Abstract
We have previously shown that propensity for weight gain, energy balance state and sex are important determinants of the neuronal response to visual food cues. It is not clear, though, whether these factors also impact the neuronal response to taste. The objective of this study was to examine the neuronal response to sweet taste during energy imbalance in men and women recruited to be obesity-prone (OP) or obesity-resistant (OR). OP (13M, 12W) and OR (12M, 12W) subjects were studied after one day of eucaloric, overfed and underfed conditions in a randomized crossover design. On each test day, fMRI was performed in the respective acute fed state while subjects received in random order 60 trials each of 1M sucrose solution (SU), or artificial saliva (AS) following a visual cue predicting the taste. The neuronal response to SU vs AS expectation was significantly greater in the amygdala, orbitofrontal cortex, putamen and insula in OR versus OP; SU receipt was not different between groups. There were also sex-based differences with men having greater neuronal response to SU vs AS receipt in the caudate than women. The results, however, were not impacted by the state of energy balance. In summary, response to expectation but not receipt of basic sweet taste was different in OR compared to OP, highlighting the importance of learning and conditioning in the propensity to gain weight. Response to sucrose taste receipt was stronger in men than women, raising questions about the effect of sex hormones on brain response to food.
Keywords: fMRI, neuroimaging, sucrose, underfeeding, overfeeding, sex
1. Introduction
Obesity continues to be a significant global public health problem despite efforts to promote healthy eating and physical activity behaviors. An important percentage of the population, however, remains normal weight despite being subjected to the same environmental forces that promote excess food intake and reduced physical activity. Understanding how these obesity-resistant (OR) individuals adapt to the obesogenic environment could lead to important advances in developing better treatment interventions for those who are prone to weight gain and obesity.
The regulation of food intake involves complex interactions between physiologic signals such as peripheral adiposity-related and meal-related hormones and higher brain circuitry important in reward, motivation, and integration of environmental cues [1]. We and others have used neuroimaging methods such as functional magnetic resonance imaging (fMRI) to study the neurocircuitry associated with energy intake regulation and the mechanisms associated with excess food intake. Obesity appears to be associated with abnormal responses to visual, gustatory and olfactory cues in brain regions known to be important in appetitive behaviors such as the hypothalamus, amygdala, hippocampus, orbitofrontal and prefrontal cortex, and insula [2–8]. We have previously found that reduced-obese and obesity-prone (OP) individuals have altered neuronal responses to visual food cues associated with altered eating-related behavior states as compared to normal weight OR individuals and that these differences in responses are impacted by the baseline state of energy balance [9–12]. While visual food-related stimuli are very important in the process of food intake, taste is also a very potent and important stimulus. Pleasant and sweet taste is associated with significant activation of brain regions important in the rewarding and hedonic properties of food and has been shown to be altered in obesity [13, 14]. Studies examining the neuronal response to sweet taste stimuli in individuals prone to weight gain and/or obesity, however, have not been well examined. We designed the present study to examine the neuronal response to sweet taste (sucrose) in individuals who self-identified themselves as being resistant to weight gain and obesity, i.e. OR, as compared to individuals who self-identified themselves as being at risk for weight gain and obesity, i.e. OP, as previously defined. Classification was based on personal and family weight history with a key feature being the reliance on self-perception of the tendency to gain weight or not [11, 12, 15–18]. Previous research suggests that overconsumption of food leads to addiction-like dopamine D2 receptor down-regulation in the striatum [19]. Human functional imaging studies are in support indicating a reduction in brain response to food receipt in OFC and striatum in obesity [20]. In addition, individuals with obesity display diminished brain response during a dopamine-related taste reward learning task in ventral striatum and insula [14]. It is uncertain whether brain function differences between obese and normal weight individuals are premorbid or whether they develop in response to overconsumption of food. In this study we examined individuals not obese, but prone or resistant to developing obesity. We hypothesized that brain function could distinguish those groups and provide information on how brain function could be involved in promoting obesity. We expected that the OP group would show decreased brain response in brain regions that process food reward with the hypothesis that lower activation in those regions would indicate the need for more food stimulation compared to OR for a similar reward system stimulation. Such a mechanism could promote overeating and obesity.
2. Methods and Procedures
2.1 Ethics Statement
All research participants provided written informed consent prior to enrolling in this study, according to the principles expressed in the Declaration of Helsinki. This study was approved by the Colorado Multiple Institutional Review Board.
2.2 Research participants
Research participants were adults aged 25–40 years (mean 30.8 ± 3.6 years) who were free of significant medical and psychiatric disease, including eating disorders as assessed by a screening medical history and physical examination, laboratory testing and questionnaires (Eating Attitudes Test [21] and Center for Epidemiologic Studies depression Scale [22]). Research participants were recruited to have a propensity to be resistant to weight gain and obesity (obesity-resistant - OR) or to be prone to weight gain and obesity (obesity-prone - OP) as previously defined [11, 12, 15–18]. In brief, OR participants had a BMI of 17–25 kg/m2 and reported no obese first degree relatives, never being overweight, weight stability, few to no attempts to lose weight, and no excessive levels of physical activity. OP participants had a BMI of 20–30 kg/m2 and reported at least one obese first degree relative, a history of past weight fluctuations, putting effort into weight regulation, but were weight stable for at least 3 months before being enrolled. Research participants were right-handed and were without MRI exclusions. A total of 49 participants, 24 OR (12 men, 12 women) and 25 OP individuals (13 men, 12 women), were studied and included in the current analyses.
2.3 Study Design and Measurements
Baseline assessments were first completed and included: anthropometric measurements (body weight, height), Three Factor Eating Questionnaire (TEFQ) [23], taste perception test (described below), and body composition (lean body mass, fat mass) measurement by dual-energy x-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp., Madison, WI, USA). Each research participant then underwent three study phases in a randomized counterbalanced manner, with each phase consisting of: 1) a 3-day baseline eucaloric diet period to ensure energy and macronutrient balance; 2) an intervention diet on day 4; and 3) a study day on day 5. The three study phases consisted of one of the following intervention diets on day 4: eucaloric (EU) diet, overfeeding (OF) by 40% above estimated energy needs, or underfeeding (UF) by 40% of baseline caloric intake. During all three study phases, the diets were made up of the same macronutrient composition: 50% carbohydrate, 30% fat, and 20% protein. Estimates of daily energy needs were made using lean body mass (as determined by DEXA) in the following equation: Resting Metabolic Rate (RMR) = (fat free mass • 23.9) + 372. The estimates were confirmed using RMR as assessed by indirect calorimetry, multiplied by an activity factor of 1.3. This method has been used successfully by our group in a number of prior studies [9, 10, 24–27]. All the food was prepared and provided by the Clinical Translational Research Center (CTRC) metabolic kitchen. Research participants presented to the CTRC each morning to be weighed, eat breakfast, and pick up the remainder of their daily meals which were packed in coolers. Research participants were asked to maintain their usual physical activity patterns and were questioned regarding activity and compliance. Research participants were asked to refrain from consuming any alcoholic or calorie-containing beverages during the study phases. Study days were scheduled during the follicular phase of their menstrual cycle in women.
2.4 Study Day
Research participants presented to the CTRC after an overnight fast of at least 10 hours. They first completed baseline (fasting) hunger and satiety ratings by visual analog scale (VAS) [10]. Hunger was rated by VAS on a line preceded by the question, "How hungry are you right now?" and anchored on the left by "not at all hungry" and by "extremely hungry" on the right. Satiety was rated by the question, “How full do you feel right now?” with the anchors "not at all" and "extremely.” Subjects were then escorted to the Brain Imaging Center at the University of Colorado where they consumed a liquid breakfast meal over 20 minutes. The caloric content of the liquid breakfast was equal to 25% of the energy provided during the intervention diet (EU, OF, or UF) and had an identical macronutrient composition (50% carbohydrate, 30% fat, and 20% protein). fMRI measures were then performed 60 minutes after the start of the meal (described below). Hunger and Satiety ratings were then repeated 30, 90, 120, 150, and 180 minutes after the meal.
2.5 Taste Perception Test
To assess general taste perceptions across groups, we applied the following taste perception test. Subjects were presented with a tray of sixteen unmarked small cups that contained solutions of four sugar (2%, 4%, 8%, 16%) and four fat (skim milk, whole milk, half n half, whipping cream) solution strengths in a 4×4 array. All cups were randomly lined up on the tray. Subjects did not know the individual content and rated blindly the solutions for pleasantness on a 100 mm visual analog scale. The scale was anchored by the descriptive ‘dislike extremely’ to ‘like extremely’ for pleasantness ratings. The results were analyzed across obesity groups and sex.
2.6 Functional Magnetic Resonance Imaging
Imaging studies were performed using a General Electric (Milwaukee, WI, USA) 3.0 T MR scanner equipped with high speed gradients (300µs rise time and maximum gradient strength 24mT/m) on three occasions in a randomized, counterbalanced manner in eucaloric, overfed and underfed conditions. Prior to functional imaging, high-resolution, T1-weighted 3D anatomical scan over 10 minutes was acquired for each subject. Functional images were then acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with TR = 2100 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. Additionally, one inversion-recovery echo-planar-image (TI=505ms) volume was acquired to improve coregistration between the echo planar images and gray matter templates used in pre-processing. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). fMRI was performed in the acute fed stated while subjects received, in random order, 1M sucrose solution (SU), no solution (NO) or artificial saliva (AS) following a unique paired visual cue (geometric shape) as conditioned stimulus (CS), predicting the taste as described previously[14]. In brief, we adapted the design used by O’Doherty et al [28]. Individuals learned to associate each taste stimulus with the unique paired CS. Each CS was presented for 2 s. With disappearance of the visual cue, simultaneously the unconditioned taste stimulus (US) was delivered and a black fixation cross appeared on a white background. The taste fluid delivery occurred over 1 s. Inter-trial interval was fixed at 6 s. Subjects were instructed to swish their tongue once, look at the fixation cross and await the next trial. For each subject, the first 9 trials were fixed with visual cue for SU (CS) followed by the delivery of SU (US) to establish an initial stable association between the CS and US [28]. All other trials (SU n=60, NO=51, AS=60) were fully randomized without predetermined order. The taste stimuli were applied using a customized programmable syringe pump (J-Kem Scientific, St Louis, MO) controlled by E-Prime Software (Psychological Software Tools, Pittsburgh, PA), and individual taste applications were triggered by the MRI scanner's radiofrequency pulse [29]. Task duration was 17 min.
2.7 Calculations and Statistical Analyses
Brain-imaging data were preprocessed and analyzed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template, and smoothed with a 3-mm FWHM Gaussian kernel. Each image sequence was manually inspected, and images with artifacts or movement > one voxel size were removed. Data were modeled with a hemodynamic response function—convolved boxcar function— using the general linear model, including temporal and dispersion derivatives, and autoregression. A 128s high-pass filter was applied to remove low-frequency fluctuation in the BOLD signal.
We developed first-level models for the two contrasts of interest: (1) trials with receipt of SU contrasted against receipt of AS (SU Receipt) and (2) trials with expectation of SU contrasted against the expectation of AS (SU Expectation).
A general linear model (GLM) whole-brain analysis was used (SPM8), the model comprising a factorial design with 3 factors: Group with 2 levels (OR and OP), Phase with 3 levels (EU, OF, UF), and Sex with 2 levels (men and women). Age, scan day (number of days since the first scan), and fat mass were included as covariates. We set a threshold of p<0.001 uncorrected and 10 voxel cluster size as significance threshold [30]. We initially tested whether the 3 Phases would be associated with significantly different brain responses as a main effect or whether there would be an interaction between phase and obesity group or gender, but that was not the case. There was also no interaction between obesity subgroup and gender irrespective of phase. We then explored the group difference main effects for OR versus OP as well as men versus women using the t-contrasts and covariates, with similarly as above, a threshold of p<0.001 uncorrected and 10 voxel cluster size as significance threshold. We used SPM8 AAL-atlas defined anatomical regions for brain areas involved in taste reward processing (insula, caudate, putamen, amygdala, midbrain, dorsolateral prefrontal cortex, anterior cingulate cortex, and orbitofrontal cortex) for small volume correction (family-wise error corrected p<0.05). We report results with and without additional multiple comparison (Bonferroni) correction applied for the number of regions small volume corrected. In addition, we conducted regression analyses between first level contrast images and results from the TFEQ, with age, scan day (number of days since the first scan), and fat mass included as covariates.
3. Results
3.1 Subject Characteristics
Twenty-four obesity-resistant (OR) and twenty-five obesity-prone (OP) research participants were studied (Table 1). Compared to OR, OP had higher BMI, body fat mass, and percent body fat but had similar fat free mass. As previously described, OP had higher scores for restraint, disinhibition, and hunger on the TFEQ than OR, but no significant group differences in ratings of hunger or satiety before or in response to the test meal were seen [31]. As shown in Table 1, women were similar to men in all characteristics except for a lower fat free mass and higher percent body fat. There were no baseline differences in taste pleasantness/perception ratings between OR and OP or between women and men.
Table 1.
Subject Characteristics.
| OR | OP | Women | Men | |
|---|---|---|---|---|
| N (women/men) | 24 (12/12) | 25 (12/13) | 24 | 25 |
| Age (years) | 30.4 ± 2.6 | 30.2 ± 3.7 | 30.4 ± 3.2 | 30.2 ± 3.3 |
| BMI (kg/m2) | 20.6 ± 1.8 | 26.5 ± 2.8a | 22.9 ± 4.1 | 24.2 ± 3.3 |
| Fat Free Mass (kg) | 50.5.2 ± 9.9 | 56.2 ± 10.7 | 45.6 ± 6.4 | 61.0 ± 8.1c |
| Fat Mass (kg) | 12.5 ± 6.1 | 23.3 ± 7.7a | 19.1 ± 9.2 | 17.0 ± 8.4 |
| Body Fat (%) | 19.0 ± 4.7 | 29.2 ± 7.4a | 27.4 ± 7.7 | 21.0 ± 7.2d |
| TFEQ | ||||
| Restraint | 5.0 ± 3.0 | 9.3 ± 4.5a | 7.7 ± 4.9 | 6.9 ± 4.0 |
| Disinhibition | 3.2 ± 2.3 | 8.0 ± 3.2a | 5.7 ± 3.8 | 5.7 ± 3.6 |
| Hunger | 4.6 ± 2.5 | 6.4 ± 3.0b | 5.0 ± 2.6 | 6.0 ± 3.1 |
Mean ± SD; OR: obese resistant; OP: obese prone; BMI: body mass index; TFEQ: Three Factor Eating Questionnaire.
p <0.001 between obesity groups
p<0.05 between obesity groups
p<0.001 between women and men
p<0.01 between women and men
3.2 Effects of Energy Imbalance
Subjects were studied on three occasions in a randomized, counterbalanced manner in eucaloric, overfed and underfed conditions. There were no significant effects of feeding condition (EU, UF, OF) on the neuronal response to either SU Receipt or SU Expectation. Furthermore, there were no significant interactions of feeding conditions with either obesity group or sex.
3.3 Obesity Prone Compared to Obesity Resistant
Greater activation was seen in the left midbrain in OR as compared to OP individuals but this did not survive Bonferroni correction (Table 2). Greater responses to SU versus AS expectation in OR as compared to OP after Bonferroni correction were seen in left amygdala, right inferior and medial orbitofrontal (OFC), right putamen and bilateral insula (Figure 1 and Table 2). There were no responses that were greater in OP as compared to OR individuals, and no interactions were seen with feeding condition.
Table 2.
Sucrose Receipt and Expectation, Obese Resistant greater than Obese Prone
| Anatomical Region | kE | Peak Level pFWE-corr |
T/Z at Peak |
Location at Peak MNI Coordinates |
|---|---|---|---|---|
| Sucrose Receipt | ||||
| Midbrain, L | 15 | 0.013 | 4.19/4.05 | −4, −22, −12 |
| Sucrose Expectation | ||||
| Amygdala, L | 14 | 0.005** | 3.96/3.84 | −28, −4, −18 |
| Caudate, R, BA 34 | 26 | 0.007 | 4.24/4.10 | 16, −4, −24 |
| Caudate, L | 40 | 0.007 | 4.25/4.11 | −18, 8, 22 |
| Anterior Cingulate Cortex, R | 24 | 0.017 | 4.09/3.96 | 12, 30, 28 |
| Anterior Cingulate Cortex, R | 24 | 0.024 | 3.99/3.87 | 6, 42, 18 |
| Anterior Cingulate Cortex, R | 55 | 0.045 | 3.78/3.68 | 2, 52, 10 |
| Anterior Cingulate Cortex, L, BA 32 | 12 | 0.042 | 3.77/3.67 | −2, 36, −8 |
| Inferior Orbitofrontal Cortex, R | 153 | 0.006** | 4.45/4.28 | 48, 20, −4 |
| Inferior Orbitofrontal Cortex, L | 143 | 0.017 | 4.14/4.00 | −32, 28, −8 |
| Medial Orbitofrontal Cortex, R | 23 | 0.005** | 4.27/4.13 | 6, 62, −4 |
| Dorsolateral Prefrontal Cortex, L, BA 10 | 66 | 0.025 | 4.33/4.18 | −46, 48, 14 |
| Middle Orbitofrontal Cortex, R, BA 11 | 23 | 0.011 | 3.98/3.86 | 26, 40, −10 |
| Superior Orbitofrontal Cortex, L | 13 | 0.028 | 3.64/3.55 | −18, 62, −4 |
| Insula, R, BA 47 | 621 | 0.001** | 4.88/4.67 | 38, 16, −4 |
| Insula, R | 15 | 0.031 | 4.00/3.88 | 42, 2, 10 |
| Insula, R | 40 | 0.043 | 3.89/3.78 | 32, −24, 20 |
| Insula, L | 198 | 0.002** | 4.75/4.56 | −38, 2, 12 |
| Insula, L | 40 | 0.037 | 3.92/3.81 | −30, −18, 18 |
| Putamen, R | 388 | 0.003** | 4.50/4.34 | 32, −16, −2 |
| Putamen, L | 13 | 0.014 | 4.05/3.93 | −32, 2, 12 |
| Putamen, L | 268 | 0.015 | 4.03/3.90 | −34, 2, 0 |
Peak level pFWE small volume corrected based on anatomical region; R, right; L, left
indicates clusters that survived additional Bonferroni correction; cluster size voxels, kE
Figure 1. Neuronal response to sucrose expectation in obesity-resistant (OR) as compared to obesity-prone (OP) individuals.
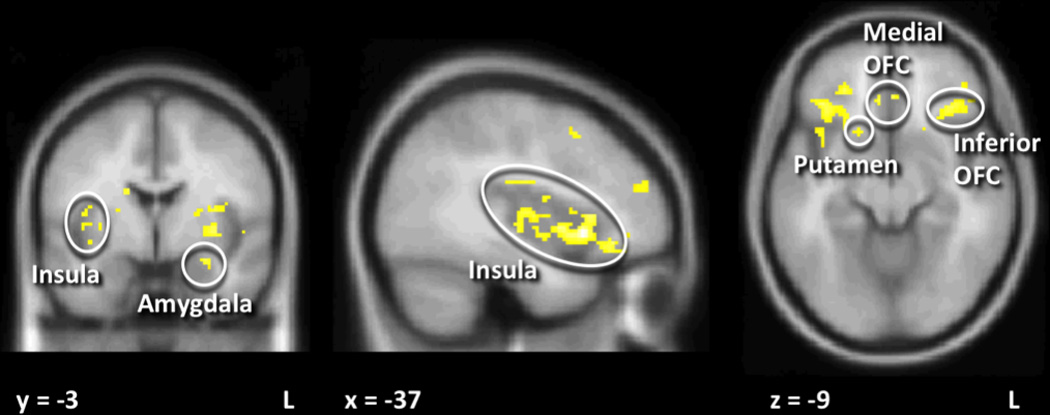
SU expectation was associated with greater activation in amygdala, inferior OFC, medial OFC, insula and putamen in OR as compared to OP individuals after correction for multiple comparisons. Statistical maps thresholded at p < 0.001 and 10 voxel threshold for visualization and overlaid onto the group average anatomical image. Data are shown in the radiological convention (right hemisphere on the left).
3.4 Sex-Based Differences
Next, we examined sex-based differences in response to SU receipt and SU expectation. As shown in Figure 2 and Table 3, men had significantly greater response to SU receipt in the right caudate nucleus. A greater response in men was also seen bilateral medial OFC, left insula and left midbrain although these differences did not survive Bonferroni correction. No sex-based differences were seen in response to SU expectation that survived multiple comparison correction. There was a trend, though, for men having greater response to SU expectation than women in bilateral anterior cingulate cortex, inferior OFC, DLPFC and insula as well as in right caudate and right medial OFC, (Table 3). There were no responses that were greater in women as compared to men, and no interactions were seen with either feeding condition or obesity group.
Figure 2. Neuronal response to sucrose (SU) receipt in men as compared to women.
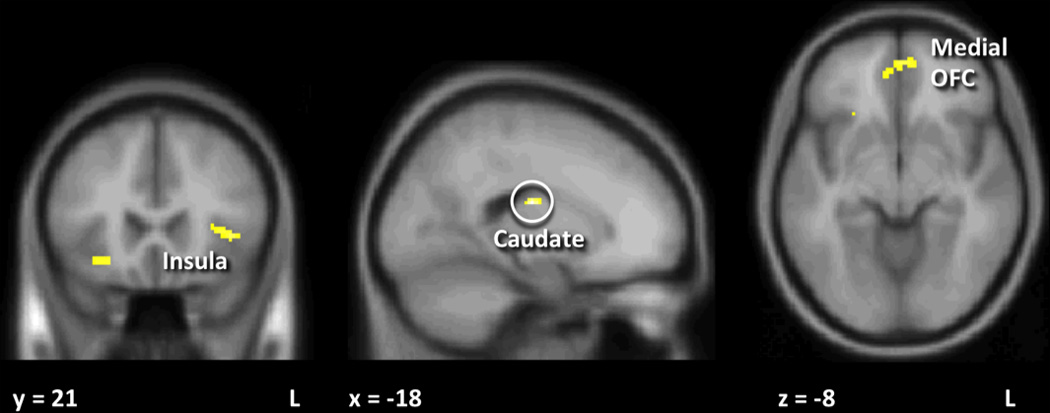
Men had significantly greater response to SU receipt than women in caudate, medial orbitofrontal cortex (OFC), and insula. Only the caudate survived multiple comparison corrections. Statistical maps thresholded at p < 0.001 and 10 voxel threshold for visualization and overlaid onto the group average anatomical image. Data are shown in the radiological convention (right hemisphere on the left).
Table 3.
Sucrose Receipt and Expectation, Males greater than Females
| Anatomical Region | kE | Peak Level pFWE-corr |
T/Z at Peak |
Location at Peak MNI Coordinates |
|---|---|---|---|---|
| Sucrose Receipt | ||||
| Caudate Nucleus, R | 21 | 0.001** | 4.68/4.49 | 20, −14, 22 |
| Medial Orbitofrontal Cortex, R | 16 | 0.033 | 3.55/3.47 | 0, 54, −8 |
| Medial Orbitofrontal Cortex, L, BA 10 | 35 | 0.023 | 3.73/3.63 | −2, 54, −8 |
| Insula, L, BA 47 | 37 | 0.045 | 3.77/3.67 | −40, 22, 2 |
| Midbrain, L | 12 | 0.012 | 4.22/4.08 | 0, −28, −14 |
| Sucrose Expectation | ||||
| Caudate Nucleus, R | 39 | 0.010 | 4.14/4.01 | 20, −2, 22 |
| Anterior Cingulate Cortex, R, BA 10 | 43 | 0.043 | 3.79/3.69 | 0, 36, 26 |
| Anterior Cingulate Cortex, L | 59 | 0.024 | 3.96/3.84 | 8, 34, 28 |
| Inferior Orbitofrontal Cortex, R | 16 | 0.016 | 4.15/4.01 | 46, 20, −4 |
| Inferior Orbitofrontal Cortex, R | 11 | 0.034 | 3.91/3.80 | 36, 44, −12 |
| Inferior Orbitofrontal Cortex, L | 96 | 0.026 | 4.00/3.88 | −40, 42, −6 |
| Medial Orbitofrontal Cortex, R, BA 11 | 12 | 0.010 | 4.08/3.95 | −4, 42, −12 |
| Dorsolateral Prefrontal Cortex, R | 23 | 0.028 | 4.28/4.14 | 32, 54, 24 |
| Dorsolateral Prefrontal Cortex, L | 146 | 0.023 | 4.36/4.20 | −46, 44, 14 |
| Dorsolateral Prefrontal Cortex, L | 352 | 0.041 | 4.19/4.05 | −38, 12, 50 |
| Insula, R, BA 45 | 47 | 0.033 | 3.97/3.85 | 36, 26, 6 |
| Insula, L, BA 13 | 17 | 0.042 | 3.88/3.77 | −36, 4, 12 |
Peak level pFWE small volume corrected based on anatomical region; R, right; L, left
indicates clusters that survived additional Bonferroni correction; cluster size voxels, kE
3.5 Correlates of neuronal response
We also examined the data without adjusting for fat mass. These models indicated that fat mass did not have a significant effect on any of the outcomes (neuronal response to SU Receipt or SU Expectation, factors of the TFEQ, or appetite ratings). There was a significant positive correlation between TFEQ Disinhibition and SU Expectation in the right insula (x=42, y=0, z=6), but significance (pFWE small volume corrected <0.029, 15 voxel cluster) did not survive correction for multiple comparisons.
4. Discussion
The neuronal response to sweet taste stimuli in individuals screened to be resistant to weight gain and obesity (OR) as compared to individuals screened to be prone to weight gain and obesity (OP) was examined in response to short-term energy imbalance. Unexpectedly, one day of 40% over- and under-feeding did not impact the neuronal response to sucrose receipt or expectation in either group. Overall, though, the neuronal response to sucrose expectation was found to be significantly attenuated in OP as compared to OR. Women across both groups showed attenuated neuronal responses to sucrose receipt in the caudate as compared to men. These results suggest that the neuronal response to expectation of basic sweet taste is down regulated in individuals prone to weight gain and obesity as compared to those resistant to obesity. Furthermore, response to sweet taste receipt is lower in women as compared to men. Fat mass did not moderate the neuronal response.
The neuronal response to sweet taste expectation was significantly attenuated or down-regulated in OP individuals in brain region circuits known to be important in the higher order regulation of food intake as it relates to reward, motivation, and environmental cues. It has been previously shown that obese individuals tend to show increased brain response to palatable food stimuli such as milk shake in the gustatory cortex, but decreased response to receipt of the taste stimuli in the striatum [14, 32] as did those at risk for weight gain [20]. Gearhardt et al showed that addictive eating behaviors were associated with increased activation in reward circuitry but reduced activation in inhibitory regions [33]. These findings are contradictory. In contrast to other studies here we used high concentration of sucrose that has less hedonic value compares to complex sugar and fat combinations such as in milkshakes. It is possible that the pure sweet taste is not as rewarding to OP individuals. OP may have been exposed to highly palatable foods to a greater extent than the OR group and they may have less anticipatory neuronal response to the simple sweet taste for that reason. Thus, learning of and conditioning to basic nutritional stimuli may be disturbed in OP individuals. This hypothesis requires further study though. Sweet taste receipt was similar between groups, but a larger sample will need to test whether there are differences that can distinguish OP and OR groups. The study did not assess neurotransmitters in the brain, and thus we know little about what systems may specifically contribute to our findings. One possibility is that dopamine receptor responsiveness is down-regulated premorbidly, or is down regulated with chronic overnutrition, as it has been suggested in animal [19] and human [34] studies and has been compared to brain mechanisms in substance use disorders [35]. Another neurotransmitter system that has been associated with reward and food intake involves the opioid system, which codes the hedonic aspects of food [36], The exact mechanisms that contribute to neuronal responses in relation to food stimuli will need further study and inclusion of neurotransmitter specific probes.
The neuronal response to sweet taste was attenuated or down-regulated in women as compared to men in the caudate. There was also a trend for less activation in women in response to sweet taste expectation in a number of other brain regions; this will need further exploration in a larger sample. Sex-based differences in the neuronal response to food-related cues have been previously shown [37–45]. Few studies, though, have examined sex-based differences in response to taste stimuli and these have been inconsistent. Similar to our findings that men have greater activation in response to sweet taste than women (or that women have reduced responses compared to men), Smeets et al found that men had greater activation in the hypothalamus, ventral striatum and medial prefrontal cortex in response to chocolate taste [42]. Other studies, though, have found greater activation in response to taste cues in women as compared to men [43, 44]. Uher et al showed that women had greater activation than men in response to chocolate milk and chicken broth without effect of hunger/satiety. Haase et al found that during satiety women showed greater activation within the posterior cingulate than men in response to sucrose. These studies, though, were of low participant number and the phase of the menstrual cycle was not taken into account in the women. The current findings that women showed reduced response to sweet taste receipt in the caudate suggests that there are sex-based differences in taste response [46]. The mechanisms responsible for these differences certainly could be attributed to differences in sex steroids [47, 48] and/or sensitivity to appetite related peptides such as leptin and ghrelin [49, 50]. Another direction of research has suggested that there is a difference in inhibition in the context of food stimulation involving insula, orbitofrontal cortex, and striatum, suggesting interactions between frontal cognitive and subcortical more drive and hedonic perception related regions [39].These sex-based differences deserve further investigation and emphasize the importance of potentially studying men and women as separate groups.
Short-term energy imbalance, however, did not impact the neuronal response to sweet taste. The effect of the state of energy balance on the neuronal response to taste has not been previously examined. We postulate that sweet taste is such a strong stimulus that one day of overfeeding or underfeeding is not sufficient to alter the response to this stimulus. It is possible that more prolonged energy restriction and/or surplus is required to impact the response to this stimulus.
A few limitations should be discussed. The study was designed to measure outcomes in “never obese” individuals who were recruited to be at risk for gain weight or to remain thin, yet there are challenges with categorizing individuals or predicting resistance or proneness to obesity before its onset. We have previously shown, however, that these categorizations as described in this study are associated with other relevant physiologic and behavioral differences. We plan to follow these research participants longitudinally which will allow us to examine baseline predictors of prospective weight gain and assess resistance or proneness to weight gain prospectively. It is possible that the group differences seen between OR and OP may be due to the OP group having a greater fat mass and not due to the categorization as defined. Adjusting the data for fat mass, however, did not impact the results, suggesting that these effects are not related to their greater adiposity. Men and women in the study showed different neuronal response; thus, it will be important to study gender groups separately with larger samples for OR and OP propensity.
5. Conclusion
Individuals screened to be prone to obesity have functional differences in brain regions central to appetite regulation as compared to individuals who appear to be resistant to obesity. The primary finding of this study was that neuronal response to basic sweet taste expectation was significantly reduced in OP as compared to OR individuals, but not the response to taste receipt. This highlights the importance of learning and conditioning to basic nutritional stimuli in the propensity to gain weight. Importantly, response to sucrose taste receipt was stronger in men than women, which raises questions about the effect of gonadal hormones on brain response to food.
Highlights.
One day of over- or under-feeding does not impact the neuronal response to sucrose
Obesity-resistance was associated with greater neuronal responses to sucrose expectation
Men had significantly greater neuronal response to sucrose receipt than women
Acknowledgments
We acknowledge and thank Debra Singel and Yiping Du of the University of Colorado Brain Imaging Center for their assistance with the fMRI studies. We also thank the dietary services and metabolic kitchen of the University of Colorado Clinical Translational Research Center. This publication was supported by NIH/NCRR Colorado CTSI Grant Number UL1 TR000154, NIH/NIDDK Nutrition Obesity Research Center Grant Number DK48520, and NIH/NIDDK Grant Number R01DK072174. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
References
- 1.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 5.McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43:253–259. doi: 10.1016/j.appet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Cornier M-A, McFadden KL, Thomas EA, Bechtell JL, Eichman LS, Bessesen DH, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiology & Behavior. 2013;110–111:122–128. doi: 10.1016/j.physbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas EA, Bechtell JL, Vestal BE, Johnson SL, Bessesen DH, Tregellas JR, et al. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite. 2013;65:96–102. doi: 10.1016/j.appet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan-Poupart S, Veldhuizen MG, Geha P, Small DM. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite. 2013;60:168–174. doi: 10.1016/j.appet.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smucny J, Cornier M-A, Eichman LC, Thomas EA, Bechtell JL, Tregellas JR. Brain structure predicts risk for obesity. Appetite. 2012;59:859–865. doi: 10.1016/j.appet.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt SL, Kealey EH, Horton TJ, Vonkaenel S, Bessesen DH. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity (Silver Spring) 2012;20:2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas EA, B J, Bessesen DH, Tregellas JR, Cornier MA. Hormonal and Metabolic Effects of Short-term Energy Imbalance in Obese-Prone as Compared to Obese-Resistant Individuals. Am J Diabetes Obes Metabol. 2014;1:1–14. [Google Scholar]
- 19.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stice E, Yokum S, Blum K, Bohon C. Weight Gain Is Associated with Reduced Striatal Response to Palatable Food. The Journal of Neuroscience. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychological medicine. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 23.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 24.Cornier MA, Bergman BC, Bessesen DH. The effects of short-term overfeeding on insulin action in lean and reduced-obese individuals. Metabolism. 2006;55:1207–1214. doi: 10.1016/j.metabol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 26.Adochio R, Leitner JW, Gray K, Draznin B, Cornier M-A. Early responses of insulin signaling to high-carbohydrate and high-fat overfeeding. Nutrition & Metabolism. 2009;6:37. doi: 10.1186/1743-7075-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CC, Adochio RL, Leitner JW, Abeyta IM, Draznin B, Cornier MA. Acute effects of different diet compositions on skeletal muscle insulin signalling in obese individuals during caloric restriction. Metabolism. 2012 doi: 10.1016/j.metabol.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 29.Frank GK, Reynolds JR, Shott ME, O'Reilly RC. Altered temporal difference learning in bulimia nervosa. Biol Psychiatry. 2011;70:728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Social cognitive and affective neuroscience. 2009;4:417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas EA, Bechtell JL, Vestal BE, Johnson SL, Bessesen DH, Tregellas JR, et al. Eating-related Behaviors and Appetite During Energy Imbalance in Obese-Prone and Obese-Resistant Individuals. doi: 10.1016/j.appet.2013.01.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural Correlates of Food Addiction. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.32. archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang G-J, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse Association Between BMI and Prefrontal Metabolic Activity in Healthy Adults. Obesity. 2008;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. Exposure to appetitive food stimuli markedly activates the human brain. NeuroImage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high vs low calorie food. Neuroreport. 2010;21:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr. 2002;75:1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 41.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Research. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–1305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 43.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behavioural Brain Research. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57:421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2012;243C:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zylan KD. Gender differences in the reasons given for meal termination. Appetite. 1996;26:37–44. doi: 10.1006/appe.1996.0003. [DOI] [PubMed] [Google Scholar]
- 47.Handa RJ, Hejna GM, Lorens SA. Androgen inhibits neurotransmitter turnover in the medial prefrontal cortex of the rat following exposure to a novel environment. Brain Research. 1997;751:131–138. doi: 10.1016/s0006-8993(96)01394-7. [DOI] [PubMed] [Google Scholar]
- 48.Barker JM, Galea LAM. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. General and Comparative Endocrinology. 2009;164:77–84. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 50.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-Dependent Decrease in the Orexigenic Potency of Ghrelin in Female Rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]


