Abstract
Chitin, a biopolymer of N-acetylglucosamine, is abundant in invertebrates and fungi, and is an important structural molecule. There has been a longstanding belief that vertebrates do not produce chitin, however, we have obtained compelling evidence to the contrary. Chitin synthase genes are present in numerous fishes and amphibians, and chitin is localized in situ to the lumen of the developing zebrafish gut, in epithelial cells of fish scales, and in at least three different cell types in larval salamander appendages. Chitin synthase gene knockdowns and various histochemical experiments in zebrafish further authenticated our results. Finally, a polysaccharide was extracted from scales of salmon that exhibited all the chemical hallmarks of chitin. Our data and analyses demonstrate the existence of endogenous chitin in vertebrates and suggest that it serves multiple roles in vertebrate biology.
In our surveys of vertebrate genomes and transcriptomes, numerous “unknown” fish and amphibian sequences were detected that exhibited striking homology to bona fide invertebrate chitin synthase genes (BlastX, e-value < 10-150). These sequences had likely been overlooked as coding for chitin synthases (CHS) during the ab initio annotation process. This list (Table S1) includes CHS genes of several fishes and a salamander, and two species (zebrafish and Xenopus) previously identified in a recent report on metazoan chitin synthase genes [1]; amniote CHS genes have not been identified. A phylogenetic tree showing the interrelationships among the identified vertebrate CHS orthologs is given in Fig. S1A.
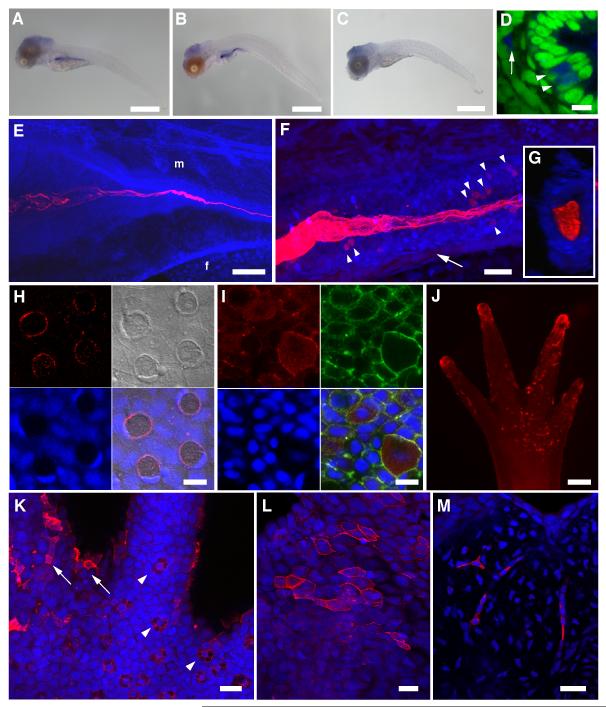
To investigate whether fish endogenously produce chitin, we employed the zebrafish (Danio rerio) model system. The zebrafish genome assembly [2] revealed the existence of four CHS genes: three closely related and linked genes on chromosome 18 and a gene on chromosome 13 (Table S1). Published Affymetrix microarray experiments (Supplemental Information) indicated that the chromosome-13 gene (DrCHS-1) is preferentially expressed during embryonic/larval development. Whole mount in situ hybridization (ISH) showed expression of DrCHS-1 in the larval gut (Fig. 1A-C), and subsequent transverse sections revealed expression in cells within the epithelial wall and in mesenchymal cells adjacent to the gut (Fig. 1D) [3].
Figure 1.
Detection of chitin in zebrafish and axolotl. (A-D) in situ hybridization for DrCHS-1. NBT/BCIP signals were observed primarily in the developing digestive tract in 5 dpf (A) and 10 dpf (B) larvae, indicating that DrCHS-1 is expressed during larval development and in the gut. A representative “sense” control larva is shown in (C). No NBT/BCIP signals were observed in the developing gut of control larvae at either 5 or 10 dpf, although background staining was observed in the cranial region. Scale bars = 500 μm. (D) The ventral region of the mid-intestine of a 10 dpf larva (transverse section, different specimen than (B)), shows DrCHS-1 expression in select cells within the columnar intestinal epithelium (arrowheads) and in mesenchymal cells adjacent to the intestinal epithelium (arrow). NBT/BCIP deposition was imaged in the infrared range with confocal microscopy (blue), and nuclei were stained with Sytox Green (green). Scale bar = 10 μm. (E-M) A chitin probe (CBD-546, shown in red) was used for affinity histochemistry on fixed whole mount specimens of zebrafish larvae (E-G) and scales (H-I), and axolotl larval forelimbs and tails (J-M). Nuclei were stained with TOTO-3 (blue), and rostral is to the left in (E) and (F). (E) 3D-rendered projection of a 4 dpf zebrafish larva. “f” and “m” represent preanal finfold and somitic muscle, respectively; the “m” designation is above the approximate junction of the middle intestine. Chitin is observed in the developing larval gut, appearing fibrillar in the lumen of the intestinal bulb and coalescing as the lumen narrows posteriorly. Scale bar = 100 μm. (F) 3D-rendered projection of the mid-intestinal lumen from a 5 dpf zebrafish larva. The fibrous nature of the chitin is obvious and several cells within the intestinal epithelial wall are observed that show strong chitin signals (arrowheads). A population of mesenchymal cells was also found to be positive for chitin signals (arrow). Scale bar = 20 μm. (G) Transverse section from a 3D volume-rendered reconstruction of the image in (F) showing chitin distributed throughout the lumen of the mid-intestine. (H) Confocal image of a field of zebrafish scale epithelium. Panels shown are: CBD-546 (top left), DIC (top right), Sytox Green nuclear counterstain (pseudocolored in blue, bottom left), and merged image (bottom right). The four circular structures with red chitin signals are goblet cells that show polarized, crescent-shaped nuclei; polygonal concentric structures on the DIC overlay are squamous epithelial cells. Aggregations of small red punctate spots probably represent extracellular chitin. (I) Maximum projection image of a field of scale epithelial cells in which chitin is prominent throughout the cytoplasm of club cells. Panels shown are: CBD-546 (top left), phalloidin-FITC (top right), TOTO-3 nuclear counterstain (bottom left), and merged image (bottom right). The scale specimen was simultaneously stained for F-actin using phalloidin-FITC to help distinguish individual cells. Scale bars = 10 μm (H, I). (J) A forelimb (palm side up) of a larval axolotl after chitin histochemistry, imaged on a stereoscope. The tips of the developing digits and the palm area that show extensive chitin signals are primarily superficial epithelial cells. Scale bar = 200 μm. (K) A field from the base of a digit from a different forelimb than shown in (J) imaged by confocal microscopy. Arrowheads indicate several specialized Leydig epidermal cells, and the arrows identify a few of the superficial epithelial cells seen in (J). (L,M) Caudal fin of larval axolotl. (L) Maximum intensity projection showing a subset of surface epithelial cells that exhibit strong chitin signals. (M) A deeper optical section showing strong chitin signals in the fibroblast-like cells of the fin mesenchyme. Scale bars = 50 μm (K,L,M). See also Figures S1, S2.
For detection of chitin in situ, we used affinity histochemistry with fluorescent probes containing a highly-specific chitin binding domain (CBD) (Supplemental Information) [4]. When applied to a zebrafish developmental series, chitin was detected as early as 3 days post-fertilization (dpf) as multiple fibers within the intestinal bulb (Fig. S2B,C); an endoderm population dorsal to the gut also showed chitin signals (Fig. S2C) which largely disappeared by 4 dpf (cf Fig. 1E). At 4-5 dpf, the fibers coalesce in the middle intestine and are distributed throughout the digestive tract (Fig. 1E-G; Supplemental movie). The bulk of this chitin is extracellular and distributed throughout the intestinal lumen, however, cellular signals were detected among cells within the lumen wall and mesenchymal cells ventral to the intestinal tract (Fig. 1F). The similarity in the DrCHS-1 ISH patterns and chitin localization (Fig. 1A-F) suggest that chitin is being synthesized by cells in and around the developing intestine and sequestered within the lumen. Validation of the robustness of our chitin detection assay is shown in Figs. S1D and S2A-C.
In order to demonstrate that chitin production is coupled with chitin synthase expression, we knocked down the DrCHS-1 gene. Two different splice-blocking morpholinos were designed to DrCHS-1 and microinjected into fertilized eggs along with their mismatch controls (Supplemental Information, Fig. S2E). The knockdowns resulted in marked reduction in chitin signals in the gut (Fig. S2F), and are consistent with the early expression profile of DrCHS-1.
We also assayed for chitin in zebrafish scales since our initial RT-PCR survey indicated expression of a zebrafish chromosome-18 CHS gene in this tissue (Fig. S2D). Fish scales are primarily comprised of a hard, partly mineralized collagenous matrix as well as partially overlying epithelium (skin). Unlike in the gut, most the chitin signals were observed intracellularly in epithelial goblet and club cells (Fig. 1H,I). The chitin in the goblet cells was largely restricted to the periphery of the cells, whereas it was distributed throughout the cytoplasm in the club cells. Our findings on zebrafish scales were corroborated via RT-PCR and chitin histochemistry on scales of juvenile Atlantic salmon, Salmo salar (Fig. S2D, Supplemental Movie).
Chitin synthase genes were also identified in two amphibian species, Xenopus and axolotl. Transcriptome data indicated that a chitin synthase gene was expressed in larval axolotls (regenerating limb, Table S1), thus appendage tissues from larvae (stage ~ 52-53) were obtained and used for chitin histochemistry. Chitin is found predominantly in superficial epidermal cells resembling squamous epithelia, Leydig epidermal cells and mesenchymal (fibroblast-like) cells (Fig. 1J-M). The strong signals on the tips of the forelimb digits (Fig. 1J) are due to superficial epidermal cells. These findings in axolotl, including chitinase validation (Fig. S2C) are similar to those seen for fish scales.
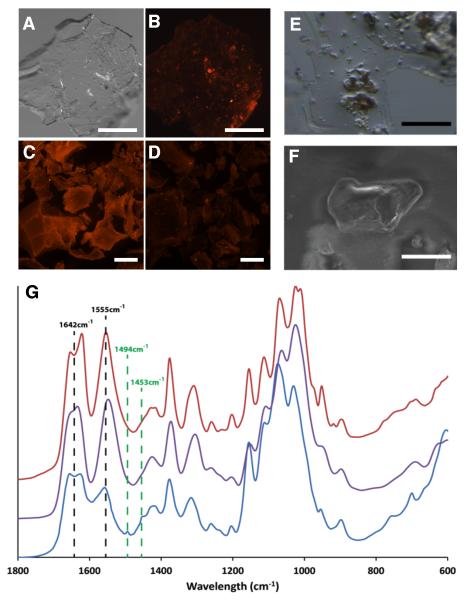
Ultimate support for the existence of vertebrate chitin is isolation of chitin per se from a vertebrate. For this experiment, we used fresh scales from Atlantic salmon and employed a fastidious chemical extraction strategy. From 60 grams of dried scales we extracted a precipitate of ~ 1.5 mg of chitinous material for analysis. This low yield and the intractability of insoluble chitin to most characterization methods, necessitated its analysis via microscopic Fourier transform infrared spectrometry (FTIR). Micrographs of the precipitate collected from salmon scales are shown in Fig. 2A-B,E-F and the FTIR spectrum of the insoluble granules confirms its identity as chitin (Fig. 2G). The fingerprint region in the FTIR spectrograph reveals a split of the amine I band into two bands at 1655 and 1626 cm-1, resulting from the two types of hydrogen bonds in the antiparallel alignment of α-chitin, though further analyses will be required to definitively rule out other isomeric forms. Our chemical results (expanded upon in Supplemental Information) demonstrate that fish scale material contains chitin and are in keeping with molecular and histochemical data of the scale epithelia of salmon and zebrafish.
Figure 2.
Extraction and analysis of chitin from scale material of Atlantic salmon. (A-D) Skin (including scales) from a specimen of farmed Atlantic salmon was subjected to chitin extraction following procedures outlined in Methods (Supplemental Experimental Procedures). After extraction, the precipitated pellets were collected, stained for chitin with the CBD-546 probe and imaged using an epifluorescent stereomicroscope. Scale bars = 500 μm. (A) A representative pellet from the chitin extraction, photographed using visible light only. (B) CBD-546 chitin detection of the same pellet as in (A), showing strong punctate signals amongst a background of unstained or weakly stained material which has been shown to be comprised largely of deacetylated chitin. Controls included purified α-chitin from shrimp shells (C) and chitosan (75-85% deacetylated shrimp shell chitin) (D). The controls demonstrate that our CBD-546 probe is very specific to intact chitin as it did not give appreciable signals with chitosan. (E) Stereomicrograph of an area containing several chitinous granules within the thin, acid-soluble film. Scale bar = 100 μm. (F) SEM image of a chitinous granule. (G) FTIR analysis of the fingerprint region of the chitinous granules (blue) and its comparison with β-chitin from squid pen (purple) and α-chitin from shrimp shells (red). Two additional resonances -- 1494 cm−1 and 1453 cm−1 -- stand out as main differences of our extracted chitin with respect to the controls and are diagnostic of aromatic ring breathing modes that were subsequently identified as polystyrene contamination of the salmon material (discussed in Supplemental Information). Scale bar = 40 μm.
First isolated from fungi over 200 years ago, chitin has since been identified from a wide range of organisms [5, 6], including fossils [7, 8]. It is an integral structural component of invertebrate organisms and its tractability to industrial-scale chemical extraction and bioengineering has enabled its use in many applications in biomedicine and technology [5, 9]. The difficulty of its extraction from vertebrates, however, likely has contributed to its assumed absence and there has been but one report of the detection of a fish chitin some 30 years ago [10]. We show here the existence of endogenous chitin in fishes and amphibians, which collectively comprise over half of all extant vertebrates. Efforts can now be focused on elucidating the underlying biology and biochemistry of vertebrate chitin.
Supplementary Material
Acknowledgments
The following individuals provided assistance, resources and/or advice: Jesse Bengtsson, Jeramiah Smith, Igor Schneider, Michael Rego, Andrew Bornstein, Paul Freddura, Andrew Nagle, John Rawls, Gail Mueller, John Cannon, Larry Dishaw, Mark Messerli, John Dowling, and Gary Litman. Yinhua Zhang (New England Biolabs) provided the plasmid used for producing the SNAP-tag-CBD fusion protein and Ronald Kwon helped with analysis of zebrafish microarray data. Support for this work was provided, in part, by NIH grants RR014085 and GM095471 (to CTA) and discretionary funds from our respective institutions. Sequencing data are deposited in NCBI under accession number KM203892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes one table (Table S1), two figures (Fig. S1, S2), one composite movie (Movie S1), Methods (Supplemental Experimental Procedures), Further Discussion, and can be found with this article online at http://XXX
References
- 1.Zakrzewski AC, Weigert A, Helm C, Adamski M, Adamska M, Bleidorn C, Raible F, Hausen H. Early divergence, broad distribution, and high diversity of animal chitin synthases. Genome biology and evolution. 2014;6:316–325. doi: 10.1093/gbe/evu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Dong PD, Stainier DY, Heath JK. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 4.JayaNandanan N, Mathew R, Leptin M. Guidance of subcellular tubulogenesis by actin under the control of a synaptotagmin-like protein and Moesin. Nat Commun. 2014;5 doi: 10.1038/ncomms4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzzarelli RAA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydrate Polymers. 2012;87:995–1012. [Google Scholar]
- 6.Wagner GP. Evolution and multi-functionality of the chitin system. In: Schierwater B, Streit B, Wagner GP, DeSalle R, editors. In Molecular Ecology and Evolution: Approaches and Applications. Burkhauser Verlag; Basel: 1994. [Google Scholar]
- 7.Cody GD, Gupta NS, Briggs DEG, Kilcoyne ALD, Summons RE, Kenig F, Plotnick RE, Scott AC. Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology. 2011;39:255–258. [Google Scholar]
- 8.Ehrlich H, Rigby JK, Botting JP, Tsurkan MV, Werner C, Schwille P, Petrasek Z, Pisera A, Simon P, Sivkov VN, et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Scientific reports. 2013;3:3497. doi: 10.1038/srep03497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzzarelli RAA. Chitin nanostructures in living organisms. In: Gupta NS, editor. In Chitin: Formation and Diagenesis. Springer; Dordrecht: 2011. pp. 1–34. [Google Scholar]
- 10.Wagner GP, Lo J, Laine R, Almeder M. Chitin in the epidermal cuticle of a vertebrate (Paralipophrys trigloides, Blenniidae, Teleostei) Experientia. 1993;49:317–319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.