Abstract
Smoking is associated with adverse effects on pregnancy and fetal development, yet 85-90% of pregnant women in medication-assisted treatment for an opioid use disorder smoke cigarettes. This review summarizes existing knowledge about smoking cessation treatments for pregnant women on buprenorphine or methadone, the two forms of medication-assisted treatment for opioid use disorder indicated for prenatal use. We performed a systematic review of the literature using indexed terms and key words to capture the concepts of smoking, pregnancy, and opioid substitution and found that only three studies met search criteria. Contingency management, an incentive based treatment, was the most promising intervention: 31% of participants achieved abstinence within the 12-week study period, compared to 0% in a non-contingent behavior incentive group and a group receiving usual care. Two studies of brief behavioral interventions resulted in reductions in smoking but not cessation. Given the growing numbers of pregnant women in medication-assisted treatment for an opioid use disorder and the negative consequences of smoking on pregnancy, further research is needed to develop and test effective cessation strategies for this group.
Keywords: cigarette smoking, opioid, pregnancy, incentives, contingency management, motivational enhancement, 5 As
1. Introduction
Approximately 16% of all pregnant women smoke cigarettes (Substance Abuse and Mental Health Services Administration, 2013). Smoking is a leading preventable risk factor for adverse pregnancy, fetal, and neonatal outcomes. Detrimental effects of smoking during pregnancy include increased rates of placental abruption, intrauterine growth restriction, preterm delivery, low birth weight, and stillbirth (Bada et al., 2002; Hammoud et al., 2005; Salihu & Wilson, 2007). Long-term effects in children born to smoking mothers may include problems with maternal-neonatal attachment, increased risk of sudden infant death syndrome, conduct problems, and an increased risk of developing tobacco and other substance use disorders later in life (Agrawal et al., 2010; Gaysina et al., 2013; Lotfipour et al., 2014; Magee et al., 2014; Salihu & Wilson, 2007; Zhang & Wang, 2013).
Alarmingly high rates of smoking (88-95%) occur in pregnant women concurrently treated with buprenorphine or methadone for an opioid use disorder (Chisolm et al., 2013; Jones et al., 2009). Medication-assisted treatment is recommended to help lessen illicit opioid use and improve pregnancy outcomes in women with an opioid use disorder (Center for Substance Abuse Treatment, 2005; National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998). However, the importance of treating co-occurring tobacco use disorder cannot be underestimated, as the negative fetal effects of smoking are potentially more severe than those associated with opioid use (Bada et al., 2002).
Babies exposed to opioids, including buprenorphine or methadone, in utero are at risk for neonatal abstinence syndrome (NAS). This is a withdrawal syndrome characterized by central nervous system hyperirritability, autonomic dysfunction, and gastrointestinal abnormalities that may appear soon after birth (Kaltenbach, Berghella, & Finnegan, 1998) and is often associated with longer hospital stays and higher cost of care (Patrick et al., 2012). Cigarette smoking can increase the duration and severity of neonatal abstinence syndrome resulting in longer hospital stays for these infants (Bakstad, Sarfi, Welle-Strand, & Ravndal, 2009; Choo, Huestis, Schroeder, Shin, & Jones, 2004; Jansson, Dipietro, Elko, & Velez, 2007, 2010; Jones et al., 2013). Moreover, babies born to heavy smokers (≥ 20 cigarettes per day) have lower birth weights and lengths compared to light smokers in medication-assisted treatment for opioid use disorder (Winklbaur et al., 2009).
In the general pregnant population, 20-45% of women spontaneously quit smoking upon becoming pregnant (Quinn, Mullen, & Ershoff, 1991; Solomon & Quinn, 2004; Woodby, Windsor, Snyder, Kohler, & Diclemente, 1999), while almost no pregnant women in medication-assisted treatment for an opioid use disorder quit spontaneously (Chisolm et al., 2013; Jones et al., 2009). The American College of Obstetricians and Gynecologists (ACOG) and the U.S. Department of Health and Human Services recommend that obstetrical providers ask all pregnant women about tobacco use and provide pregnancy-tailored counseling based on the “5 A's” counseling model (Albrecht, Phelan, & Melvin, 2011; Fiore et al., 2008). Used as it was designed, the “5 A's” is associated with improvement in cessation rates of 30-70% among pregnant women in general (Jordan, Dake, & Price, 2006). However, in actual practice many obstetricians are more likely to deliver the first two of the five A's (ask and advise) and less likely to include the other three (assess, assist and arrange), which may impact effectiveness (Jordan et al., 2006).
Other behavioral smoking cessation interventions studied among the general population of pregnant women include cognitive behavioral therapy, motivational interviewing, interventions based on the stages of change, feedback on fetal nicotine exposure, the measurement of nicotine by-products, and incentive-based treatment (Fiore et al., 2008; Lumley et al., 2009). Counseling interventions appear to be more effective than usual obstetrical care (RR 1.44, 95% CI 1.19 to 1.75), and incentive-based treatment is more effective than less intensive interventions (RR 3.64, 95% CI 1.84 to 7.23) (Chamberlain et al., 2013) with incentive based treatment also associated with improvements in fetal growth and birth outcomes (Higgins et al., 2012).
Results of research on smoking cessation pharmacotherapies with pregnant smokers have been mixed (Brose, McEwen, & West, 2013; Coleman, Chamberlain, Davey, Cooper, & Leonardi-Bee, 2012; Swamy et al., 2009) and concerns remain about the safety of these medications during pregnancy (Albrecht et al., 2011; Alwan et al., 2010; Chisolm, Brigham, Tuten, Strain, & Jones, 2010; Swamy et al., 2009). Fiore and colleagues (2008) reviewed three existing randomized controlled trials of nicotine replacement therapy during pregnancy. Two found no significant improvement over nonpharmacologic approaches; one study comparing nicotine replacement and cognitive behavioral therapy (CBT) to CBT alone found improvement with the addition of nicotine replacement, however, the study was halted due to an increase in preterm birth rate in the nicotine treatment group (Pollack, et al 2007).
Although rates of spontaneous smoking cessation in pregnant women on medication-assisted treatment are extremely low (Chisolm et al., 2013; Jones et al., 2009), those engaged in substance abuse treatment may be interested in smoking cessation, (Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997; Haug, Stitzer, & Svikis, 2001) and pregnancy can be a time when women are particularly motivated to stop smoking (Lumley et al., 2009). Pregnant women on methadone or buprenorphine are also a reachable population with regular contact with substance treatment providers. Moreover, studies in patients with substance use disorders suggest that smoking cessation treatment increases rates of substance abstinence and does not negatively impact the treatment of the primary substance use disorder (Dunn, Sigmon, Reimann, Heil, & Higgins, 2009; Reid et al., 2008; Shoptaw et al., 2002), all indicating that pregnancy is an ideal time for clinicians to treat women with tobacco use disorder.
Given the current rise in illicit opioid use during pregnancy (Patrick et al., 2012), concurrent cigarette smoking during pregnancy is becoming a growing problem with great public health significance (Haug et al., 2001; Jones et al., 2009; Tong, Jones, Dietz, D'Angelo, & Bombard, 2009). Effective treatments are needed. The purpose of this systematic review, therefore, is to summarize research on interventions to treat tobacco use disorder in pregnant women in medication-assisted treatment for an opioid use disorder.
2. Methods
Two librarians (including co-author H. Blunt) searched the following databases up to November 4, 2013: MEDLINE (PubMed, from 1946); Cochrane Library (Wiley, issues 11 and 12, 2013); Web of Science (Web of Knowledge, from 1900); PsycINFO (Proquest, from 1806); CINAHL (EBSCO from 1981); Dissertations & Theses (Proquest, from 1861).
The search included indexed terms and text words to capture the concepts of smoking, pregnancy, and opioid substitution. There were no language or study design restrictions. The search strategy was adjusted for the syntax appropriate for each database. (See Appendix for full search strategies.)
Searches returned a total of 268 results. Two review authors (S. Akerman and S. Heil) independently screened titles and abstracts for inclusion. We included trials of any study type and design (full text, those published as abstracts, and unpublished data) evaluating any treatment for smoking in pregnant women in opioid medication-assisted treatment. We included adult pregnant women (>18 years old) with a diagnosis of opioid use disorder/opioid dependence in medication-assisted treatment with buprenorphine, methadone, or Levacetylmethadol (LAAM).
The two above-mentioned authors independently screened for inclusion all studies identified as a result of the search protocol. There were no disagreements requiring consultation with a third person. Reviewers identified and excluded duplicates and collated multiple reports of the same study. They examined reference lists of included papers for additional relevant studies revealing no additional studies that met inclusion criteria.
3. Results
Three articles met inclusion criteria; one uncontrolled cohort study and two randomized controlled studies. These are summarized in Table 2. The cohort study evaluated a six-week smoking cessation group treatment within a comprehensive outpatient substance abuse treatment program that included methadone maintenance therapy for 44 pregnant and 47 non-pregnant parenting women. Consistent with ACOG and US DHHS recommendations, the group included implementation of the 5 A's technique for assessment of tobacco use as well as education about the risks of tobacco use and benefits of cessation, identification of patient motivators to quit, and coping skills. Daily self-reported cigarette use in the pregnant methadone maintained women decreased by 49 % from week one of the intervention to the three-month follow-up. This finding was not statistically different from the 32% decrease reported by the non-pregnant group, suggesting comparable efficacy for this intervention in methadone maintained pregnant and non-pregnant parenting women, but overall poor efficacy in terms of cessation. Authors of this study note that its limitations include the lack of a control condition, reliance on self-reported data (subject to recall bias and responding based on social desirability) as well as the small sample size (Holbrook & Kaltenbach, 2011).
A randomized trial evaluated a four-session motivational enhancement therapy for smoking cessation compared to usual care among pregnant smokers on methadone (Haug, Svikis, & Diclemente, 2004). Motivational Enhancement Therapy (MET) is a systematic intervention based on the principles of motivational psychology designed to promote a change in behavior (Miller, Zweben, DiClemente, & Rychtarik, 1995). Usual care consisted of provider advice to quit smoking during pregnancy. The intervention included an intake assessment, education, and interactive, personalized feedback regarding behaviors as well as follow up for 63 pregnant opioid-dependent smokers on methadone maintenance. Women in the MET group were no more likely to quit smoking compared to those receiving standard practitioner advice. Although participants in both groups self-reported a decrease in smoking, carbon monoxide and cotinine levels increased. Thirty-five percent of participants in the MET group moved forward on the change continuum (e.g., from pre-contemplation to contemplation) versus 15% in the standard care group. This suggests that the intervention may have increased their motivation for quitting but this intervention was insufficient.
A second randomized trial evaluated 12 weeks of contingency management for smoking cessation in pregnant women on medication-assisted treatment for an opioid use disorder. Pregnant women (n=102) in a methadone treatment program were randomly assigned to one of three treatment arms: contingent behavioral incentives; non-contingent behavioral incentives; or treatment as usual for smoking cessation, consisting of verbal and written information about the adverse effects of smoking on mother and infant along with routine assessment of smoking status and positive feedback about efforts to abstain at each obstetric appointment. Those in the contingent behavioral incentives group met three times weekly to receive escalating monetary incentives if they met target smoking reduction goals as measured by breath carbon monoxide. Goals escalated from graduated reduction to abstinence over 12 weeks. Those in the non-contingent behavioral incentives group received monetary vouchers based on a predetermined schedule not linked to smoking behavior; and those in the treatment as usual group did not receive any vouchers. In the group receiving contingent behavioral incentives, almost half (48%) met a target of 75% reduction (as measured by reduced breath CO) and nearly one third (31%) were abstinent (CO < 4 p.p.m.) by week 12. In the non-contingent behavior intervention group, 0% achieved a 75% reduction and 0% achieved abstinence. In the treatment as usual group, 2% achieved a 75% reduction and 0% achieved abstinence. Birth outcomes between groups were not significantly different, though the authors suggest that in a larger sample, the contingent behavioral incentive group may have had fewer preterm and low birth weight babies when compared to the other two groups. Participants typically began the intervention in the second trimester and authors suggest that an earlier intervention may improve birth outcomes (Tuten, Fitzsimons, Chisolm, Nuzzo, & Jones, 2012).
4. Discussion
Despite the extremely high prevalence and adverse consequences of smoking in pregnant women in medication-assisted treatment for an opioid use disorder, only three studies have evaluated smoking cessation interventions in this population. Incentive based treatment (i.e. contingency management) was the only effective intervention, demonstrating significant effects on both smoking abstinence and reduction (Tuten et al., 2012). These results are consistent with the literature experimentally documenting the efficacy of contingency management in promoting smoking abstinence in the general pregnant population (Lumley et al., 2009; Lussier, Heil, Mongeon, Badger, & Higgins, 2006) and in non-pregnant subjects in medication-assisted treatment (Dunn et al., 2010), and also with the large extant literature on contingency management interventions for substance use disorders (Lussier et al., 2006).
The brief behavioral interventions were promising but did not lead to abstinence (Haug et al., 2004; Holbrook & Kaltenbach, 2011). Several brief behavioral interventions have been shown to have significant but modest effects (e.g. 10% abstinence) in non- pregnant methadone-maintained smokers (Reid et al., 2008; Zirakzadeh, Shuman, Stauter, Hays, & Ebbert, 2013). However, these studies did not have sufficient power to demonstrate the impact of interventions with small effects. Overall, these studies suggest that more intensive interventions are needed to help people with an opioid use disorder quit smoking.
This systematic review is limited by the small number of available studies and the relatively small sample sizes in each of them. Moreover, none of the studies reported controlling for the trimester of pregnancy, which may be of relevance in prenatal smoking cessation (Heil et al., 2014).
Little is known about the etiology of the very high rates of smoking in pregnant women in opioid medication-assisted treatment for an opioid use disorder and why an intensive intervention such as contingency management might be required to help these women quit smoking. Data from non-pregnant patients indicate that people smoke more when exposed to opioid substitution medications (Chait & Griffiths, 1984; Mello, Lukas, & Mendelson, 1985; Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002; Richter et al., 2007; Schmitz, Grabowski, & Rhoades, 1994) and smoke less when detoxified from them (Patrick et al., 2014), suggesting a pharmacological interaction between nicotine and medications used in opioid medication-assisted treatment that may perpetuate smoking (Chait & Griffiths, 1984; Elkader, Brands, Selby, & Sproule, 2009; Frosch, Nahom, & Shoptaw, 2002; McCool & Paschall Richter, 2003; Mello et al., 1985; Mutschler et al., 2002; Patrick et al., 2014; Richter et al., 2007; Schmitz et al., 1994; Shoptaw et al., 2002; Spiga, Schmitz, & Day, 1998; Stein, Anderson, & Niaura, 2006). Furthermore, compared to healthy controls, people in medication-assisted treatment for an opioid use disorder demonstrate a blunted subjective response to non-drug pleasurable activities (Hill et al., 2013; Lubman et al., 2009) and decreased fMRI activity in response to a non-drug reward in the ventral striatum (Gradin, Baldacchino, Balfour, Matthews, & Steele, 2014), a key component of the dopamine mediated brain reward circuitry (Volkow, Wang, Fowler, & Tomasi, 2012). Exposure to drugs of abuse, while initially reinforcing, in part due to an increase in dopamine in the brain reward system (Di Chiara & North, 1992; Tobler, O'Doherty, Dolan, & Schultz, 2007), may lead to alteration of the dopamine response after chronic exposure, (Volkow et al., 2010) resulting in drug rewards having more (and non-drug rewards having less) incentive value (Hyman, Malenka, & Nestler, 2006; Koob & Le Moal, 2001; Nazzaro, Seeger, & Gardner, 1981; Robinson & Berridge, 1993). Medication-assisted treatment decreases illicit opioid use (Mattick, Kimber, Breen, & Davoli, 2008), but does not appear to ameliorate the alteration in reward processing seen in drug dependent individuals. While the neurobiological effects of nicotine are complex, nicotine administration causes dopamine release in the ventral striatum (Brody et al., 2004) and enhances sensitivity to non-drug rewards (Attwood, Penton-Voak, & Munafo, 2009; Barr, Pizzagalli, Culhane, Goff, & Evins, 2008; Chaudhri et al., 2006; Donny et al., 2003; Kenny & Markou, 2006; Thiel, Sanabria, & Neisewander, 2009). Taken together, these studies suggest that future research should explore the role of the dopamine mediated brain reward circuitry in the etiology of the high rates of cigarette smoking in people in medication-assisted treatment for an opioid use disorder.
People who use substances such as alcohol (Griffiths, Bigelow, & Liebson, 1976; Henningfield, Chait, & Griffiths, 1983) and stimulants (Rush et al., 2005; Sigmon, Tidey, Badger, & Higgins, 2003) also have high rates of smoking, and there is no consensus on treating tobacco use disorder in pregnant smokers with these substance use disorders (Lundquist, Seward, Byatt, Tonelli, & Kolodziej, 2012). However, treatment for these primary substance use disorders during pregnancy is abstinence (Keegan, Parva, Finnegan, Gerson, & Belden, 2010) whereas medication-assisted treatment is the recommended treatment for opioid use disorder in pregnancy (“ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy,” 2012). The possibility of an interaction between nicotine and opioid medications and the role of the dopamine mediated brain reward circuitry must be explored among pregnant women, as the prevalence of opioid use disorder in pregnancy is increasing and pregnant women are increasingly being treated with medication-assisted treatment.
Clinicians may also prove to be a barrier to smoking cessation. Pregnancy is a time in which women become motivated to decrease and stop substance use. However, clinicians often do not consider smoking cessation to be a priority in patients with cooccurring substance use disorders (Fuller et al., 2007), or they may not realize that smoking cessation interventions do not negatively impact the treatment of the primary substance use disorder and may actually improve outcomes (Dunn et al., 2009; Winhusen et al., 2013). The benefits of reducing or eliminating illicit opioid use and the corresponding illicit-drug-using lifestyle are clear and include decreased rates of infectious disease, less fetal exposure to drug withdrawal, better maternal nutrition and more contact with healthcare providers (Winklbaur et al., 2008). In addition to the obvious advantages of reducing illicit opioid use, concurrent tobacco cessation has the potential to further improve outcomes.
5. Conclusions
Smoking is a significant problem in pregnant women in medication-assisted treatment with growing economic and public health implications and should be addressed as part of a comprehensive substance treatment program. Incentive based treatment is a promising intervention, consistent with the literature experimentally documenting the efficacy of contingency management in promoting smoking abstinence in the general pregnant population. More research is needed to understand the etiology of smoking in pregnant women in medication-assisted treatment for an opioid use disorder and to develop effective interventions to improve maternal and neonatal outcomes.
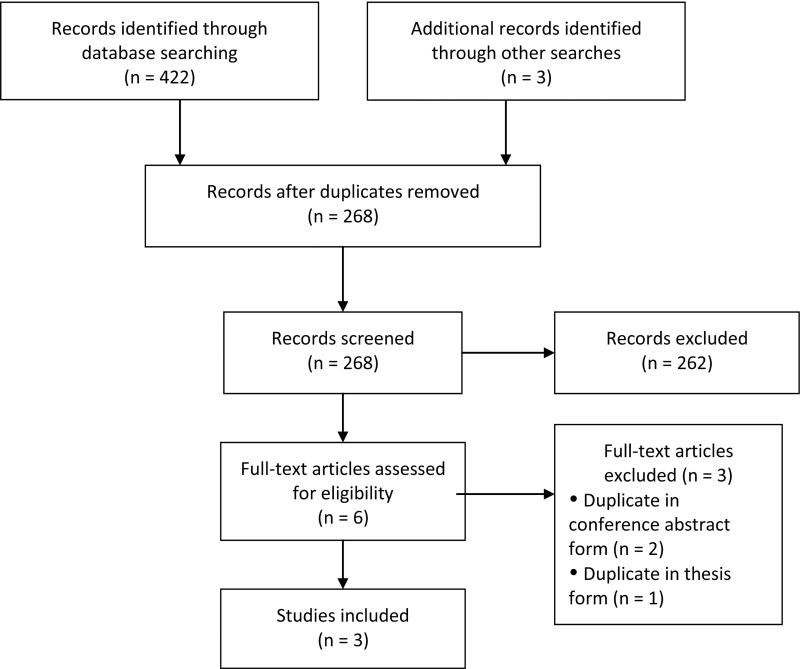
Figure 1.
Study Identification Flow Diagram, adapted from (Moher, Liberati, Tetzlaff, & Altman, 2009)
Table 1.
Summary of Studies Evaluating Smoking Cessation Treatment in Pregnant Women in Medication-Assisted Treatment for an Opioid Use Disorder
| Authors | N | N includes | Type of Treatment | Classification | Brief summary of findings | Biochemical Validation |
|---|---|---|---|---|---|---|
| Holbrook et al. (2011) (Holbrook & Kaltenbach, 2011) | 91 | Methadone maintained pregnant (N=44) and non-pregnant (N=47) cigarette smokers | 6 week group smoking cessation intervention, training in coping skills for smoking cessation | Prospective | The number of self reported daily cigarettes decreased by 49% for pregnant patients and 32% for nonpregnant patients at 3-month followup | Not done |
| Haug et al. (2004) (Haug et al., 2004) | 63 | Methadone maintained pregnant cigarette smokers (≤26 weeks gestational age) | 10 week intervention in two groups: 1.Motivational Enhancement Therapy (MET) (N=30) 2.Standard care (N=33) |
Randomized controlled trial | No difference in smoking between the two groups. 1.MET condition more likely to have progressed on the stage continuum than were SC women (35% vs. 15%) 2.SC group was more likely to have regressed on the stage continuum than were the MET participants (30% vs. 4%) |
Breath carbon monoxide, urine cotinine |
| Tuten et al. (2012) (Tuten et al., 2012) | 102 | Methadone maintained pregnant cigarette smokers (mean gestational age at entry = 16.6 weeks) | 12 week outpatient intervention in three groups: 1.Contingent behavioral incentive (N=42) 2.Noncontingent behavioral incentive (N=28) 3.Treatment as usual (N=32) |
Randomized controlled trial | 1.Contingent behavioral incentive group: 48% met a target of 75% reduction in smoking, 31% were abstinent by week 12 2.Non-contingent behavior intervention group: 0% achieved 75% reduction and 0% achieved abstinence 3.Treatment as usual group: 2% achieved a 75% reduction, 0% achieved abstinence |
Breath carbon monoxide, urine cotinine |
Acknowledgements
This work was funded in part by NIH grants CA168778 (to MFB, PI), DA032533, DA034699 (to AIG, PI) and UL1TR001086 (to AIG, PI; pilot funding via this award to SCA), DA031928 (to SHH, PI) and HD075669 (to SHH, co-investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank librarians Heather Blunt and Karen Odato for assistance with the literature search.
Appendix: Search Strategies
| PubMed | ||
|---|---|---|
| #20 | Search (#17 AND #18 AND #19) | 122 |
| #19 | Search Pregnancy[mesh] OR “Pregnancy trimesters”[mesh] OR “Pregnant women”[mesh] OR “Pregnancy complications”[mesh] OR “Prenatal Exposure Delayed Effects”[Mesh] OR “Maternal Exposure”[Mesh] OR “fetus”[mesh] OR Placenta[mesh] OR “Neonatal abstinence syndrome”[mesh] OR Pregnant[tiab] OR pregnancy[tiab] OR pregnancies[tiab] OR prenatal[tiab] OR Fetus[tiab] OR foetus[tiab] OR foetal[tiab] OR fetal[tiab] OR Placenta*[tiab] OR “in utero”[tiab] OR maternal[tiab] OR perinatal[tiab] | 1010082 |
| #18 | Search Buprenorphine[mesh] OR Suboxone[supplementary concept] OR “Opiate substitution treatment”[mesh] OR “Opioid-Related Disorders/drug therapy”[mesh] OR “Opioid-Related Disorders/rehabilitation”[mesh] OR Methadone[mesh] OR “narcotic antagonists”[mesh] OR “narcotic antagonists”[pharm action] OR LAAM[tiab] OR “l-alpha-acetylmethydol”[tiab] OR “l-alpha-acetylmethadol”[tiab] OR “levo-alpha-acetylmethadol”[tiab] OR “levo-alpha-acetylmethydol”[tiab] OR Opioid substitut*[tiab] OR opioid replace*[tiab] OR Opiate substitut*[tiab] OR opiate replace*[tiab] OR Methadone[tiab] OR Buprenorphine[tiab] OR subutex[tiab] OR buprenex[tiab] OR butrans[tiab] OR suboxone[tiab] OR narcotic antagonist*[tiab] OR Butorphanol[tiab] OR Naltrexone[tiab] OR Naloxone[tiab] OR narcan[tiab] | 52180 |
| #17 | Search Smoking[mesh:noexp] OR “Tobacco use disorder”[mesh] OR “Tobacco use cessation”[mesh] OR “Smoking cessation”[mesh] OR Tobacco[mesh] OR “Tobacco products”[mesh] OR “Tobacco use cessation products”[mesh] OR Nicotine[mesh] OR Nicotine[tiab] OR Tobacco*[tiab] OR smok*[tiab] OR cigar*[tiab] OR e-cigar*[tiab] OR snuff[tiab] | 268927 |
Note: all searches performed on November 4, 2013
PsycINFO (Proquest)
((SU.EXACT.EXPLODE(“Adolescent Pregnancy”) OR SU.EXACT.EXPLODE(“Placenta”) OR SU.EXACT.EXPLODE(“Pregnancy”) OR SU.EXACT.EXPLODE(“Perinatal Period”) OR SU.EXACT.EXPLODE(“Neonatal Period”) OR SU.EXACT.EXPLODE(“Fetus”) OR SU.EXACT.EXPLODE(“Prenatal Exposure”)) OR ab(Pregnant OR pregnancy OR pregnancies OR prenatal OR Fetus OR foetus OR foetal OR fetal OR Placenta* OR “in utero” OR maternal OR perinatal) OR ti(Pregnant OR pregnancy OR pregnancies OR prenatal OR Fetus OR foetus OR foetal OR fetal OR Placenta* OR “in utero” OR maternal OR perinatal)) AND ((SU.EXACT.EXPLODE(“Smoking Cessation”) OR SU.EXACT(“Nicotine Withdrawal”) OR SU.EXACT.EXPLODE(“Smokeless Tobacco”) OR SU.EXACT(“Tobacco Smoking”) OR SU.EXACT.EXPLODE(“Nicotine”) OR SU.EXACT.EXPLODE(“Nicotine Withdrawal”) OR SU.EXACT.EXPLODE(“Tobacco Smoking”)) OR ab(Nicotine OR Tobacco* OR smok* OR cigar* OR snuff) OR ti(Nicotine OR Tobacco* OR smok* OR cigar* OR e-cigar* OR snuff)) AND ((SU.EXACT.EXPLODE(“Naltrexone”) OR SU.EXACT.EXPLODE(“Nalorphine”) OR SU.EXACT.EXPLODE(“Methadone Maintenance”) OR SU.EXACT.EXPLODE(“Naloxone”) OR SU.EXACT.EXPLODE(“Methadone”) OR SU.EXACT.EXPLODE(“Narcotic Antagonists”)) OR ab(LAAM OR “l-alpha-acetylmethydol” OR “l-alpha-acetylmethadol” OR “levo-alpha-acetylmethadol” OR “levo-alpha-acetylmethydol” OR Opioid substitut* OR opioid replace* OR Opiate substitut* OR opiate replace* OR Methadone OR Buprenorphine OR subutex OR buprenex OR butrans OR suboxone OR narcotic antagonist* OR Butorphanol OR Naltrexone OR Naloxone OR narcan) OR ti(LAAM OR “l-alpha-acetylmethydol” OR “l-alpha-acetylmethadol” OR “levo-alpha-acetylmethadol” OR “levo-alpha-acetylmethydol” OR Opioid substitut* OR opioid replace* OR Opiate substitut* OR opiate replace* OR Methadone OR Buprenorphine OR subutex OR buprenex OR butrans OR suboxone OR narcotic antagonist* OR Butorphanol OR Naltrexone OR Naloxone OR narcan))
Dissertations & Theses (Proquest)
(ab(Nicotine OR Tobacco* OR smok* OR cigar* OR snuff OR e-cigar*) OR ti(Nicotine OR Tobacco* OR smok* OR cigar* OR snuff OR e-cigar*)) AND (ab(Pregnant OR pregnancy OR pregnancies OR prenatal OR Fetus OR foetus OR foetal OR fetal OR Placenta* OR “in utero” OR maternal OR perinatal) OR ti(Pregnant OR pregnancy OR pregnancies OR prenatal OR Fetus OR foetus OR foetal OR fetal OR Placenta* OR “in utero” OR maternal OR perinatal)) AND (ab(LAAM OR “l-alpha-acetylmethydol” OR “l-alpha-acetylmethadol” OR “levo-alpha-acetylmethadol” OR “levo-alpha-acetylmethydol” OR Opioid substitut* OR opioid replace* OR Opiate substitut* OR opiate replace* OR Methadone OR Buprenorphine OR subutex OR buprenex OR butrans OR suboxone OR narcotic antagonist* OR Butorphanol OR Naltrexone OR Naloxone OR narcan) OR ti(LAAM OR “l-alpha-acetylmethydol” OR “l-alpha-acetylmethadol” OR “levo-alpha-acetylmethadol” OR “levo-alpha-acetylmethydol” OR Opioid substitut* OR opioid replace* OR Opiate substitut* OR opiate replace* OR Methadone OR Buprenorphine OR subutex OR buprenex OR butrans OR suboxone OR narcotic antagonist* OR Butorphanol OR Naltrexone OR Naloxone OR narcan))
CINAHL (Ebsco)
| # | Query | Results |
|---|---|---|
| S10 | S3 AND S6 AND S9 | 39 |
| S9 | S7 OR S8 | 49,529 |
| S8 | nicotine OR Tobacco* OR smok* OR cigar* OR snuff OR e-cigar* | 49,529 |
| S7 | (MH “Smoking+”) OR (MH “Tobacco+”) OR (MH “Nicotine Withdrawal”) OR (MH “Nicotine”) | 33,793 |
| S6 | S4 OR S5 | 5,232 |
| S5 | LAAM OR “l-alpha-acetylmethydol” OR “l-alpha-acetylmethadol” OR “levo-alpha-acetylmethadol” OR “levo-alpha-acetylmethydol” OR Opioid substitut* OR opioid replace* OR Opiate substitut* OR opiate replace* OR Methadone OR Buprenorphine OR subutex OR buprenex OR butrans OR suboxone OR narcotic antagonist* OR Butorphanol OR Naltrexone OR Naloxone OR narcan | 5,165 |
| S4 | (MH “Buprenorphine”) OR (MH “Methadone”) OR (MH “Narcotic Antagonists+”) | 4,232 |
| S3 | S1 OR S2 | 126,586 |
| S2 | Pregnant OR pregnancy OR pregnancies OR prenatal OR Fetus OR foetus OR foetal OR fetal OR Placenta* OR “in utero” OR maternal OR perinatal | 121,502 |
| S1 | (MH “Prenatal Exposure Delayed Effects”) OR (MH “Pregnancy+”) OR (MH “Pregnancy Trimesters+”) OR (MH “Expectant Mothers”) OR (MH “Pregnancy Complications+”) OR (MH “Maternal Exposure”) OR (MH “Fetus+”) OR (MH “Placenta+”) OR (MH “Neonatal Abstinence Syndrome”) | 99,804 |
Cochrane Library (Wiley)
| ID | Search Hits |
|---|---|
| #1 | MeSH descriptor: [Smoking] this term only 4902 |
| #2 | MeSH descriptor: [Tobacco Use Disorder] explode all trees 666 |
| #3 | MeSH descriptor: [Tobacco Use Cessation] explode all trees 2835 |
| #4 | MeSH descriptor: [Tobacco Use Cessation Products] explode all trees 126 |
| #5 | MeSH descriptor: [Smoking Cessation] explode all trees 2780 |
| #6 | MeSH descriptor: [Tobacco] explode all trees 206 |
| #7 | MeSH descriptor: [Tobacco Products] explode all trees 20 |
| #8 | MeSH descriptor: [Nicotine] explode all trees 1448 |
| #9 | Nicotine or Tobacco* or smok* or cigar* or snuff or e-cigar* 17279 |
| #10 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 17279 |
| #11 | MeSH descriptor: [Pregnancy Complications] explode all trees 7162 |
| #12 | MeSH descriptor: [Pregnancy] explode all trees 5428 |
| #13 | MeSH descriptor: [Pregnancy Trimesters] explode all trees 1357 |
| #14 | MeSH descriptor: [Pregnant Women] explode all trees 77 |
| #15 | MeSH descriptor: [Prenatal Exposure Delayed Effects] explode all trees 225 |
| #16 | MeSH descriptor: [Maternal Exposure] explode all trees 29 |
| #17 | MeSH descriptor: [Fetus] explode all trees 1414 |
| #18 | MeSH descriptor: [Placenta] explode all trees 268 |
| #19 | MeSH descriptor: [Neonatal Abstinence Syndrome] explode all trees 29 |
| #20 | Pregnant or pregnancy or pregnancies or prenatal or Fetus or foetus or foetal or fetal or Placenta* or “in utero” or maternal or perinatal 31031 |
| #21 | #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 31492 |
| #22 | MeSH descriptor: [Buprenorphine] explode all trees 597 |
| #23 | MeSH descriptor: [Opiate Substitution Treatment] explode all trees74 |
| #24 | MeSH descriptor: [Opiate Substitution Treatment] explode all trees74 |
| #25 | MeSH descriptor: [Opioid-Related Disorders] explode all trees and with qualifiers: [Drug therapy - DT] 333 |
| #26 | MeSH descriptor: [Opioid-Related Disorders] explode all trees and with qualifiers: [Rehabilitation - RH] 629 |
| #27 | MeSH descriptor: [Methadone] explode all trees 861 |
| #28 | MeSH descriptor: [Narcotic Antagonists] explode all trees 817 |
| #29 | LAAM or “l-alpha-acetylmethydol” or “l-alpha-acetylmethadol” or “levo-alpha-acetyl methadol” or “levo-alpha-acetylmethydol” or Opioid substitut* or opioid replace* or Opiate substitut* or opiate replace* or Methadone or Buprenorphine or subutex or buprenex or butrans or suboxone or narcotic antagonist* or Butorphanol or Naltrexone or Naloxone or narcan 5940 |
| #30 | #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 6042 |
| #31 | #10 and #21 and #30 in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Methods Studies, Technology Assessments and Economic Evaluations 88 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACOG Committee Opinion No. 524 Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. doi: 10.1097/AOG.0b013e318256496e. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Scherrer JF, Grant JD, Sartor CE, Pergadia ML, Duncan AE, Xian H. The effects of maternal smoking during pregnancy on offspring outcomes. Prev Med. 2010;50(1-2):13–18. doi: 10.1016/j.ypmed.2009.12.009. doi:10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht S, Phelan S, Melvin C. A clinican's guide to helping pregnant women quit smoking. American College of Obstetricians and Gynecologists; Washington, DC: 2011. Committee Opinion #471. Smoking cessation during pregnancy. [Google Scholar]
- Alwan S, Reefhuis J, Botto LD, Rasmussen SA, Correa A, Friedman JM. Maternal use of bupropion and risk for congenital heart defects. Am J Obstet Gynecol. 2010;203(1):52, e51–56. doi: 10.1016/j.ajog.2010.02.015. doi: 10.1016/j.ajog.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Attwood AS, Penton-Voak IS, Munafo MR. Effects of acute nicotine administration on ratings of attractiveness of facial cues. Nicotine Tob Res. 2009;11(1):44–48. doi: 10.1093/ntr/ntn006. doi: 10.1093/ntr/ntn006. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, Wright LL, Maza PL. Gestational cocaine exposure and intrauterine growth: maternal lifestyle study. Obstet Gynecol. 2002;100(5):916–924. doi: 10.1016/s0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15(3):128–134. doi: 10.1159/000210042. doi:10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63(11):1061–1065. doi: 10.1016/j.biopsych.2007.09.015. doi:10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Mandelkern MA. Smoking-induced ventral striatum dopamine release. JAMA Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.04.017. doi: 10.1016/j.drugalcdep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment, CSAT Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs, Treatment Improvement Protocol (TIP) Series. 2005;43 Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK64164/ [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. J Pharmacol Exp Ther. 1984;229(3):636–640. [PubMed] [Google Scholar]
- Chamberlain C, O'Mara-Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, Thomas J. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev, 10. 2013:CD001055. doi: 10.1002/14651858.CD001055.pub4. doi:10.1002/14651858.CD001055.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184(3-4):353–366. doi: 10.1007/s00213-005-0178-1. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chisolm MS, Brigham EP, Tuten M, Strain EC, Jones HE. The relationship between antidepressant use and smoking cessation in pregnant women in treatment for substance abuse. Am J Drug Alcohol Abuse. 2010;36(1):46–51. doi: 10.3109/00952990903544844. doi: 10.3109/00952990903544844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm MS, Fitzsimons H, Leoutsakos JM, Acquavita SP, Heil SH, Wilson-Murphy M, Jones HE. A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. Nicotine Tob Res. 2013;15(7):1297–1304. doi: 10.1093/ntr/nts274. doi: 10.1093/ntr/nts274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75(3):253–260. doi: 10.1016/j.drugalcdep.2004.03.012. doi:10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend. 1997;44(2):123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev, 9. 2012:CD010078. doi: 10.1002/14651858.CD010078. doi:10.1002/14651858.cd010078. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992;13(5):185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann E, Heil SH, Higgins ST. Effects of Smoking Cessation on Illicit Drug Use among Opioid Maintenance Patients: A Pilot Study. J Drug Issues. 2009;39(2):313–328. doi: 10.1177/002204260903900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18(1):37. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkader AK, Brands B, Selby P, Sproule BA. Methadone-nicotine interactions in methadone maintenance treatment patients. J Clin Psychopharmacol. 2009;29(3):231–238. doi: 10.1097/JCP.0b013e3181a39113. doi: 10.1097/JCP.0b013e3181a39113. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Leitzke C. Treating tobacco use and dependence: 2008 update: Clinical practice guideline. US Deparment of Health and Human Services. Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. Journal Subst Abuse Treat. 2002;23(4):425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, McCarty D. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. J Subst Abuse Treat. 2007;32(1):53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Harold GT. Maternal Smoking During Pregnancy and Offspring Conduct Problems: Evidence From 3 Independent Genetically Sensitive Research Designs. JAMA Psychiatry. 2013;70(9):956–963. doi: 10.1001/jamapsychiatry.2013.127. doi:10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Baldacchino A, Balfour D, Matthews K, Steele JD. Abnormal Brain Activity During a Reward and Loss Task in Opiate Dependent Patients Receiving Methadone Maintenance Therapy. Neuropsychopharmacology. 2014;39(4):885–894. doi: 10.1038/npp.2013.289. doi: 10.1038/npp.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25(3):279–292. doi: 10.1901/jeab.1976.25-279. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192(6):1856–1863. doi: 10.1016/j.ajog.2004.12.057. doi:10.1016/j.ajog.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Haug NA, Stitzer ML, Svikis DS. Smoking during pregnancy and intention to quit: a profile of methadone-maintained women. Nicotine Tob Res. 2001;3(4):333–339. doi: 10.1080/14622200110050493. doi: 10.1080/14622200110050493. [DOI] [PubMed] [Google Scholar]
- Haug NA, Svikis DS, Diclemente C. Motivational enhancement therapy for nicotine dependence in methadone-maintained pregnant women. Psychol Addict Behav. 2004;18(3):289–292. doi: 10.1037/0893-164X.18.3.289. doi: 10.1037/0893-164x.18.3.289. [DOI] [PubMed] [Google Scholar]
- Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST. Examining the timing of changes in cigarette smoking upon learning of pregnancy. Prev Med. 2014 doi: 10.1016/j.ypmed.2014.06.034. doi: 10.1016/j.ypmed.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Cigarette smoking and subjective response in alcoholics: effects of pentobarbital. Clin Pharmacol Ther. 1983;33(6):806–812. doi: 10.1038/clpt.1983.110. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, Bernstein IM. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med. 2012;55:S33–S40. doi: 10.1016/j.ypmed.2011.12.016. doi:10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Han D, Dumouchel P, Dehak N, Quatieri T, Moehs C, Blum K. Long Term Suboxone™ Emotional Reactivity As Measured by Automatic Detection in Speech. PLoS One. 2013;8(7):e69043. doi: 10.1371/journal.pone.0069043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook AM, Kaltenbach KA. Effectiveness of a smoking cessation intervention for methadone-maintained women: a comparison of pregnant and parenting women. Int J Pediatr. 2011;2011:567056. doi: 10.1155/2011/567056. doi:10.1155/2011/567056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, Elko A, Velez M. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonatal Med. 2007;20(9):677–685. doi: 10.1080/14767050701490327. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, Elko A, Velez M. Infant autonomic functioning and neonatal abstinence syndrome. Drug Alcohol Depend. 2010;109(1-3):198–204. doi: 10.1016/j.drugalcdep.2010.01.004. doi: 10.1016/j.drugalcdep.2010.01.004; 10.1016/j.drugalcdep.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Heil SH, O'Grady KE, Martin PR, Kaltenbach K, Coyle MG, Fischer G. Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant patient samples. Am J Drug Alcohol Abuse. 2009;35(5):375–380. doi: 10.1080/00952990903125235. doi:10.1080/00952990903125235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Heil SH, Tuten M, Chisolm MS, Foster JM, O'Grady KE, Kaltenbach K. Cigarette smoking in opioid-dependent pregnant women: Neonatal and maternal outcomes. Drug Alcohol Depend. 2013;131(3):271–277. doi: 10.1016/j.drugalcdep.2012.11.019. doi: 10.1016/j.drugalcdep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan TR, Dake JR, Price JH. Best practices for smoking cessation in pregnancy: do obstetrician/gynecologists use them in practice? J Womens Health (Larchmt) 2006;15(4):400–441. doi: 10.1089/jwh.2006.15.400. doi: 10.1089/jwh.2006.15.400. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. J Addict Dis. 2010;29(2):175–191. doi: 10.1080/10550881003684723. doi: 10.1080/10550881003684723;10.1080/10550881003684723. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. doi: 10.1038/sj.npp.1300905. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. doi: 10.1016/s0893-133x(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Ferguson E, Leonard G, Miettunen J, Perron M, Pike GB, Paus T. Maternal Cigarette Smoking during Pregnancy Predicts Drug Use Via Externalizing Behavior in Two Community - based Samples of Adolescents. Addiction, Epub ahead of print. 2014 doi: 10.1111/add.12665. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, MacKenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66(2):205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev(3) 2009:CD001055. doi: 10.1002/14651858.CD001055.pub3. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist RS, Seward G, Byatt N, Tonelli ME, Kolodziej ME. Using a multidisciplinary approach for pregnant women with nicotine dependence and co-occurring disorders. J Dual Diagn. 2012;8(2):158–167. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta - analysis of voucher - based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Magee SR, Bublitz MH, Orazine C, Brush B, Salisbury A, Niaura R, Stroud LR. The Relationship Between Maternal-Fetal Attachment and Cigarette Smoking Over Pregnancy. Matern Child Health J. 2014;18(4):1017–1022. doi: 10.1007/s10995-013-1330-x. doi: 10.1007/s10995-013-1330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008 Apr;16(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. doi:10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McCool RM, Paschall Richter K. Why do so many drug users smoke? J Subst Abuse Treat. 2003;25(1):43–49. doi: 10.1016/s0740-5472(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology (Berl) 1985;86(4):417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. NIAAA Project MATCH monograph series; Rockville, MD: 1995. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine Tob Res. 2002;4(2):223–228. doi: 10.1080/14622200210124012. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, National Institutes of Health Effective medical treatment of opiate addiction. JAMA : the journal of the American Medical Association. 1998;280(22):1936–1943. [PubMed] [Google Scholar]
- Nazzaro JM, Seeger TF, Gardner EL. Morphine differentially affects ventral tegmental and substantia nigra brain reward thresholds. Pharmacol Biochem Behav. 1981;14(3):325–331. doi: 10.1016/0091-3057(81)90398-1. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Dunn KE, Badger GJ, Heil SH, Higgins ST, Sigmon SC. Spontaneous reductions in smoking during double-blind buprenorphine detoxification. Addict Behav. 2014;39(9):1353–1356. doi: 10.1016/j.addbeh.2014.04.023. doi:10.1016/j.addbeh.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA : the journal of the American Medical Association. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. doi:10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- Quinn VP, Mullen PD, Ershoff DH. Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addict Behav. 1991;16(1-2):29–40. doi: 10.1016/0306-4603(91)90037-i. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Burgess C. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35(1):68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Richter KP, Hamilton AK, Hall S, Catley D, Cox LS, Grobe J. Patterns of smoking and methadone dose in drug treatment patients. Exp Clin Psychopharmacol. 2007;15(2):144–153. doi: 10.1037/1064-1297.15.2.144. doi: 10.1037/1064-1297.15.2.144. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl) 2005;181(4):781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83(11):713–720. doi: 10.1016/j.earlhumdev.2007.08.002. doi:10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug Alcohol Depend. 1994;34(3):237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of d-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl) 2003;167(4):393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Solomon LJ, Quinn VP. Spontaneous quitting: self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004;6(Suppl 2):S203–S216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- Spiga R, Schmitz J, Day J., 2nd. Effects of nicotine on methadone self-administration in humans. Drug Alcohol Depend. 1998;50(2):157–165. doi: 10.1016/s0376-8716(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Niaura R. Nicotine Replacement Therapy: Patterns Of Use After A Quit Attempt Among Methadone - Maintained Smokers. J Gen Intern Med. 2006;21(7):753–757. doi: 10.1111/j.1525-1497.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, SAMHSA . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings (Vol. NSDUH Series H-46, HHS) Rockville, MD.: 2013. [Google Scholar]
- Swamy GK, Roelands JJ, Peterson BL, Fish LJ, Oncken CA, Pletsch PK, Pollak KI. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. Am J Obstet Gynecol. 2009;201(4):354, e351–354, e357. doi: 10.1016/j.ajog.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O'Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97(2):1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in Smoking Before, During, and After Pregnancy: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 Sites, 2000-2005. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [PubMed] [Google Scholar]
- Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction. 2012;107(10):1868–1877. doi: 10.1111/j.1360-0443.2012.03923.x. doi: 10.1111/j.1360-0443.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, 2nd, Penn P, Ghitza U. A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers. J Clin Psychiatry. 2013 doi: 10.4088/JCP.13m08449. doi: 10.4088/JCP.13m08449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Baewert A, Jagsch R, Rohrmeister K, Metz V, Aeschbach Jachmann C, Fischer G. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. Eur Addict Res. 2009;15(3):150–156. doi: 10.1159/000216466. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103(9):1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- Woodby LL, Windsor RA, Snyder SW, Kohler CL, Diclemente CC. Predictors of smoking cessation during pregnancy. Addiction. 1999;94(2):283–292. doi: 10.1046/j.1360-0443.1999.94228311.x. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang X. Maternal smoking and increased risk of sudden infant death syndrome: a meta-analysis. Leg Med (Tokyo) 2013;15(3):115–121. doi: 10.1016/j.legalmed.2012.10.007. doi: 10.1016/j.legalmed.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Zirakzadeh A, Shuman C, Stauter E, Hays JT, Ebbert JO. Cigarette Smoking in Methadone Maintained Patients: An Up-to-Date Review. Curr Drug Abuse Rev. 2013;6(1):77–84. doi: 10.2174/1874473711306010009. [DOI] [PubMed] [Google Scholar]