Abstract
Obesity, a risk factor for kidney stones and chronic kidney disease (CKD), is effectively treated with bariatric surgery. However, it is unclear if surgery alters stone or CKD risk. To determine this we studied 762 Olmsted County, Minnesota residents who underwent bariatric surgery and matched them with equally obese control individuals who did not undergo surgery. The majority of bariatric patients underwent standard Roux-en-Y gastric bypass (RYGB) (78%), with the remainder having more malabsorptive procedures (very long limb RYGB or biliopancreatic diversion/duodenal switch; 14%), or restrictive procedures (laparoscopic banding or sleeve gastrectomy; 7%). Mean age was 45 years with 80% female. The mean preoperative BMI was 46.7 kg/m2 for both cohorts. Rates of kidney stones were similar between surgery patients and controls at baseline, but new stone formation significantly increased in surgery patients (11.0%) compared to controls (4.3%) during 6.0 years of follow up. After malabsorptive and standard surgery, the comorbidity-adjusted hazard ratio of incident stones was significantly increased to 4.15 and 2.13, respectively but not significantly changed for restrictive surgery. The risk of CKD significantly increased after the malabsorptive procedures (adjusted hazard ratio of 1.96). Thus, while RYGB and malabsorptive procedures are more effective for weight loss, both are associated with increased risk of stones, while malabsorptive procedures also increase CKD risk.
Keywords: Bariatric surgery, hyperoxaluria, nephrolithiasis, obesity
Introduction
Utilization of bariatric surgery continues to be high in the United States. Recent large, randomized trials confirm that patients have sustained weight loss, less mortality, and a decrease in obesity-related complications, such as diabetes, hypertension, and obstructive sleep apnea 1, 2. Thus, the number of bariatric procedures performed annually in the United States has increased from 12,775 into a peak of 135,985 in 2004; rates have since plateaued.
In 2008, about 70% of bariatric procedures were Roux-en-Y gastric bypass (RYGB) 3, the preferred procedure, because it is associated with acceptably low morbidity and improved absolute and sustained weight lost compared to restrictive procedures (mainly adjustable gastric banding). Recently, sleeve gastrectomy has been reported to have an efficacy between that of gastric banding and RYGB.3 RYGB is still viewed as a more durable and effective procedure, especially in cases of severe obesity, and still represented 56% of procedures in 2012.3 The number of existing persons in the United States with RYGB procedures performed between 1998 and 2008 can be estimated to be approximately 830,000.4 We reported previously a high incidence of hyperoxaluria and kidney stones amongst patients after RYGB for obesity5. Others have made similar observations in other patient cohorts.6-8 The risk of hyperoxaluria and perhaps kidney stones may be less with other forms of bariatric surgery 9-11. However, the risk of kidney stones and/or CKD with bariatric surgery remains unclear, because these studies were either not population-based or lacked controls with similar obesity and comorbidities that did not undergo bariatric surgery.
Thus, in the current study we used the resources of the Rochester Epidemiology Project 12 to conduct a population-based study to compare the incidence of stones in patients after bariatric surgery to comorbidity-matched obese controls.
Results
There were 2683 patients with a history of bariatric surgery at Mayo Clinic during the study period. After excluding those without research authorization (n=63), Olmsted County residency (n=1832), or preoperative BMI greater than 35 kg/m2 (n=26), there were 762 bariatric surgery patients studied. There were 13,256 Olmsted County residents with a BMI >35 kg/m2 during the study period. After excluding those with bariatric surgery (n=699) and subjects who refused research authorization (n=63), some 12,494 potential controls remained. With 1:1 matching, we were able to identify controls for 759 of the 762 bariatric surgery patients.
Among the bariatric operations performed, most (n=591, 78%) were standard RYGB procedures (Table 1). The majority of standard RYGB operations before 2007 were open procedures (n=188), while laparoscopic procedures predominated after 2004 (n=404). When a greater amount of weight loss was deemed desirable, procedures typically more malabsorptive in nature were performed, including very, very long limb RYGB (VLLRYGB, n=55) or biliopancreatic diversion/duodenal switch (BPD-DS; n=50). At our institution, a relatively small number of restrictive procedures, including laparoscopic banding (n=43) or laparoscopic sleeve gastrectomy (n=13), were completed during the years of the study. Mean (SD) age at the time of bariatric surgery was 44.7 (11.2) years, 80% were female, and mean preoperative BMI was 46.7(7.9) kg/m2; due to matching these were the similar in controls (Table 2). Baseline comorbidities, including hypertension, diabetes, osteoarthritis, and sleep apnea, were more common in bariatric surgery patients than obese controls (Table 2). CKD at baseline was similar between both groups (10.4% versus 8.7%, p=0.26).
Table 1. Types of bariatric operations 2000-2011.
| Malabsorptive procedure | RYGB | Restrictive | Other | |
|---|---|---|---|---|
| Total Number | 105 | 591 | 56 | 7 |
| Number by year | ||||
| 2000-3 | 43 | 127 | 0 | 0 |
| 2004-7 | 36 | 273 | 15 | 1 |
| 2008-11 | 26 | 191 | 41 | 6 |
| Years of follow-up (mean (SD)) | 6.2 (3.7) | 6.2 (3.1) | 3.9 (1.6) | 3.1 (2.6) |
| Age at surgery (mean (SD)) | 44.1 (11.0) | 44.9 (11.2) | 42.7 (12.0) | 52.3 (9.3) |
| Sex (% Female) | 70.5% | 82.9% | 78.6% | 57.1% |
| BMI kg/m2 (mean (SD)) | 56.3 (8.4) | 45.3 (6.5) | 43.6 (7.6) | 45.4 (10.0) |
Abbreviations: Abbreviations: BMI: body mass index; RYGB Roux-en-Y gastric bypass; SD: standard deviation
Table 2. Demographics of bariatric patients and controls.
| Bariatric patients (n=759) | Controls (n=759) | P value* | |
|---|---|---|---|
| Demographics | |||
| Age, y (mean, SD) | 44.7 (11.2) | 44.7 (11.2) | matched |
| Female Sex | 80.6% | 80.6% | matched |
| BMI, kg/m2 (mean, SD) | 46.7 (7.9) | 46.7 (7.8) | matched |
| Years of follow-up (mean, SD) | 6.9 (3.4) | 7.0 (3.3) | 0.42 |
| Hypertension | 52.3% | 47.2% | 0.05 |
| Diabetes | 27.1% | 22.8% | 0.05 |
| Arthritis | 56.1% | 8.8% | <0.001 |
| Sleep apnea | 56.8% | 30.4% | <0.001 |
| CKD events | |||
| Prevalence at baseline | 10.4% | 8.7% | 0.26 |
| New (Incident) | 7.9% | 9.6% | 0.24 |
| Nephrolithiasis events | |||
| Prevalence at baseline | 4.3% | 4.0% | 0.70 |
| New (Incident) stones | 11.1% | 4.3% | <0.01 |
Abbreviations: BMI: body mass index; CKD: chronic kidney disease; SD: standard deviation
P-value from chi-square test (nominal factors), rank sum test (continuous factors), and logrank test (time to incident CKD and stones).
Nephrolithiasis at baseline was of similar frequency between the bariatric surgery patients and controls (4.0% versus 4.2%, p=0.70). In contrast, over a mean of 6.0 (3.2) years of followup, new (incident) stone events were more common in bariatric surgery patients than obese controls (11.1% vs 4.3%) (P<0.01, Table 2). Kaplan-Meier plots confirmed that kidney stone events increased among operated patients within the first two years, reaching approximately 14% at 10 years compared to 7% of controls at that time point. Among those stones analyzed, calcium oxalate stones were most common in the bariatric patients pre procedure (73%) and obese control patients (65% before or after the incident date combined; Table 3). However, calcium oxalate stones were even more common after bariatric surgery representing 94% of those analyzed (P=0.02 for comparison of the stone distribution of the post bariatric group vs obese stone formers using Fishers exact test). Stone risk varied by type of procedure, being highest for patients with a malabsorptive procedure, intermediate for standard RYGB, and lowest for restrictive procedures, which were similar to obese controls (Figure 1). Baseline diabetes, osteoarthritis, sleep apnea, and being a bariatric surgery case were all significant risk factors for incident stones (Table 4). In multivariable models, only diabetes, RYGB, and malabsorptive procedures remained statistically significant risk factors. Patients with a history of a prior stone (prevalent) at the time of bariatric surgery were more likely to form a stone after surgery than those without a prior stone history (42% vs 14% at 10 years; HR=4.1, p<0.001). However, the risk of prevalent obese patients forming a second stone was slightly higher (52% at 10 years). Thus, this observation likely reflects the known tendency for stone event risk to increase as the number of prior events increases 13, and does not suggest that bariatric surgery disproportionately augments stone risk among those with past stone events.
Table 3. Available stone composition of bariatric and obese patients, before and after the incident date.
| Bariatric cohort | Obese cohort | ||
|---|---|---|---|
| Prevalent | Incident | Prevalent and Incident | |
| Hydroxyapatite | 3 (10%) | 2 (3%) | 7 (31%) |
| Calcium oxalate | 21 (73%) | 63 (94%) | 15 (65%) |
| Struvite | 2 (7%) | 1 (1.5%) | 1 (4%) |
| Uric Acid | 3 (10%) | 1 (1.5%) | 0 (0%) |
| Not available | 4 | 17 | 40 |
Values expressed as total number and % of those available
Figure 1. Risk of new onset nephrolithiasis after bariatric surgery.
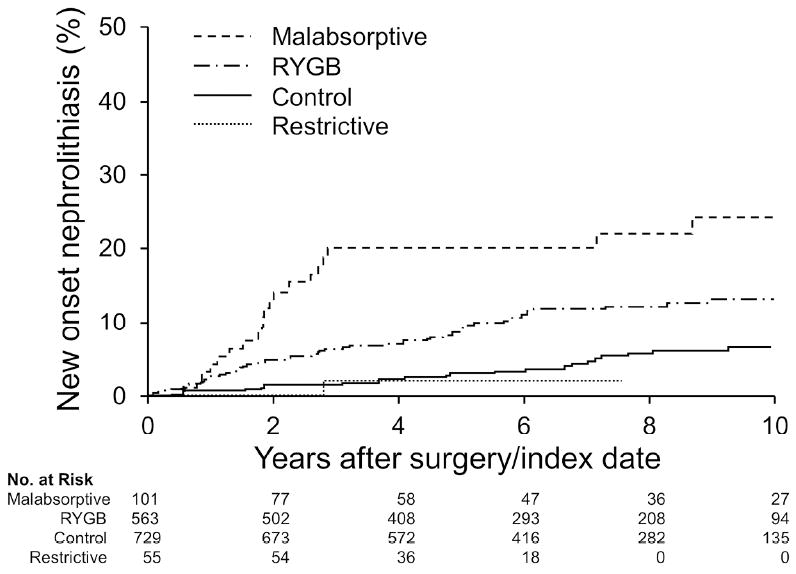
The risk of incident stones was greater after RYGB or malabsorptive bariatric procedures, compared to matched obese controls (P<0.001 overall). Patients with restrictive procedures were not at increased risk.
Table 4. Univariable and multivariable models of hazard ratios for kidney stones.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Risk Factor | HR | 95% CI | P value | HR | 95% CI | P value |
| Age at surgery (per 10 yr) | 1.04 | 0.88-1.23 | 0.65 | 0.99 | 0.82-1.20 | 0.93 |
| Male Sex | 1.11 | 0.70-1.76 | 0.64 | 1.00 | 0.62-1.61 | 0.99 |
| Hypertension | 0.89 | 0.61-1.28 | 0.52 | 1.11 | 0.73-1.69 | 0.62 |
| Diabetes | 0.51 | 0.35-0.75 | 0.0005 | 0.55 | 0.37-0.82 | 0.004 |
| Arthritis | 0.46 | 0.31-0.65 | <0.001 | 0.77 | 0.50-1.2- | 0.25 |
| Sleep apnea | 0.67 | 0.47-0.97 | 0.034 | 1.00 | 0.66-1.50 | 0.99 |
| Type of bariatric surgery | ||||||
| Control (referent) | 1.00 | na | na | 1.00 | na | na |
| RYGB | 2.49 | 1.63-3.81 | <0.001 | 2.13 | 1.30-3.49 | 0.003 |
| Malabsorptive | 5.23 | 3.02-9.05 | <0.001 | 4.15 | 2.16-8.00 | <0.001 |
| Restrictive | 0.50 | 0.07-3.69 | 0.50 | 0.46 | 0.06-3.45 | 0.45 |
Abbreviations: HR: hazard ratio; CI” confidence interval; na: not applicable; RYGB Roux-en-Y gastric bypass
Overall, bariatric surgery was not a risk factor for developing CKD (HR=0.95, CI 0.67-1.35). However, when evaluated by type of bariatric procedure, those patients with malabsorptive procedures were at increased risk (Figure 2). In multivariable modeling that controlled for DM and HTN, the HR remained increased for those undergoing the malabsorptive procedure (Table 5).
Figure 2. Risk of new onset CKD after bariatric surgery.
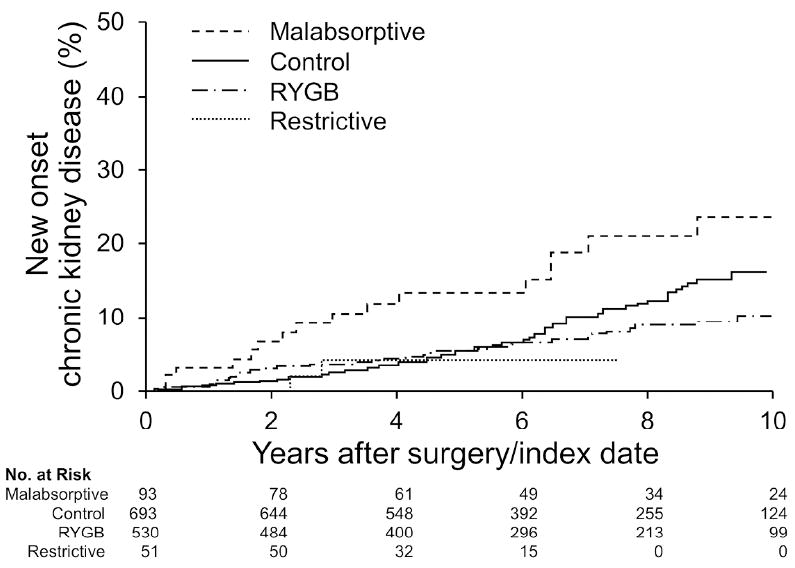
The risk of incident CKDwas greater after malabsorptive bariatric procedures compared to matched obesecontrols (P=0.004 overall). Patients were not at increased CKD risk after RYGB orrestrictive procedures.
Table 5. Univariable and multivariable models of hazard ratios for CKD.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Risk Factor | HR | 95% CI | P value | HR | 95% CI | P value |
| Age at surgery (per 10 yr) | 1.44 | 1.23-1.70 | <0.001 | 1.23 | 1.02-1.48 | 0.03 |
| Male Sex | 1.86 | 1.27-2.74 | 0.0014 | 1.40 | 0.93-2.10 | 0.11 |
| Hypertension | 0.47 | 0.33-0.66 | <0.001 | 0.70 | 0.47-1.04 | 0.08 |
| Diabetes | 0.28 | 0.20-0.39 | <0.001 | 0.34 | 0.24-0.49 | <0.001 |
| Arthritis | 0.98 | 0.68-1.40 | 0.91 | 1.05 | 0.67-1.65 | 0.83 |
| Sleep apnea | 0.72 | 0.52-1.02 | 0.06 | 1.10 | 0.75-1.61 | 0.63 |
| Type of bariatric surgery | ||||||
| Control (referent) | 1.00 | na | na | 1.00 | na | na |
| RYGB | 0.71 | 0.48-1.05 | 0.09 | 0.70 | 0.44-1.12 | 0.14 |
| Malabsorptive | 1.91 | 1.14-3.20 | 0.01 | 1.96 | 1.06-3.64 | 0.03 |
| Restrictive | 0.61 | 0.15-2.49 | 0.49 | 0.73 | 0.18-3.04 | 0.67 |
Abbreviations: HR: hazard ratio; CI” confidence interval; na: not applicable; RYGB Roux-en-Y gastric bypass
Urinary supersaturation profiles were available after bariatric surgery for 55 patients with follow-up stones and 248 without follow-up stones, as well as 20 obese controls with follow-up stones (Table 6). For the bariatric surgery group, data are presented broken down as < 8 mos after surgery (first follow-up visits) and > 8 mos after surgery (later visits). Urine oxalate excretion increased with time after surgery (< 8 mos verus > 8 mos P<0.001, and was most prominent in the post bariatric patients who developed stones > 8 months after surgery (0.70(0.37) (stone) vs 0.47(0.34) mmol/day (no stone); P<0.001). Urine citrate was also lower in this group (448 (340) (stone) vs 610(417) mg/day (no stone), P<0.001), resulting in higher overall CaOx supersaturations (2.12(0.92) (stone) vs 1.50(0.95) DG (no stone), P<0.001). Urine volumes were appreciable lower in the < 8 mos group regardless of stone status, resulting in higher supersaturations despite lower oxalate excretion at this time period. Increasing urine oxalate excretion was characteristic of the post bariatric stone forming group, with oxalate excretions appreciably higher than the non-stone forming post bariatric surgery patients and the obese stone formers (Figure 3a). Overall, CaOx SS tended to decrease in the non-stone forming post bariatric group, but remained high in those patients who experienced stone formation after surgery (Figure 3b).
Table 6. 24 hour Urine Chemistries by stone status.
| Post Bariatric Surgery | Obese stone formers | ||||
|---|---|---|---|---|---|
| Time after surgery/index date | <8 months | >8 months | 43 (25) months (Mean (SD)) | ||
| No stone | Stone | No stone | Stone | ||
| Number w/ Labs | 136 | 13 | 112 | 42 | 20 |
| Oxalate, mmol | 0.35 (0.18) | 0.29 (0.20) | 0.47 (0.34) @ | 0.70 (0.37)* | 0.39 (0.20)*** |
| Calcium, mg | 152 (90) | 163 (73) | 138 (99) | 135 (83) | 170 (107) |
| Citrate, mg | 690 (397) | 739 (485) | 610 (417) | 448 (340)& | 638 (340) |
| Uric acid, mg | 434 (153) | 477 (190) | 466 (176) | 447 (183) | 497 (186) |
| pH | 5.9 (0.5) | 6.0 (0.6) | 6.0 (0.5) | 5.8 (0.5) | 6.1 (0.8) |
| Sodium, mEq | 122 (62) | 127 (81) | 165 (88) @ | 182 (101) | 152 (75) |
| Chloride, mEq | 115 (61) | 121 (74) | 155 (92) @ | 183 (107) | 147 (71) |
| Potassium, mEq | 41 (22) | 35 (19) | 51 (25) @ | 46 (28) | 59 (30)* |
| Phosphorous, mg | 629 (293) | 568 (335) | 823 (390) @ | 800 (304) | 704 (333) |
| Magnesium, mg | 89 (39) | 88 (44) | 134 (62) @ | 127 (56) | 115 (51) |
| Sulfate, mMol | 11.4 (7.3) | 9.9 (5.5) | 14.7 (7.0) @ | 14.6 (6.5) | 16.7 (7.9) |
| Creatinine, mg | 1228 (383) | 1308 (573) | 1269 (449) | 1297 (465) | 1329 (186) |
| Volume, ml | 1380 (572) | 1094 (141) | 1970 (1039) @ | 1793 (140) | 1834 (784) |
| CaOx SS, DG | 2.02 (0.77) | 2.36 (0.44) | 1.50 (0.95) @ | 2.12 (0.92)* | 1.69 (0.38)** |
| CaP (Apatite) SS, DG | 2.98 (2.03) | 3.80 (2.12) | 2.69 (2.02) | 2.11 (2.43) | 2.98 (2.77) |
| CaP (Brushite) SS, DG | 0.73 (1.42) | 0.12 (1.18) | 1.11 (1.39) | 1.34 (1.67) | 1.01 (1.69) |
| Uric Acid SS, DG | 0.90 (2.73) | 1.32 (2.86) | 0.10 (3.08) | 0.82 (2.46) | 0.21 (3.45) |
Abbreviations: DG, Delta Gibbs; RGYB, Roux-en-Y gastric bypass.
P<0.001 vs no stone at > 8 mos
P<0.05 vs no stone
P=0.01 vs no stone at <8 mos
P<0.05 versus RYGB stone formers
P<0.005 versus RYGB stone formers
Figure 3. Changes in urine oxalate and CaOx SS after surgery.
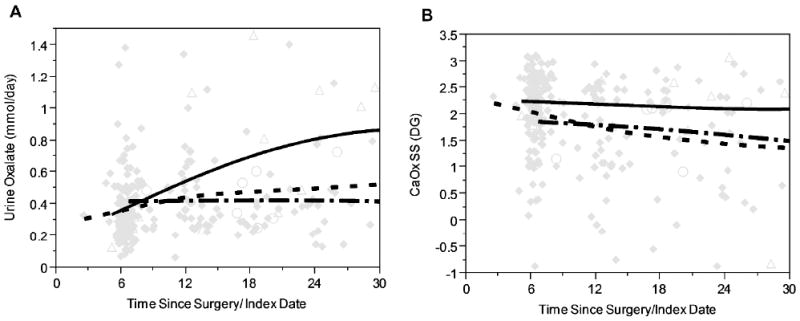
Panel A: Urinaryoxalate increased subtly in all cases over time after bariatric surgery (◆ solid diamonds, - - - - dashed line), and more dramatically in those that developed stones (Δ open triangles, —— solid line). Mean urine oxalate was at the upper limit of the referencevalue (0.46 mmol/day) at all time points in obese controls that developed stones (○ open circles, - · - · - · - dash-dot line). Panel B: At all time points CaOx SS was highest in the post bariatric surgery patients that developed stones (Δ open diamonds, —— solid line), but still at or above the reference mean (1.77 DG) in both obese controls with stones (○ open circles, - · - · - dash-dot line) as well as post bariatric surgery patients without stones (◆ solid diamonds, - - - - dashed line).
Discussion
The current study provides strong objective evidence that the risk of kidney stones is approximately doubled in patients after RYGB compared to matched, non-operated, obese controls. This risk depends on the choice of specific type of bariatric surgery, being greatest in malabsorptive procedures, intermediate in standard RYGB, and least in restrictive procedures. Although it has been recognized that hyperoxaluria and kidney stones can occur after RYGB, these are some of the first data to quantify the risk of stones in comparison to a group of obese, non-operated controls. Among those operated patients with urinary data available, hyperoxaluria was increasingly common although not marked until approximately 18 months after the procedure. Overall, however, urinary saturations for calcium oxalate were increased at all-time points after bariatric surgery. As expected, the urinary profile of obese controls with new onset stones differed, with hyperoxaluria being less of a feature.
Previous studies suggested that hyperoxaluria develops in up to one half of individuals after certain forms of bariatric surgery (e.g., RYGB) 5-8 but not others (e.g., restrictive procedures such adjustable lap band) 9, 10, and that patients with hyperoxaluria after bariatric surgery are at increased risk for nephrolithiasis 14. Perhaps the best previous quantitative data was estimated from a review of a database of private insurance claims which contained 4639 patients who were matched to obese controls14. Over a median follow-up of about 4 years, 7.65% of bariatric patients had a stone compared to 4.63% of obese controls (OR 1.71). Mean time from bariatric surgery to the stone event was 1.5 years, and RYGB patients were also more likely to have a surgical intervention for stones (OR 3.65). In contrast, laparoscopic adjustable gastric band surgery or sleeve gastrectomy did not appear to increase stone risk 11, 15. These findings are consistent with our current study. These studies were not population based and also were not controlled for other risk factors (e.g., diabetes, hypertension).
Enteric hyperoxaluria occurs in association with fat malabsorption and is believed to develop when oxalate in the diet is delivered to the colon without sufficient calcium. This pathophysiology occurs when gastrointestinal disorders affect the mucosa of the ileum, when the ileum has been resected (or bypassed) leading to short bowel syndrome, or in association with pancreatic insufficiency. More recently, a large number of patients have been recognized with hyperoxaluria and calcium oxalate stones after bariatric procedures performed for weight loss 5, 16. Most commonly, these procedures are the RYGB, with or without other features to lead to relatively more malabsorption to enhance weight loss potential. Two examples in our current cohort are the BPD-DS and VLLRYGB. It has been suspected that these patients might be at increased risk for oxalate-related complications due to a greater likelihood and degree of fat malabsorption 5, 17. Indeed, the current study provides strong objective evidence that the more malabsorptive procedures have a greater risk of kidney stones than standard RYGB.
The mechanism(s) by which RYGB patients develop hyperoxaluria has yet to be fully explained (especially because the entire ileum is in continuity with the enteric stream), but it seems likely that the length of the common channel, at least in the small group of bariatric patients who underwent the distal malabsorptive so called VLLRYGB or the BPD-DS, may predispose to clinically important fat malabsorption, leading to enteric hyperoxaluria. Previous studies have suggested that the extent of hyperoxaluria corresponds with the degree of steatorrhea 18, as observed in the various disease conditions associated with fat malabsorption, including inflammatory bowel disease, ileal resection, and jejuno-ileal bypass 19-22. Interestingly, none of the 31 patients in our published Mayo Clinic series who were seen in stone clinic with nephrolithiasis after RYGB reported symptoms of diarrhea. In the one stone clinic patient in whom fat malabsorption was assessed, 72 hour fecal fat excretion was increased (57 g; normal < 7 g), despite the absence of diarrhea. In a single case series, steatorrhea was reported in 18 of 45 patients after biliopancreatic bypass for obesity, although clinical symptoms were not reported 23.
Not all patients with gastrointestinal disorders characterized by fat malabsorption develop kidney stones. A recent study identified 51 patients with fat malabsorption and compared 10 stone formers to 41 non-stone formers 24. The stone forming group was characterized by greater urinary oxalate excretion (0.66 versus 0.38 mmol per day), less urinary citrate excretion (309 versus 607 mg/day), and greater calcium oxalate supersaturation (relative supersaturation for CaOx of 8.16 versus 3.94). Interestingly, fecal elastase, serum beta-carotene, and vitamin E were all less in the stone former group, suggesting that the degree of fat malabsorption was a key differentiating feature. Our data are consistent with the paradigm that the degree of fat malabsorption is a key determinant of kidney stone risk. Because fecal fat collections are difficult to achieve, it will be interesting to see if some panel of markers such as these can be used to identify those bariatric patients at greatest risk of stone formation. In addition, it would be interesting to extend these measurements to a group of idiopathic calcium oxalate stone formers in order to determine if subclinical fat malabsorption is often or ever a contributing factor in that patient group as well.
Kidney stones are not the only potential consequence of enteric hyperoxaluria after RYGB. Cases of oxalate nephropathy after RYGB have been clearly documented25, 26. The prevalence and risk factors for this severe outcome are less certain. In our clinics, several patients with enteric hyperoxaluria after bariatric surgery have progressed to kidney failure regardless of prescribed therapy 26. The presence of CKD prior to RYGB may be an important predisposing factor 25. In the current cohort the malabsorptive procedures, but not other forms of bariatric surgery appeared to increase the risk of new onset CKD. Clearly, only a relatively small percentage of the vast number of patients undergoing bariatric surgery has developed CKD and/or end stage kidney disease (ESKD) to date. Reliable methods to detect which of this patient group would benefit most from earlier identification and aggressive management to prevent renal failure are clearly needed.
Limitations of this study include its retrospective nature and lack of randomization. Further, the results apply primarily to Caucasians. In addition, kidney stones, CKD, and comorbidities were determined by diagnoses codes, and lab data were available for only a subset of all patients with or without kidney stones. The incidence and prevalence of CKD post bariatric surgery might also have been underestimated due to the effects of weight loos on creatinine generation and serum creatinine levels. It is also possible that kidney stones or chronic kidney disease may have been more readily diagnosed in the bariatric surgery group if they were having more medical care and diagnostic procedures. However, we were able to study a population based, matched cohort of obese patients with and without bariatric surgery. Furthermore, detailed lab data was available in a sizable number of post bariatric surgery patients, including many without kidney stones.
In conclusion, the current study suggests that obese patients who undergo RYGB have an increased risk of kidney stones that is approximately double that in obese, non-operated controls. Approximately 1 in five individuals will develop a stone within 10 years after these bariatric procedures. Urinary risk factors include hyperoxaluria, low urine volume, and hypocitraturia. Patients with malabsorptive bariatric procedures appear at greatest risk for stones, but are also at increased risk for new onset CKD. Future studies are needed to develop better treatment strategies and identify those at greatest risk for this complication.
Methods
All patients with a history of bariatric surgery at Mayo Clinic between the years of 2000 and 2011 were obtained from our institutional bariatric surgical registry that included the type and date of procedure. Patients without research authorization, Olmsted County residency, or preoperative BMI greater than 35 kg/m2 were excluded. Using the Rochester Epidemiology Project 12 controls were sampled from among all Olmsted County residents with a BMI greater than 35 kg/m2 who were seen at a clinic visit at Mayo Clinic at a clinic visit during the study period. Using validated matching algorithms 27 surgery cases were individually matched to controls exactly on sex and index year (BMI date in control closest to pre-operative BMI in surgery patient) and on BMI within ±3. Further, penalty weights were assigned to large age and BMI differences. With these criteria 759 of the 762 cases were matched with 96% having an age within 5 years.
Over 95% of the Olmsted County population is seen by a local health care provider in any 3 year period12, and thus the Rochester Epidemiology Project was used to capture follow-up kidney stone and CKD events in both bariatric surgery patients and controls. Kidney or bladder stones were identified using the International Classification of Diseases (ICD)-9 codes 592, 594, and 274.11 as reported previously 28. The initial dates of comorbidities (hypertension, diabetes, sleep apnea, osteoarthritis, chronic kidney disease (CKD)) were identified from ICD-9 codes as described previously 28. Clinical laboratory tests including results of 24-hour urine studies were captured from the electronic medical record. In the bariatric surgery group, urine chemistries were obtained as part of a routine follow-up visits beginning approximately 6 months post-surgery or potentially at the time of a nephrology stone clinic visit if they developed stones. Urine studies from obese controls were only available at the time of a nephrology stone clinic visit.
Statistical Analyses
The associations of bariatric surgery with a subsequent kidney stone event and CKD were assessed using Kaplan-Meier plots and Cox proportional hazards models with adjustments for age, sex, and other baseline comorbidities. Subjects with prevalent kidney stones were excluded from the analyses of new onset stones. Risk factor effects are presented as hazard ratios of the event rates and 95% CI’s. All tests were two-sided with alpha level 0.05. All analyses used SAS v9.3 software (SAS Institute, Cary, NC).
Acknowledgments
This study was supported by the Mayo Clinic O’Brien Urology Research Center (U54 DK100227), the Rare Kidney Stone Consortium (U54KD083908), a member of the NIH Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK and the National Center for Advancing Translational Sciences (NCATS), the Mayo Hyperoxaluria Center
Footnotes
Disclosure
The authors have no financial interests to disclose.
References
- 1.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. The New England journal of medicine. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA : the journal of the American Medical Association. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NT, Nguyen B, Gebhart A, et al. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. Journal of the American College of Surgeons. 2013;216:252–257. doi: 10.1016/j.jamcollsurg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen NT, Masoomi H, Magno CP, et al. Trends in use of bariatric surgery, 2003-2008. Journal of the American College of Surgeons. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 6.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Duffey BG, Alanee S, Pedro RN, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. Journal of the American College of Surgeons. 2010;211:8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. Journal of the American College of Surgeons. 2008;206:1145–1153. doi: 10.1016/j.jamcollsurg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Penniston KL, Kaplon DM, Gould JC, et al. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol. 2009;182:2340–2346. doi: 10.1016/j.juro.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Semins MJ, Asplin JR, Steele K, et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology. 2010;76:826–829. doi: 10.1016/j.urology.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Chen T, Godebu E, Horgan S, et al. The effect of restrictive bariatric surgery on urolithiasis. Journal of endourology / Endourological Society. 2013;27:242–244. doi: 10.1089/end.2012.0408. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Rule AD, Lieske JC, Li X, et al. The ROKS Nomogram for Predicting a Second Symptomatic Stone Episode. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matlaga BR, Shore AD, Magnuson T, et al. Effect of gastric bypass surgery on kidney stone disease. JUrol. 2009;181:2573–2577. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Semins MJ, Matlaga BR, Shore AD, et al. The effect of gastric banding on kidney stone disease. Urology. 2009;74:746–749. doi: 10.1016/j.urology.2009.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieske JC, Kumar R, Collazo-Clavell ML. Nephrolithiasis After Bariatric Surgery for Obesity. Semin Nephrol. 2008;28:163–173. doi: 10.1016/j.semnephrol.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puzziferri N, Blankenship J, Wolfe BM. Surgical treatment of obesity. Endocrine. 2006;29:11–19. doi: 10.1385/ENDO:29:1:11. [DOI] [PubMed] [Google Scholar]
- 18.McLeod RS, Churchill DN. Urolithiasis complicating inflammatory bowel disease. JUrol. 1992;148:974–978. doi: 10.1016/s0022-5347(17)36794-0. [DOI] [PubMed] [Google Scholar]
- 19.Andersson H, Bosaeus I. Hyperoxaluria in malabsorptive states. Urol Int. 1981;36:1–9. doi: 10.1159/000280387. [DOI] [PubMed] [Google Scholar]
- 20.Modigliani R, Labayle D, Aymes C, et al. Evidence for excessive absorption of oxalate by the colon in enteric hyperoxaluria. Scand J Gastroent. 1978;13:187–192. doi: 10.3109/00365527809181746. [DOI] [PubMed] [Google Scholar]
- 21.Stauffer JQ. Hyperoxaluria and intestinal disease. The role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. AmJ Dig Dis. 1977;22:921–928. doi: 10.1007/BF01076170. [DOI] [PubMed] [Google Scholar]
- 22.Earnest DL, Johnson G, Williams HE, et al. Hyperoxaluria in patients with ileal resection: an abnormality in dietary oxalate absorption. Gastroenterology. 1974;66:1114–1122. [PubMed] [Google Scholar]
- 23.Ocon Breton J, Perez Naranjo S, Gimeno Laborda S, et al. Effectiveness and complications of bariatric surgery in the treatment of morbid obesity. Nutr Hosp. 2005;20:409–414. [PubMed] [Google Scholar]
- 24.Siener R, Petzold J, Bitterlich N, et al. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology. 2013;81:17–24. doi: 10.1016/j.urology.2012.07.107. [DOI] [PubMed] [Google Scholar]
- 25.Nasr SH, D’Agati VD, Said SM, et al. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson WK, Houghton SG, Milliner DS, et al. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surgery for Obesity and Related Diseases. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Bergstralh EJ, Kosanke JL, Jacobsen SJ. Software for optimal matching in observational studies. Epidemiology. 1996;7:331–332. [PubMed] [Google Scholar]
- 28.Rule AD, Bergstralh EJ, Melton LJ, 3rd, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]


