Abstract
Heavy prenatal alcohol exposure results in a range of deficits, including both volumetric and functional changes in brain regions involved in response inhibition such as the prefrontal cortex and striatum. The current study examined blood oxygen level-dependent (BOLD) response during a stop signal task in adolescents (ages 13–16 y) with histories of heavy prenatal alcohol exposure (AE, n = 21) and controls (CON, n = 21). Task performance was measured using percent correct inhibits during three difficulty conditions: easy, medium, and hard. Group differences in BOLD response relative to baseline motor responding were examined across all inhibition trials and for each difficulty condition separately. The contrast between hard and easy trials was analyzed to determine whether increasing task difficulty affected BOLD response. Groups had similar task performance and demographic characteristics, except for full scale IQ scores (AE < CON). The AE group demonstrated greater BOLD response in frontal, sensorimotor, striatal, and cingulate regions relative to controls, especially as task difficulty increased. When contrasting hard vs. easy inhibition trials, the AE group showed greater medial/superior frontal and cuneus BOLD response than controls. Results were unchanged after demographics and FAS diagnosis were statistically controlled. This was the first fMRI study to utilize a stop signal task, isolating fronto-striatal functioning, to assess response inhibition and the effects task difficulty in adolescents with prenatal alcohol exposure. Results suggest that heavy prenatal alcohol exposure disrupts neural function of this circuitry, resulting in immature cognitive processing and motor-association learning and neural compensation during response inhibition.
Keywords: fMRI, Fetal alcohol spectrum disorders (FASD), Fetal alcohol syndrome (FAS), Prenatal alcohol exposure, Response inhibition
1. Introduction
A common clinical observation in neurodevelopmental populations is the inability to withhold strong response tendencies that are contextually or socially inappropriate. Response inhibition (RI) involves suppressing initial prepotent responses, stopping ongoing responses, and guarding a period of delay from competing responses [3]. RI represents a necessary precursor of goal-directed behavior [3,47] that is distinguishable from other executive functions [26,27]. Clinical and preclinical studies support RI deficits following heavy prenatal alcohol exposure [17,37] through increased perseveration [54], impaired inhibitory control [43], and hyperactivity [53]. Children with histories of this exposure exhibit behavioral disinhibition, resulting in increased secondary deficits such as disruptive disorders, academic failure, and social complications [41,48,62,64]. RI deficits may contribute to global executive dysfunction [2], a hallmark deficit in alcohol-affected children (reviewed in [37]).
Neuroimaging studies illustrate alcohol’s teratogenicity on frontal–subcortical structure and function; macro- and microstructural aberrations exist in frontal, subcortical, and white matter regions [33,78]. Caudate and putamen volumes are disproportionately reduced in alcohol-exposed children and correlate with IQ and perseverative errors during inhibition [24,56]. Electrophysiological and functional MRI (fMRI) findings of Go/No-Go (GNG) performance support altered frontal–striatal functioning in children with prenatal alcohol exposure [5–7,68]. Event-related potential (ERP) findings indicated that alcohol-exposed youth exhibited slower wave latencies and smaller wave amplitudes during GNG than controls, suggesting impaired early visual processing and stimulus discrimination, increased cognitive effort, and alternate frontal inhibitory recruitment [5–7,68]. fMRI findings of greater BOLD response in medial and middle frontal regions during GNG relative to performance-matched controls corroborate inefficient frontal functioning in this population although differences in subcortical recruitment during RI are conflicting [23,49].
Since executive control, which includes RI, relates to behavioral dysfunction in alcohol-affected children [41,62,75], understanding the neuropathology underlying RI deficits in this population will increase specificity of known deficits and promote effective interventions. The current study examined RI using the stop-signal task (SST), which may measure different components of RI than GNG. Though generally assumed to measure similar aspects of RI, recent literature indicates benefits of the SST over GNG to assess RI [60,69]. Despite some commonalities, the SST and GNG utilize disparate neural systems [69]; the SST involves frontal–striatal circuits whereas GNG is frontal-parietal dependent. Distinct neural patterns suggest that GNG provides a measure of stimulus learning and action selection relative to SST, which may more accurately measure action cancellation, or RI [63,69]. Discrete task design may contribute to these differences in as much as the SST requires inhibition of ongoing, initiated motor responses instead of action withholding [63,69].
Since prefrontal and striatal brain regions may be especially sensitive to heavy prenatal alcohol exposure, the current investigation of neural correlates of motor RI utilizing the SST in this population may further elucidate compensatory mechanisms utilized by this population. Given previous findings of disrupted frontostriatal structure and function (of greater BOLD response during GNG performance) in alcohol-exposed compared to nonexposed youth, we expected greater BOLD response in alcohol-exposed adolescents relative to controls in prefrontal, subcortical, and cingulate regions, which are recruited during SST performance [23,49,59,63,69].
2. Materials and methods
Two groups (N = 42) of adolescents between 13 and 16 years of age were recruited to the Center for Behavioral Teratology at San Diego State University: those with histories of heavy prenatal alcohol exposure (the AE group) and controls (the CON group). Both male and female subjects were eligible for participation and were recruited through referrals from area healthcare providers, other professionals, and community outreach. Estimates of full scale IQ (FSIQ), from the Wechsler Intelligence Scale for Children (WISC-IV) [77], and socioeconomic status, from the Hollingshead Four Factor Index of Social Status [31], were collected as part of a larger project. Informed assent and consent were obtained prior to participation and subject incentive was provided to all subjects. The Institutional Review Board (IRB) at San Diego State University and University of California San Diego approved all procedures.
2.1. Subjects
The AE group (n = 21) comprised adolescents with histories of heavy prenatal alcohol exposure, defined as an average of ≥14 drinks per week or ≥4 alcoholic drinks per occasion at least once per week during gestation. Prenatal exposure was confirmed retrospectively through medical history, birth records, social services records, and maternal report and questionnaires, when available. In most cases, precise measures of alcohol consumption were unavailable. In these cases, mothers were reported to be “alcoholic” or alcohol abusing or dependent during pregnancy. All subjects were evaluated by a dysmorphologist with expertise in Fetal Alcohol Syndrome (FAS) (KLJ). Seven (33%) subjects in the AE group were diagnosed with FAS [32,40]. Additionally, seventeen (81%) subjects in the AE group met DSM-IV criteria for attention deficit/hyperactivity disorder (ADHD) as determined by the clinician-assisted National Institute of Mental Health Diagnostic Interview Schedule for Children (C-DISC-4.0) [65].
The CON group (n = 21) consisted of adolescents with minimal or no prenatal alcohol exposure, defined as an average of ≤1 drink per week and never more than 2 drinks on a single occasion during gestation. Controls were excluded if they met subclinical or clinical criteria for ADHD. Exclusion criteria for both groups were history of significant head injury or loss of consciousness >30 min, non-fluent English speaker, psychiatric (i.e., active psychosis, pervasive developmental disorder) or physical (i.e., neurological disorders) disability preventing participation, MRI contradictions (i.e., metal in body, claustrophobia), or adopted from abroad after 5 years of age or ≤2 years before assessment.
2.2. Mock scan procedure
Prior to MRI scanning, all subjects underwent a mock MRI at the Center for Behavioral Teratology, San Diego State University. Mock procedures were the similar to actual fMRI protocol and included a pre-training session, a 10-min mock anatomical scan during which subjects watched a movie and were trained to remain still, and an 8 min 20 s SST. Mock procedures have successfully decreased data loss resulting from motion artifact in this population (unpublished findings).
2.3. Stop-signal fMRI task
The event-related SST employed in the current study, based on that described by Matthews et al., has been successfully used to examine neural correlates of RI in other adolescent populations [35,36]. During the task (shown in Fig. 1), visual stimuli were projected at a visual angle of approximately 85° onto a white projection screen at the foot of the MRI bed. Stimuli were white capital letters (X or O) displayed on a black background. Subjects were told to press the right button as quickly and as accurately as possible whenever an “O” appeared, the left button whenever an “X” appeared, and not to press either button whenever they heard a tone during a trial. Stimuli appeared at the beginning of each trial, which lasted 1300 ms or until the subject responded. Trials were separated by a 200 ms interstimulus interval (blank screen). Subjects performed six blocks of 48 trials (12 Stop and 36 Go trials in each block). Stop trials were pseudorandomized and counterbalanced. The Go trials involved only motor responding to an “X” or an “O” and were considered the baseline condition. Task instructions were presented for 12 s between blocks and total task duration was 8 min 20 s.
Fig. 1.

Stop-signal fMRI paradigm instructions. Task based on paradigm described by Matthews et al. [35,36].
Prior to scanning, subjects performed a behavioral practice session consisting of 4 SST blocks to obtain a mean reaction time (MRT). Each subjects’ MRT was used to determine individual inhibition stimulus presentation onset (presentation of the tone after the presentation of the “X” or “O”) as follows: 0, 100, 200, 300, 400, and 500 ms less than the MRT. From these six trial types, three trial difficulties were designated: easy (400–500 ms), medium (200–300 ms), and hard (0–100 ms). Individual response latencies were used to denote the period of inhibitory processing and provided a naturally jittered reference function.
2.4. Image acquisition
Images were acquired at the University of California, San Diego Keck Center for fMRI on a 3 T General Electric Signa Excite whole body system with a body transmit coil and an eight-channel receiver head coil (Milwaukee, WI). Subjects wore earplugs and headphones and foam padding was placed around their heads in attempt to reduce noise and movement and promote comfort during the scan session. The SST was the second functional scan collected as part of a larger neuroimaging battery for which total scan time lasted approximately 60 min (total functional scan time was 20 min). Subjects watched a computer projection, displayed at the foot of the scanner bed, of a movie (during the structural scan) and the SST (during the functional scan).
A 15 s localizer scan was used to ensure correct head placement at the start of the scan, followed by a high-resolution sagittally collected structural image that was used for co-registration with the functional protocol. Structural parameters were as follows: TR = 8000 ms, TE = 3.1 ms, flip angle = 12°, 256 × 192 matrix, 1 mm slice thickness, FOV = 24 cm, and acquisition time of 7 min 24 s. Functional images were collected axially using echo planar imaging with the following parameters: TR = 2000 ms, TE = 32 ms, flip angle = 90°, matrix size = 64 mm × 64 mm, FOV = 24 cm, 4.0 mm slice thickness, 20 slices for whole brain coverage, 256 repetitions, and acquisition time of 8 min 20 s.
3. Statistical analyses
3.1. Demographic data
Demographic variables were analyzed using chi-square and independent t-tests in SPSS version 19.0 [67].
3.2. Task performance data
Group (AE vs. CON) differences in SST performance were examined using independent t-test and repeated measures ANOVA techniques with Group entered as the independent variable. Only subjects with >75% “go” trial accuracy and ≥25% successful stop trials were included in analyses, as these cut-offs have been used in other neurodevelopmental populations [46]. One subject in the AE group was excluded from analysis because of poor task performance and is not included in the current analyses.
3.3. fMRI data
Analysis of BOLD response
All functional image processing was conducted using the Analysis of Functional Neuroimages (AFNI) software package [12]. Slices of each echo planar imaging (EPI) repetition were temporally aligned and motion was corrected by co-registering each repetition to a stable base image using a 6-parameter 3-D algorithm [13]. The time series of motion parameters was used in multiple regressor analysis of individual data to control for spin history effects to increase power in detecting task-related BOLD response [66]. The absolute values of the motion parameters were averaged and compared to determine any group differences in motion during the tasks. Subjects were excluded if the average in any of the motion parameters exceeded 2 standard deviations from the population mean, assuring that differences between groups were not due to increased movement in the clinical group [51,71]. Two subjects in the CON group were excluded from analysis because of excessive motion and are not included in the current analyses.
A multivariate regressor approach was used to relate fluctuations in BOLD signal intensity to changes in task demands across the time series [74]. A 0–1 reference function was convolved with a gamma variate function modeling a prototypical hemodynamic response to shift the regressor according to the hemodynamic delay (6–8 s) [21] and to account for the temporal dynamics of the hemodynamic response (typically 12–16 s) [10]. The convolved time series was normalized and used as a regressor of interest. A series of regressors of interest and the motion regressors were entered into the AFNI program 3dDeconvolve to determine the height of each regressor for each subject, controlling for linear trends and motion correction previously applied. Spatial smoothing using a filter with full-width half-maximum (FWHM) of approximately 1–2-voxel size (i.e., 5 mm) is reported to yield the highest detection power [18]. The spatially smoothed data was then transformed manually in AFNI into standard coordinates based on the high-resolution MR image [29,70]. The key dependent measure produced was the voxel-wise normalized relative percent signal change that represents BOLD response to each condition. In addition to examining BOLD response during the Go (baseline) condition, the following contrasts were examined: All-stop (all inhibition trials relative to baseline), Easy (easy trials relative to baseline), Medium (medium trials relative to baseline, Hard (hard trials relative to baseline), and Hard–Easy (hard trials relative to easy trials).
3.4. fMRI group-level analyses
The voxel-wise percent signal difference was subjected to a mixed model ANOVA, with condition (active vs. comparison) and group (AE vs. CON) as independent factors. All voxel-wise mixed ANOVA results were subjected to a threshold adjustment method based on Monte Carlo simulations in the AFNI program to guard against false positive areas of activation [20]. Given spatial smoothing using a FWHM of 5.0 mm, a voxel-wise a priori probability of .05, a cluster volume of 832 μl with connectivity radius of 4.0 mm has a 5% chance of occurring under the null hypothesis. For the whole-brain analyses only clusters ≥832 μl (i.e., 14 contiguous voxels each with effect p < .05) were interpreted as significant. BOLD response data for the resulting clusters were exported into SPSS for further analysis and outlier identification.
4. Results
4.1. Demographics
As shown in Table 1, groups were similar (ps > 368) on age, sex, race, ethnicity, handedness, SES, and number of TRs removed due to motion artifact during processing. As expected, the AE group had significantly lower FSIQ scores than controls [F(1,32) = 17.19, p < .001].
Table 1.
Demographic and behavioral data for the alcohol-exposed (AE) and control (CON) groups.
| Variable | AE (n = 21) | CON (n = 21) | Statistic |
|---|---|---|---|
| Handedness [N (% Right)] | 20 (95.2) | 21 (100) | X2 (df = 1) = 1.02, p = .311 |
| FAS [N (%)] | 7 (35) | 0 (0) | – |
| ADHD diagnosis [N (% positive)] | 17 (81.0) | 0 (0) | – |
| Sex [N (% Males)] | 12 (57.1) | 12 (57.1) | X2 (df = 1) = .00, p = 1.00 |
| Race [N (% White)] | 12 (57.1) | 13 (61.9) | X2 (df = 11) = 11.71, p = .386 |
| Ethnicity [N (% Hispanic)] | 7 (33.3) | 6 (28.6) | X2 (df = 1) = .11, p = 739 |
| Age at scan [M (SD)] | 14.70 (1.27) | 15.09 (1.51) | F(1, 41) = .83, p = .368 |
| Socioeconomic status (SES) [M (SD)] | 45.88 (11.2) | 48.67 (10.86) | F(1, 41) = .67, p = .418 |
| Days between mock MRI and actual MRI [M (SD)] | 14.52 (15.42) | 14.81 (15.65) | F(1, 41) = .004, p = .953 |
| FSIQ [M (SD)]* | 88.37 (11.96) | 106.61 (11.71) | F(1, 41) = 17.19, p < .001 |
| Pre-training mean reaction time (ms) [M (SD)] | 664.05 (117.41) | 646.81 (119.130) | F(1, 41) = .23, p = .638 |
| Scanner mean reaction time (ms) [M (SD)] | 571.24 (70.52) | 576.34 (79.87) | F(1, 38) = .44, p = .835 |
| % Successful Go trials [M (SD)] | 95.85 (4.33) | 94.75 (4.48) | F(1, 41) = .65, p = .425 |
| % Successful inhibits (averaged across easy, medium, hard) [M (SD)] | 69.50 (11.90) | 70.374 (10.93) | F(1, 41) = 1.06 p = .807 |
| % Successful easy inhibits [M (SD)] | 91.28 (10.19) | 95.049 (6.92) | F(1, 41) = 1.96, p = .169 |
| % Successful medium inhibits [M (SD)] | 78.97 (12.25) | 76.98 (14.83) | F(1, 41) = .22, p = .639 |
| % Successful hard inhibits [M (SD)] | 38.29 (19.58) | 39.09 (16.38) | F(1, 41) = .02, p = .886 |
| Number of repetitions removed due to motion artifact [M (SD)] | 11.52 (7.17) | 12.09 (6.44) | F(1, 41) = .10, p = .753 |
p < .001.
4.2. Task performance
Groups had similar pre-scan (p = .638) and scan MRT (p = .835), indicating similar vigilance and reaction times during task conditions, and similar percent successful inhibits during easy (p = .169), medium (p = .639), and hard (p = .886) trials (see Table 1). As expected from task design, both groups committed significantly greater errors during hard versus easy trials [F(1, 40) = 493.39, p < .001]. However, nonsignificant group [F(1,40) = .40, p = .533] and group × condition [F(1,40) = .36, p = .550] effects indicated similar accuracy rates for each group, regardless of trial difficulty.
4.3. Stop-signal task BOLD response
Between group fMRI response
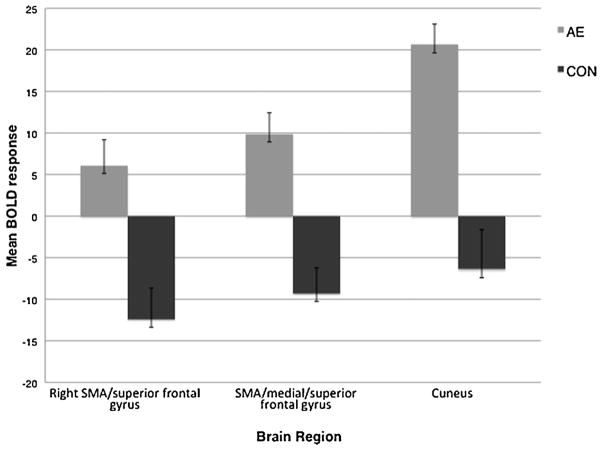
Group differences in task-related BOLD response are shown in Table 2. Groups differed (CON > AE) during baseline (motor responding to an “X” or an “O”) BOLD response in right precentral and left pre- and postcentral gyri. The AE group showed greater BOLD response in the All-stop contrast (All-stop trials relative to baseline) compared to controls in bilateral postcentral, right precentral, left putamen/globus pallidus, left supplementary motor area (SMA)/middle cingulate, and right middle cingulate. To assess BOLD response at each difficulty level, groups were compared on Easy, Medium, and Hard trials relative to baseline. Groups did not differ on BOLD response contrast during the Easy contrast (easy trials relative to baseline). During Medium trials relative to baseline (the Medium contrast), the AE group had more response in left insula/superior temporal and right middle/medial temporal gyri than the CON group. During Hard contrast (hard trials relative to baseline), the AE group had greater BOLD response than the CON group in the left pre- and postcentral gyri, right pre-and postcentral/supramarginal gyrus, left middle cingulate/SMA, left thalamus/caudate, and left anterior/middle cingulate. To determine the effect of increasing task difficulty groups were compared on the Hard–Easy contrast (hard trials relative to easy trials), and the AE group demonstrated greater response in the medial/superior frontal gyrus and cuneus (see Figs. 2 and 3).
Table 2.
Whole brain functional MRI (fMRI) results comparing BOLD response in the alcohol-exposed (AE) group relative to the control (CON) group during the Stop-signal task (cluster level α < .05, with voxel level α < .001).
| Stop-signal contrast | Anatomical region | t (1, 41) | Effect size | Brodmann area | Volume (μl) | Talairach coordinates (reported in LPI)
|
||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Baseline (motor responding) | R precentral gyrus | 4.65 | .351 | 6 | 1408 | 48.9 | −2.8 | 31.8 |
| L pre- and postcentral gyrus | 4.75 | .361 | 6, 4 | 1408 | −45.1 | −11.0 | 32.0 | |
| All-stop | L postcentral gyrus | 4.63 | .349 | 4 | 2304 | −47.0 | −13.0 | 34.8 |
| R precentral gyrus | 4.51 | .337 | 6 | 1344 | 48.7 | −6.9 | 35.0 | |
| L putamen/lentiform nucleus | 4.31 | .317 | – | 1088 | −27.9 | −7.1 | −.9 | |
| L SMA/middle cingulate | 3.62 | .232 | 6, 24 | 1088 | −7.2 | −.5 | 49.2 | |
| R middle cingulate | 4.26 | .312 | 24 | 896 | 12.0 | −13.9 | 38.6 | |
| Medium | L insula/superior temporal gyrus | 5.11 | .395 | 38 | 1024 | −35.5 | −2.0 | −8.5 |
| R middle/medial temporal gyrus | 5.35 | .417 | 21, 20 | 960 | 50.0 | 2.7 | −22.9 | |
| Hard | L postcentral gyrus | 4.85 | .371 | 3, 4 | 5312 | −41.9 | −19.3 | 43.6 |
| R pre- and postcentral/supramarginal gyrus | 5.13 | .397 | 6, 40 | 3712 | 53.0 | −15.8 | 29.9 | |
| L middle cingulate/SMA | 4.063 | .292 | 24, 6 | 2688 | −8.3 | −2.0 | 45.0 | |
| L precentral gyrus | 4.40 | .326 | 6 | 1024 | −25.5 | −4.5 | 29.8 | |
| L thalamus/caudate | 3.84 | .269 | – | 960 | −13.7 | −9.0 | 14.7 | |
| L middle/anterior cingulate | 4.51 | .337 | 24 | 960 | .4 | −2.1 | 32.3 | |
| Hard–easy | R SMA/superior frontal gyrus | 4.18 | .304 | 6 | 1344 | 10.0 | 19.0 | 56.0 |
| SMA/medial/superior frontal gyrus | 4.17 | .303 | 6, 24, 32 | 1088 | −2.0 | 7.0 | 44.0 | |
| Cuneus | 3.62 | .247 | 18 | 1024 | 6.0 | −81.0 | 12.0 | |
Fig. 2.
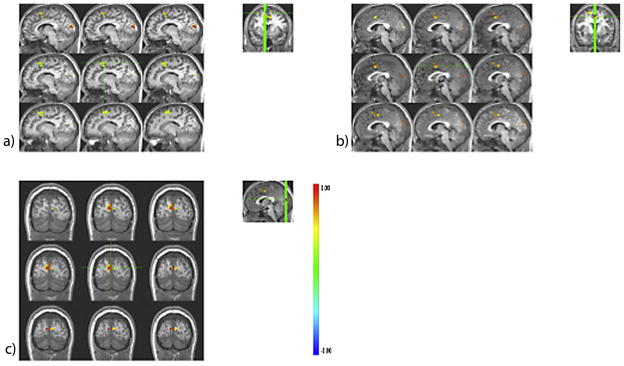
Group differences in stop-signal task blood oxygen level-dependent (BOLD) response (α < .05, corrected) during the Hard–Easy contrast (Hard inhibit trials relative to Easy inhibit trials) in the (a) right supplementary motor area (SMA)/superior frontal gyrus, (b) medial/superior frontal gyrus/SMA, and (c) cuneus. In all regions, adolescents with histories of heavy prenatal alcohol exposure (the AE group) exhibited greater BOLD response compared to controls (the CON group). Coordinates are displayed in Table 2.
Fig. 3.
Difference in BOLD response (i.e., beta weights) in clusters with significant between-group differences during the Hard–Easy contrast (Hard inhibit trials relative to Easy inhibit trials). Data are presented as mean ± standard error of the mean for the alcohol-exposed (AE, n = 21) and control (CON, n = 21) groups.
Within group fMRI response
To explore the nature of group differences, single sample t-tests were conducted within each group separately. Alpha was adjusted using Bonferroni correction (α = .05/2) and only effects with p ≤ .025 (corrected) were considered significant (see Table 3).
Table 3.
Significant single-sample t-test results for the alcohol-exposed (AE) and control (CON) groups (cluster level α < .025, with voxel level α < .001) on the stop-Signal functional MRI (fMRI) task.
| Stop-signal contrast | Anatomical region | Brodmann area | Volume (μl) | Talairach coordinates (reported in LPI)
|
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| The CON group | ||||||
| Baseline (motor responding) | L pre/postcentral gyrus | 4, 3 | 6656 | −43.3 | −17.6 | 43.5 |
| L lingual gyrus/cuneusa | 17, 18 | 5632 | −3.8 | −86.5 | −1.2 | |
| SMA | 6 | 2304 | −1.6 | −.9 | 53.2 | |
| L superior/middle temporal gyrus | 22 | 2240 | −55.0 | −14.7 | 9.0 | |
| L putamen | – | 1664 | −24.9 | −6.8 | 10.6 | |
| R superior temporal/supramarginal gyrus | 40 | 1408 | 58.2 | −33.7 | 18.5 | |
| R superior temporal gyrus | 22 | 1216 | 50.8 | −13.0 | 4.2 | |
| R precentral gyrus | 6 | 1152 | 48.7 | −4.1 | 34.0 | |
| All-stop | L pre/postcentral gyrusa | 4 | 8896 | 45.4 | −15.9 | 39.3 |
| R pre/postcentral gyrusa | 6, 4 | 3648 | 47.7 | −10.0 | 36.1 | |
| L putamena | – | 2688 | −31.2 | −18.0 | 9.0 | |
| R angular/inferior parietal gyrus | 40 | 2432 | 34.1 | −55.7 | 42.0 | |
| R middle temporal gyrus | 21 | 1088 | 50.7 | −34.9 | −2.1 | |
| L cuneus/middle occipital gyrusa | 18 | 1088 | −22.5 | −84.1 | 16.2 | |
| Insulaa | 13 | 960 | −43.9 | −10.1 | 12.5 | |
| Easy | L postcentral gyrusa | 4 | 6336 | −48.2 | −13.8 | 34.3 |
| L posterior cingulate/precuneusa | 31 | 4672 | −8.0 | −56.2 | 17.6 | |
| R inferior parietal lobule | 40 | 3264 | 37.2 | −49.1 | 41.5 | |
| R middle cingulate gyrus | 32 | 3136 | 3.1 | 25.9 | 33.1 | |
| R precentral gyrusa | 4 | 3136 | 46.9 | −11.1 | 40.5 | |
| R middle temporal gyrus | 22 | 2240 | 48.2 | −32.9 | −1.4 | |
| R posterior cingulatea | 30 | 1792 | 14.4 | −52.0 | 9.7 | |
| L middle/superior frontal gyrusa | 8 | 1344 | −3.3 | 24.1 | 40.0 | |
| L paracentral lobulea | 6 | 1280 | −11.6 | −24.6 | 59.8 | |
| R cingulate | 23 | 1216 | .9 | −24.2 | 27.4 | |
| R inferior/middle frontal gyrus | 9 | 1088 | 38.5 | 3.9 | 33.2 | |
| R middle/superior frontal gyrus | 10 | 960 | 31.1 | 47.0 | 14.9 | |
| R middle frontal gyrus | 9 | 960 | 38.0 | 34.5 | 28.3 | |
| L cerebellum | – | 896 | −27.7 | −75.0 | −38.0 | |
| L parahippocampal gyrusa | – | 896 | −22.3 | −15.0 | −11.7 | |
| L inferior parietal lobule | 7 | 896 | −30.0 | −55.9 | 41.4 | |
| Medium | L postcentral gyrusa | 4 | 5248 | −48.1 | −13.2 | 38.0 |
| L superior gyrus/rolandic operculuma | 42, 22 | 2432 | −52.5 | −8.5 | 9.2 | |
| R precentral gyrusa | 6 | 2178 | 45.1 | −9.8 | 41.2 | |
| SMAa | 6 | 1344 | −2.0 | −4.8 | 53.7 | |
| L putamena | – | 960 | −26.3 | −10.1 | 3.5 | |
| R superior temporal gyrus/rolandic operculuma | 22, 43 | 960 | 52.7 | −9.5 | 9.3 | |
| Hard | L pre/postcentral gyrusa | 6, 1, 4a | 3200 | −42.9 | −16.7 | 46.1 |
| L cuneus/middle occipital gyrusa | 18 | 1792 | −20.3 | −84.1 | 17.3 | |
| L cuneus/calcarine gyrusa | 18, 31 | 1152 | −3.6 | −68.6 | 18.2 | |
| R cuneus/calcarine gyrusa | 31 | 896 | 19.1 | −62.4 | 18.9 | |
| R angular gyrus | 39, 7 | 896 | 36.9 | −62.7 | 38.9 | |
| Hard–easy | R cingulatea | 32, 6 | 6464 | 3.0 | 22.1 | 36.3 |
| R insula/inferior frontal gyrusa | 13 | 3456 | 39.0 | 19.1 | 10.1 | |
| R middle/superior frontal gyrusa | 10 | 2495 | 30.1 | 47.0 | 17.1 | |
| Cerebellar vermisa | – | 1472 | .8 | −51.4 | −22.3 | |
| The AE group | ||||||
| Baseline | Middle cingulatea | 31 | 3392 | 3.4 | −30.3 | 36.8 |
| R superior frontal gyrusa | 8 | 896 | 15.7 | 27.0 | 37.7 | |
| All-stop | R thalamus | – | 896 | 14.0 | −5.0 | 8.0 |
| Medium | R inferior frontal gyrus | 45 | 896 | 46.3 | 23.3 | 6.3 |
| Hard | R middle/superior temporal gyrus | 22 | 1984 | 49.9 | −40.9 | 10.1 |
| R thalamus | – | 1024 | 17.5 | −10.5 | 14.8 | |
| R inferior parietal/supramarginal gyrus | 40 | 896 | 56.0 | −28.7 | 24.9 | |
| Hard–easy | Paracentral lobule | 6, 5 | 1216 | .3 | −29.4 | 53.5 |
| L precuneus/postcentral gyrus | 7 | 1024 | −15.0 | −42.0 | 63.2 | |
| L postcentral gyrus | 2, 3 | 960 | −47.6 | −21.3 | 41.3 | |
Indicates negative BOLD response.
4.4. Relation between BOLD response and demographic data
Age
Since cognitive abilities are expected to develop throughout adolescence, correlations were run between subject age and performance and extracted BOLD response clusters. No significant correlations were found between age and performance measures across groups (p ≥ .298), or within the AE (p ≥ .693) or CON (p ≥ .281) groups separately, with the exception of MRT (r = −.345, p = .025) and successful inhibits during Hard trials in the CON group (r = .436, p = .048). For the All-stop contrast, age was significantly associated with BOLD response across groups in the left postcentral gyrus (r = −.332, p = .032), putamen/lentiform nucleus (r = −.411, p = .007), and SMA/middle cingulate (r = −.425, p = .005). When groups were examined separately, age was significantly associated with BOLD response in the left SMA/middle cingulate of the AE group (r = −.441, p = .045) and the left putamen/lentiform nucleus of the CON group (r = −.573, p = .007) during the All-stop contrast. Across groups, age was also significantly associated with BOLD response in the cuneus during the Hard contrast (r = −.369, p = .016) and the left pre- and postcentral gyrus during baseline (r = .314, p = .043). Therefore, older children exhibited less BOLD response in these regions during SST performance, except during baseline (motor responding) whereby younger controls exhibited less BOLD response in the cuneus compared to older controls. Initial BOLD response analyses were rerun with age entered as a model covariate and remained relatively unchanged; group differences on BOLD response remained statistically significant (ps ≤ .002) with age entered into the ANCOVA.
Socioeconomic status (SES)
Across groups, SES correlated significantly with performance during medium difficulty trials (r = .38, p = .013), but was not significantly associated with performance on Go, easy, or hard trials, or with any extracted BOLD cluster values for any of the contrasts (r < −.25, p > .110). SES was not significantly associated with any performance or BOLD response values in the AE or the CON (r < −.32, p > .158) groups separately. Groups remained similar on medium difficulty trials after SES was entered as a model covariate.
Sex
There were no significant sex or group × sex interaction effects on performance measures or BOLD response for any of the contrasts. Further, group effects remained significant when sex and group × sex effects were entered as model covariates.
Handedness
Given initial inclusion of 1 left-handed subject in the AE group, previous analyses were repeated using only the right-handed subjects. Performance (p > .225), and BOLD (p < .001) results remained similar when left-handed subjects were excluded.
FSIQ
As research has indicated that covarying for FSIQ in populations with developmental disabilities is statistically and theoretically inappropriate [15], FSIQ was not considered as a covariate for the analyses. However, we examined the correlations between IQ and BOLD and task performance in the groups separately. IQ was unrelated to behavioral performance and BOLD response in both groups (p > .08).
4.5. Relation between BOLD response and diagnosis of FAS
Although less established in the neuroimaging literature, neuropsychological evidence indicates similar deficits in children with heavy prenatal exposure to alcohol regardless of FAS diagnosis [38,73]. Alcohol-exposed subjects with (FAS; n = 7) and without (noFAS; n = 14) FAS did not differ on performance (ps ≥ .197) or extracted BOLD cluster means (ps > .141), with the exception of significant differences (FAS > noFAS) in the Hard contrast in left postcentral gyrus (p = .028) and left middle cingulate/SMA (p = .019). Trend-level differences (FAS > noFAS) were also found during the All-stop contrast in the left SMA/middle cingulate (p = .099) and during the Hard contrast in the right pre- and post-central and supramarginal gyri (p = .082) and left thalamus/caudate (p = .070).
4.6. Medication effects
Some subjects in the AE group (n = 7; 33%) were unable to abstain from psychostimulant medications during testing (methylphenidate, n = 4; dextroamphetamine sulfate, n = 2; dexmethylphenidate hydrochloride, n = 1). Though the literature on psychostimulant effects in youth with prenatal alcohol exposure is scarce [22,50], psychostimulants affect BOLD response in similar neurodevelopmental populations, including ADHD (e.g., [52]). Therefore, analyses were repeated with medicated subjects excluded from the AE group. With the exception of a trend-level group difference on easy difficulty trials (p = .091; medication usage was associated with better performance), results for SST performance (p ≤ .478) and BOLD (p < .003) values between the AE and CON groups remained similar to those reported above.
4.7. BOLD response and SST performance
Correlations between performance during the SST and BOLD response were examined. Across groups, percent accuracy of Go trials (motor responses to X’s and O’s) was significantly positively correlated with BOLD response during the All-stop contrast in the left postcentral gyrus, right precentral gyrus, and left SMA/middle cingulate (rs = .346–.366, ps = .017–.025) and during the Hard contrast in bilateral pre- and postcentral gyri and left middle cingulate/SMA (rs = .307–.428, ps = .005–.048).
In the AE group, Go trial accuracy was significantly positively associated with BOLD response during the All-stop contrast in the left postcentral gyrus and left SMA/middle cingulate (r = .480, p = .028), during the Hard contrast in the left pre- and postcentral gyri (rs = .457–.571, ps = .007–.037), and during the Hard–Easy contrast in the cuneus (r = −.453, p = .039). Percent successful inhibits during medium difficulty trials significantly negatively correlated with BOLD response in the left putamen during the All-stop contrast (r = .435, p = .049). MRT was significantly positively associated with BOLD response in the left pre- and postcentral gyri during baseline (r = .531, p = .013) and in the left precentral gyrus during the Hard contrast (r = −.500, p = .021).
In the CON group, Go trial accuracy was significantly positively associated with BOLD response during the All-stop contrast in the right precentral gyrus (r = .444, p = .044). Percent successful inhibits of hard trials correlated significantly with BOLD response during the Hard contrast in the left middle cingulate/SMA (r = .483, p = .026) and precentral gyrus (r = .448, p = .042), and during the Hard–Easy contrast in the SMA/medial/superior frontal region (r = .436, p = .048). MRT was significantly associated with BOLD response during the All-stop contrast in the left SMA/middle cingulate cortex (r = .453, p = .039) and during the Hard contrast in the left postcentral gyrus (r = .550, p = .010), right pre- and postcentral/supramarginal gyrus (r = .485, p = .026), and left middle/anterior cingulate cortex (r = .574, p = .007).
5. Discussion
The current study examined neural mechanisms of RI in adolescents with heavy prenatal alcohol exposure using the SST paradigm. This is the first study to utilize the SST to measure RI and to examine increasing task difficulty on BOLD response during RI in alcohol-exposed adolescents. Current findings supported hypotheses, of neural response patterns commensurate with neural compensation in the AE group, and prior neuroimaging findings [23,49]. Relative to controls, the AE group showed greater BOLD response in brain regions associated with RI, including frontal, cingulate, middle temporal, and striatal areas [59,63,69]. Greater response in the right medial superior frontal regions confirms findings implying compensation for immature and inefficient frontal lobe function during inhibitory processing in the AE group [23]. Aberrant frontro-striatal functioning in the AE group also supports clinical reports of impulsivity, behavioral dysregulation, and disruptive disorders in alcohol-exposed adolescents [37]. Results in the control group support findings in typically developing youth [4,58].
While structural and functional neuroimaging studies indicate striatal sensitivity to alcohol’s teratogenicity [39,45,56], this is the first study to show greater striatal and thalamic response in adolescents with prenatal alcohol exposure during RI specifically. These findings likely result from task differences as the SST recruits fronto-striatal networks to a greater extent than GNG paradigms [63,69]. The striatum (i.e., caudate and putamen) and thalamus comprise components of fronto-striatal circuitry; both regions connect with primary motor and prefrontal areas. Greater recruitment of these regions during RI, particularly in the presence of significant associations between BOLD response and task performance, suggests alternate neural strategies and compensatory mechanisms following heavy prenatal alcohol exposure. The putamen is implicated in higher-order cognitions including inhibition and attention [9,42,59,60,63,69] as are the thalamus and caudate, which facilitate decision-making and performance monitoring processes [30]. Greater striatal recruitment in the AE group may reflect compensatory mechanisms resulting from volumetric reductions of basal ganglia nuclei following heavy prenatal alcohol exposure [39,45]. However, future research is needed to fully elucidate which processes result in neural compensation in this population.
Greater BOLD response in primary motor and somatosensory areas in the AE group during RI corroborates prior findings of altered pre- and postcentral functioning in this population [11,33,44]. Results suggest that alcohol-exposed adolescents utilize primary motor and somatosensory areas to a lesser extent than controls during simple motor responding. When taken together with the negative correlations observed between BOLD response during baseline and MRT in the AE group, results further imply that slower motor responses to Go stimuli correspond with less neural recruitment during motor processing. However, despite lower response in these regions during motor responding (baseline), the AE group demonstrated greater BOLD response than controls in pre- and postcentral gyri across trial difficulty levels (All-stop relative to baseline) and the SMA during the Hard trials relative to baseline. These findings substantiate earlier results of aberrant BOLD response in alcohol-exposed youth relative to controls in somatosensory regions during cued RI [49].
Motor, premotor, and supplementary motor regions facilitate planning, preparation, initiation, and control of voluntary movement [19,55,72]. Primary motor cortex functioning also contributes to implicit motor sequence learning and sensory-motor associations (reviewed in [61]). Increased BOLD response in the AE group, despite similar reaction times and SST performance as controls, implies immature motor control and suggests that adolescents with heavy prenatal alcohol exposure may require increased neural resources when learning, initiating, and inhibiting motor responses. This hypothesis was further supported by the positive correlations observed between SST performance and BOLD response in the current study. This may be especially true for adolescents with FAS, as the comparison between heavily exposed youth with and without FAS indicated significant differences in somatosensory motor areas and middle cingulate. Adolescents with FAS may have even greater neural dysfunction and utilize alternate neural pathways compared to alcohol-exposed adolescents without FAS, although these results should be considered preliminary given the small sample size in this comparison and may reflect other factors such as exposure levels.
The current task allowed for a novel investigation of increasing task demands in adolescents with and without heavy prenatal alcohol exposure. The AE group recruited a greater number of RI-related brain regions during hard inhibition trials (relative to baseline) as opposed to those found during the medium difficulty trials relative to baseline, including the left middle and dorsal anterior cingulate and thalamus and caudate. Greater BOLD response in brain regions associated with auditory processing (i.e., insula and temporal regions) in the AE group may suggest aberrant auditory stimulus processing relative controls. As task difficulty increased from easy to hard (the Hard–Easy comparison), the AE group exhibited greater BOLD response in RI-associated regions, specifically the right SMA, medial superior prefrontal gyri and cuneus [1,69], compared to controls. These results extend previous findings of abnormal brain functioning in alcohol-exposed youth [23,49] to include the middle cingulate, which is involved in executive control processes, such as error detection, attention control, and response selection [8]. The middle cingulate cortex also has connections with the superior frontal gyrus, which is associated with visuospatial processing, motor control, and memory processing [69]. Increased recruitment of superior frontal and middle cingulate regions in the alcohol-exposed group suggests that heavy prenatal alcohol exposure disrupts prefrontal connectivity, thus resulting in inefficient neural functioning of these regions during RI. This may be particularly the case as significant associations between BOLD response and SST performance were only observed for controls.
5.1. Limitations
Although our sample was representative of other studies of fetal alcohol spectrum disorders and our study had many strengths, some methodological limitations of the current investigation should be addressed. One factor that needs to be considered in all studies of prenatal alcohol exposure is the probability of other teratogenic exposures. Children with other exposures were not excluded from the current study, and although we do not have precise data for all subjects in the AE group, at least 57% were also exposed to maternal smoking (vs. 5% of controls) and 67% were also exposed to other drugs (vs. 0% of controls). Combined alcohol and nicotine exposure in utero is shown to have deleterious effects on brain volumes [14], although little is known about functional outcomes of these combined effects. Similarly, other drug exposure could have independent effects on brain function, although effects of prenatal alcohol exposure are typically greater than those of other drugs (e.g., [57]). However, we cannot rule out the effect that these other exposures may have had on our results. Further, a third of the subjects in the AE group were unable to abstain from psychoactive medication during testing. Despite our efforts to control for medication effects through follow-up analyses (which indicated similar findings after excluding medicated subjects), long-term medication exposure could be moderating BOLD response in alcohol-exposed youth as shown in other populations (e.g., [52]). The effects of stimulant medication in children with heavy prenatal alcohol exposure are not known and the literature suggests highly variable medication outcomes in this population (see [22,50]). Future research would benefit from an fMRI investigation of psychostimulant medication effects on neural response in children with heavy prenatal alcohol exposure. Additionally, the currently employed task did not allow for an in-depth comparison between successful and failed inhibits. Future research would benefit from elucidating neural mechanisms contributing to successful inhibition following heavy prenatal alcohol exposure.
The majority of our AE group (81.0%) met DSM-IV criteria for ADHD, which is consistent with estimates suggesting that ADHD occurs in 60–90% of heavily exposed children [37]. While parent-rated behavior is worse for heavily exposed youth with ADHD relative to those without ADHD [28,76], this is not the case for performanced-based neurocognition [25]. It is unlikely that the presence of ADHD in the AE group accounted for observed group differences since RI in nonexposed children with ADHD is typically associated with BOLD hypoactivation (for a review see [16]), although an ADHD diagnosis could have attenuated current findings. Future literature would benefit from an in depth analysis comparing neural response in alcohol-exposed children with and without ADHD to understand whether this disorder alters brain functioning in this population. Since these clinical populations can be distinguished on BOLD response during other executive control domains (e.g., spatial working memory) [34], an fMRI comparison of these two clinical populations during RI would elucidate pathological differences contributing to behavioral dysregulation and might facilitate identification of exposed youth.
6. Conclusion
The current results are novel in several ways. This is the first study to utilize the SST in adolescents with heavy prenatal alcohol exposure compared to behaviorally and demographically similar controls and the first study to examine the effect of increasing task demands on brain functioning during RI in this population. In general, findings suggest that youth with heavy prenatal alcohol exposure exhibit immature and inefficient neural patterns during cognitive and motor control. Results extend understanding of damage following heavy prenatal alcohol exposure to RI-related brain regions, including prefrontal and subcortical gray matter structures. Specifically, results suggest that components of fronto-striatal circuitry, especially the striatum, thalamus, and prefrontal functioning, may be especially disrupted by heavy prenatal exposure to alcohol. These findings are clinically relevant as they suggest functional abnormality of brain regions associated with behavioral initiation, monitoring, and inhibition. Functional neuropathology during SST performance explains why alcohol-exposed adolescents are often described as being unable to suppress contextually and socially inappropriate responses, have frequent legal concerns, and have increased risk for substance use disorders [37]. Interventions should consider targeting behavioral monitoring and sensory-motor association learning and control as these could possibly increase contextually appropriate and adaptive behaviors in this population. For instance, multimodal interventions, such as those targeting motor and auditory behaviors simultaneously, could be used to increase on-line information processing in this population. Also, using a more cognitively demanding laboratory task of RI (outside of the scanning environment) will provide additional details about the nature of the response inhibition deficit in this population. In combination, such research could help further explain how neural dysfunction resulting from prenatal alcohol exposure impacts behavioral outcomes and inhibitory deficits.
HIGHLIGHTS.
Examined neural function of response inhibition in prenatal alcohol exposure.
Exposure resulted in greater prefrontal and subcortical activation.
Greater BOLD was especially related to increased task difficulty.
Exposed youth with FAS trended towards greater BOLD response than those without FAS.
Results suggest that this exposure disrupts fronto-striatal circuitry.
Acknowledgments
Research described in this paper was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant number AA019605. Additional funding was provided through grant T32 AA013525. The authors extend gratitude to the families and children who graciously participate in our studies and to the members of the Center for Behavioral Teratology for ongoing assistance and support, particularly the efforts of Amy Flink, Heather Holden, Eileen Moore, and Jill Vander Velde.
Footnotes
Conflicts of interest
None to declare.
References
- 1.Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. ACER. 2005;29:337–46. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- 2.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Barkley RA. Response inhibition in attention-deficit hyperactivity disorder. Ment Retard Dev Disabil Res Rev. 1999;5:177–84. [Google Scholar]
- 4.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burden MJ, Westerlund A, Muckle G, Dodge N, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. The effects of maternal binge drinking during pregnancy on neural correlates of response inhibition and memory in childhood. ACER. 2011;35:69–82. doi: 10.1111/j.1530-0277.2010.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, Klorman R, Nelson CA, Avison MJ, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. ACER. 2010;34:617–27. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 7.Burden MJ, Andrew C, Saint-Amour D, Meintjes EM, Molteno CD, Hoyme HE, Robinson LK, Khaole N, Nelson CA, Jacobson JL, Jacobson SW. The effects of fetal alcohol syndrome on response execution and inhibition: an event-related potential study. ACER. 2009;33:1994–2004. doi: 10.1111/j.1530-0277.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- 8.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–46. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DJ, Leckman JF, Pauls D. Neuropsychiatric disorders of childhood: Tourette’s syndrome as a model. Acta Paediatr Suppl. 1997;422:106–11. doi: 10.1111/j.1651-2227.1997.tb18357.x. [DOI] [PubMed] [Google Scholar]
- 11.Coles CD, Li Z. Functional neuroimaging in the examination of effects of prenatal alcohol exposure. Neuropsychol Rev. 2011;21:119–32. doi: 10.1007/s11065-011-9165-y. [DOI] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–8. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw P, Zwart F, Schrama R, van Engeland H, Durston S. Prenatal exposure to cigarette smoke or alcohol and cerebellum volume in attention-deficit/hyperactivity disorder and typical development. Translational Psychiatry. 2012;2:e84. doi: 10.1038/tp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–7. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- 18.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 19.Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–31. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 21.Frackoviak RJ, Friston KJ, Frith CD, Mazziotta JC. Human brain function. New York: Academic Press; 1997. [Google Scholar]
- 22.Frankel F, Paley B, Marquardt R, O’Connor M. Stimulants, neuroleptics, and children’s friendship training for children with fetal alcohol spectrum disorders. J Child Adol Psychop. 2006;16:777–89. doi: 10.1089/cap.2006.16.777. [DOI] [PubMed] [Google Scholar]
- 23.Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. ACER. 2007;31:1415–24. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 24.Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP. Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. ACER. 2012;36:1932–41. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass L, Ware AL, Crocker N, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN the CIFASD. Neuropsychological deficits associated with heavy prenatal alcohol exposure are not exacerbated by ADHD. Neuropsychology. 2013;27:713–24. doi: 10.1037/a0033994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman-Rakic PS. Specification of higher cortical function. Head Trauma Rehabil. 1993;8:13–23. [Google Scholar]
- 27.Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 28.Graham DM, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN the CIFASD. Prenatal alcohol exposure, attention-deficit/hyperactivity disorder, and sluggish cognitive tempo. ACER. 2013;37:E338–46. doi: 10.1111/j.1530-0277.2012.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child’s Nervous Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 31.Hollingshead AB. Unpublished working paper. 1975. Four factor index of social status. [Google Scholar]
- 32.Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:e1734–8. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- 33.Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–18. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malisza KL, Buss JL, Bolster RB, de Gervai PD, Woods-Frohlich L, Summers R, Clancy CA, Chudley AE, Longstaffe S. Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD: a functional magnetic resonance imaging study. J Neurodev Disord. 2012;4:12. doi: 10.1186/1866-1955-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport. 2005;16:755–60. doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- 37.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–53. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 39.Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. ACER. 1996;20:1088–93. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å, Autti-Rämö I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP the CIFASD. Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010;44:635–41. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. Ame J Drug Alcohol Abuse. 2008;34:423–31. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- 42.McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–82. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol. 2001;23:453–62. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 44.Miller MW. Effects of prenatal exposure to alcohol on the distribution and time of origin of corticospinal neurons in the rat. J Comp Neurol. 1987;257: 372–82. doi: 10.1002/cne.902570306. [DOI] [PubMed] [Google Scholar]
- 45.Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. ACER. 2011;35:1404–17. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- 46.Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- 47.Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–98. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 48.Nigg JT. Response inhibition and disruptive behaviors: toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Ann N Y Acad Sci. 2003;1008:170–82. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien JW, Norman AL, Fryer SL, Tapert SF, Paulus MP, Jones KL, Riley EP, Mattson SN. Effect of predictive cuing on response inhibiton in children with heavy prenatal alcohol exposure. ACER. 2013;37:644–54. doi: 10.1111/acer.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Malley KD, Koplin B, Dohner VA. Psychostimulant clinical response in fetal alcohol syndrome. Can J Psychiatry. 2000;45:90–1. [PubMed] [Google Scholar]
- 51.Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- 52.Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, 3rd, Xiong J, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry. 2006;163:1052–60. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- 53.Riley EP, Shapiro NR, Lochry EA. Nose-poking and head-dipping behaviors in rats prenatally exposed to alcohol. Pharmacol Biochem Behav. 1979;11:513–9. doi: 10.1016/0091-3057(79)90034-0. [DOI] [PubMed] [Google Scholar]
- 54.Riley EP, Lochry EA, Shapiro NR, Baldwin J. Response perseveration in rats exposed to alcohol prenatally. Pharmacol Biochem Behav. 1979;10:255–9. doi: 10.1016/0091-3057(79)90097-2. [DOI] [PubMed] [Google Scholar]
- 55.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–36. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 56.Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 2012;33:920–37. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O’Connor MJ, Bookheimer SY, Sowell ER. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54:3067–75. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–75. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 59.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–99. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 61.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 62.Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–52. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- 63.Sebastian A, Pohl MF, Kloppel S, Feige B, Lange T, Stahl C, Voss A, Klauer KC, Lieb K, Tuscher O. Disentangling common and specific neural subprocesses of response inhibition. Neuroimage. 2012;64 C:601–15. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- 65.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV. (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Skudlarski P, Constable RT, Gore JC. ROC analysis of statistical methods used in functional MRI: individual subjects. Neuroimage. 1999;9:311–29. doi: 10.1006/nimg.1999.0402. [DOI] [PubMed] [Google Scholar]
- 67.SPSS. SPSS 19.0 for Mac OS X. Chicago, IL: 2010. [Google Scholar]
- 68.Steinmann TP, Andrew CM, Thomsen CE, Kjar TW, Meintjes EM, Molteno CD, Jacobson JB, Jacobson SW, Sorensen HB. An auditory Go/No-Go study of event-related potentials in children with fetal alcohol spectrum disorders. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:789–92. doi: 10.1109/IEMBS.2011.6090181. [DOI] [PubMed] [Google Scholar]
- 69.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–65. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 70.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human bra 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 71.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. 2003;90:3330–40. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- 73.Vaurio L, Crocker N, Mattson SN. Fetal alcohol spectrum disorders. In: Davis AS, editor. The handbook of pediatric neuropsychology. New York: Springer Publishing Company; 2010. pp. 877–86. [Google Scholar]
- 74.Ward BD. Deconvolution analysis of fMRI data. Milwaukee: Biophysics Research Institute, Medical College of Wisconsin; 2002. [Google Scholar]
- 75.Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN the CIFASD. Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. ACER. 2012;36:1431. doi: 10.1111/j.1530-0277.2011.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ware AL, O’Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN the CIFASD. The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. ACER. 2013;37:507–16. doi: 10.1111/j.1530-0277.2012.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wechsler D. Manual for the Wechsler intelligence scale for children-fourth edition integrated. 4. San Antonio: Pearson; 2004. [Google Scholar]
- 78.Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21:133–47. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]