women at late midlife and therefore the results may not be generalized to men or other age groups. Regardless, this is the first study to evaluate associations between physical performance measures and vascular health indices in a cohort of multi-ethnic community-dwelling women at relatively younger ages compared with previous studies among elderly.
It is important to understand the temporal nature of the associations between the progression in physical functioning limitations and changes in subclinical vascular disease and thus longitudinal assessments are critical. CVD and physical functioning limitation are two important conditions that significantly increase as women age. It is crucial to understand how each condition impacts the other to better design appropriate prevention strategies.
In conclusion, the current data suggest that poor performance in simple non-invasive objective physical functioning tests, such as walking speed, may be an early indicator of structural changes in vascular health at late midlife. These tests, which can be performed in a few minutes, could be included among measures used to gauge CVD risk in women.
Supplementary Material
Highlights.
Poor performance in simple non-invasive physical functioning tests may be an early indicator of structural changes in vascular health in late midlife women.
Independent of potential covariates, women with a slower walking speed had a wider carotid adventitial diameter and tended to have a higher probability of a high burden of carotid plaque.
Acknowledgments
Acknowledgement of Grant Support
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495).
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Disclosures
None
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 2.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–25. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 4.Inzitari M, Naydeck BL, Newman AB. Coronary artery calcium and physical function in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2008;63:1112–8. doi: 10.1093/gerona/63.10.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamer M, Kivimaki M, Lahiri A, et al. Walking speed and subclinical atherosclerosis in healthy older adults: the Whitehall II study. Heart. 2010;96:380–4. doi: 10.1136/hrt.2009.183350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–202. doi: 10.1161/01.STR.0000181752.16915.5c. [DOI] [PubMed] [Google Scholar]
- 7.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Memorial Fund Q. 1976;54:439–67. [PubMed] [Google Scholar]
- 8.Ross CE, Bird CE. Sex stratification and health lifestyle: Consequence for men’s and women’s perceived health. J Health Soc Behav. 1994;355:161–78. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions—United States, 1991–1992. MMWR Morb Mortal Wkly Rep. 1994;43:730–31. 737–39. [PubMed] [Google Scholar]
- 10.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression Rates of Carotid Intima-media Thickness and Adventitial Diameter during the Menopausal Transition. Menopause. 2013;20:8–14. doi: 10.1097/gme.0b013e3182611787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowers M, Crawford S, Sternfed B, et al. SWAN: a multicenter multiethnic community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: biology and pathology. New York (NY): Academic Press; 2000. pp. 175–88. [Google Scholar]
- 12.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clinical Physiology. 1991;11:565–77. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 13.Thompson T, Sutton-Tyrrell K, Wildman RP, et al. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis and rheumatism. 2008;58:835–42. doi: 10.1002/art.23196. [DOI] [PubMed] [Google Scholar]
- 14.Sutton-Tyrrell K, Wolfson SK, Jr, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23:215–20. doi: 10.1161/01.str.23.2.215. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Matsui H, Ito Y, Fujiwara A, Inano K. Low-density lipoprotein cholesterol can be chemically measured: a new superior method. The Journal of laboratory and clinical medicine. 1998;132:195–201. doi: 10.1016/s0022-2143(98)90168-8. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 18.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 19.Jensen-Urstad K, Jensen-Urstad M, Johansson J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke. 1999;30:1572–6. doi: 10.1161/01.str.30.8.1572. [DOI] [PubMed] [Google Scholar]
- 20.Crouse JR, Goldbourt U, Evans G, et al. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27:69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW, O’Rourke MF, editors. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 3. Philadelphia, Pa: Lea & Febiger; 1990. pp. 77–142.pp. 216–69.pp. 283–359.pp. 398–437. [Google Scholar]
- 22.Liao ZY, Peng MC, Yun CH, et al. Relation of carotid artery diameter with cardiac geometry and mechanics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2012;1:e003053. doi: 10.1161/JAHA.112.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bild DE, Fitzpatrick A, Fried LP, et al. Age-related trends in cardiovascular morbidity and physical functioning in the elderly: the Cardiovascular Health Study. J Am Geriatr Soc. 1993;41:1047–56. doi: 10.1111/j.1532-5415.1993.tb06451.x. [DOI] [PubMed] [Google Scholar]
- 24.Pettee KK, Larouere BM, Kriska AM, et al. Associations among walking performance, physical activity, and subclinical cardiovascular disease. Prev Cardiol. 2007;10:134–40. doi: 10.1111/j.1520-037x.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 25.Davey Smith G, Shipley MJ, Batty GD, Morris JN, Marmot M. Physical activity and cause-specific mortality in the Whitehall study. Public Health. 2000;114:308–15. doi: 10.1038/sj.ph.1900675. [DOI] [PubMed] [Google Scholar]
- 26.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–4. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 27.Heliopoulos I, Artemis D, Vadikolias K, Tripsianis G, Piperidou C, Tsivgoulis G. Association of ultrasonographic parameters with subclinical white-matter hyperintensities in hypertensive patients. Cardiovasc Psychiatry Neurol. 2012;2012:616572. doi: 10.1155/2012/616572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman AB, Gottdiener JS, Mcburnie MA, et al. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 29.Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause. 2012;19:1186–92. doi: 10.1097/gme.0b013e3182565740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.