Abstract
Attention/vigilance impairments are present in individuals with schizophrenia across psychotic and remitted states and in their first-degree relatives. An important question is whether deficits in attention/vigilance can be consistently and reliably measured across sites varying in many participant demographic, clinical, and functional characteristics, as needed for large-scale genetic studies of endophenotypes.
We examined Continuous Performance Test (CPT) data from Phase 2 of the Consortium on the Genetics of Schizophrenia (COGS-2), the largest-scale assessment of cognitive and psychophysiological endophenotypes relevant to schizophrenia. CPT data from 2251 participants from five sites were examined. A perceptual-load vigilance task (the Degraded Stimulus CPT or DS-CPT) and a memory-load vigilance task (CPT - Identical Pairs or CPT-IP) were utilized.
Schizophrenia patients performed more poorly than healthy comparison subjects (HCS) across sites, despite significant site differences in participant age, sex, education, and racial distribution. Patient-HCS differences in signal/noise discrimination (d’) in the DS-CPT varied significantly across sites, but averaged a medium effect size. CPT-IP performance showed large patient-HCS differences across sites. Poor CPT performance was independent of or weakly correlated with symptom severity, but was significantly associated with lower educational achievement and functional capacity. Current smoking was associated with poorer CPT-IP d’. Patients taking both atypical and typical antipsychotic medication performed more poorly than those on no or atypical antipsychotic medications, likely reflecting their greater severity of illness.
We conclude that CPT deficits in schizophrenia can be reliably detected across sites, are relatively independent of current symptom severity, and are related to functional capacity.
Keywords: schizophrenia, endophenotype, attention, Continuous Performance Test, cognition, functional capacity, genetics
INTRODUCTION
Attentional deficits are a core feature of schizophrenia (Braff, 1993; Keefe and Harvey, 2012; Kraepelin, 1919; Nuechterlein, 1977; Nuechterlein and Dawson, 1984). Sustained focused attention (Cornblatt and Keilp, 1994a; Nuechterlein, 1991) is one aspect of attention that appears to be separable from other neurocognitive factors in schizophrenia in factor analytic studies (Nuechterlein et al., 2004). It is also the aspect of attention that has yielded the most extensive research on attentional dysfunction as an endophenotype for schizophrenia (Agnew-Blais and Seidman, 2013; Chen, 2013; Cornblatt and Malhotra, 2001; Nuechterlein et al., 1998), leading to its selection for the Consortium on the Genetics of Schizophrenia (COGS) family study (COGS-1) and current (COGS-2) case-control study (Gur et al., 2007).
Sustained focused attention is frequently measured in schizophrenia through forms of the Continuous Performance Test (CPT), rapidly paced vigilance tasks in which letters or numbers are presented in a quasi-random sequence, with the task of responding only to occasional targets (Cornblatt and Keilp, 1994b; Nuechterlein, 1991; Rosvold et al., 1956; Seidman et al., 1998). CPTs evaluate ability to maintain continuous, focused readiness to detect and respond to selected target stimuli over a prolonged time period. The most extensive research on sustained attention in schizophrenia has involved a visual CPT version with a perceptual load (the Degraded Stimulus CPT, or DS-CPT) (Nuechterlein, 1983; Nuechterlein et al., 1983) and one with a working-memory load (CPT, Identical Pairs Version, or CPT-IP) (Cornblatt et al., 1988b). Because these CPT versions burden sustained focused attention through demands on quite different cognitive processes, both were included in COGS. Either burdening perceptual processes or working memory appears to increase the sensitivity of a CPT to detect abnormalities associated with vulnerability to schizophrenia (Cornblatt et al., 1989; Cornblatt et al., 1988b; Nuechterlein, 1983; Nuechterlein et al., 1998).
Prior research supported CPT deficits as an endophenotype for schizophrenia in several ways. Within individuals with schizophrenia, the deficit is present in psychotic and remitted states (Asarnow and MacCrimmon, 1978; Nuechterlein et al., 1992; Wohlberg and Kornetsky, 1973). Both parents and siblings of schizophrenic probands have been found to show hit rate and signal/noise discrimination deficits on CPT versions with high perceptual loads (Asarnow et al., 2002; Chen et al., 2004; Chen et al., 1998; Grove et al., 1991; Maier et al., 1992; Nuechterlein et al., 1998; Saoud et al., 2000) or high working memory loads (Appels et al., 2003; Finkelstein et al., 1997; Franke et al., 1994; Laurent et al., 1999; Mirsky et al., 1992), with a few exceptions (Egan et al., 2000; Jones et al., 2001). Indeed, a meta-analysis of cognitive deficits in unaffected first-degree relatives of schizophrenia patients (Snitz et al., 2006) concluded that, when studies were restricted to those with age-matched groups and symmetrical exclusion criteria, CPT deficits showed the largest effect sizes of 24 cognitive variables (d =.56−.66). Furthermore, CPT deficits showed at least moderate heritability in earlier small samples (Chen et al., 1998; Cornblatt et al., 1988b; Grove et al., 1991), confirmed in the large COGS-1 family sample (Greenwood et al., 2007). Initial results of association and linkage analyses also show promising relationships with genes or chromosomal loci that may have relevance to schizophrenia (Chen, 2013; Greenwood et al., 2011; Greenwood et al., 2013).
Despite these encouraging results, no endophenotype study before COGS had directly examined CPT deficits across several sites differing substantially in the demographic, symptomatic, and functional characteristics of schizophrenia patients, so the extent to which the severity of CPT deficit would generalize across these varying characteristics of participants across sites was unknown. In this article, using the COGS-2 sample, we examine the degree to which CPT performance did vary across site and the extent to which patient-control differences were affected. In addition, taking advantage of the large COGS-2 sample of schizophrenia patients, we examine the relationship of CPT performance to symptom severity, mental status scores, functional capacity, and real-life functioning with much more statistical power than provided by small, single-site studies.
METHODS
Participants
Participants were recruited from five sites. All were 18-65 years old. All patients met DSM-IV criteria for schizophrenia or schizoaffective disorder, depressed type. Healthy comparison subjects (HCS) were required to have no psychotic disorder or Cluster A Axis II disorder and no current mood disorder. Further information about recruitment procedures and inclusion/exclusion criteria are provided in the introductory article for this theme issue (Swerdlow et al., 2015).
Procedures
To establish and maintain standardization in assessments across sites, all interviewers for symptoms and functioning and all testers for endophenotypes were initially trained at the University of California, San Diego (UCSD) by senior COGS investigators. Equipment and testing room specifications were standardized. All testers were observed during practice assessments by the quality assurance leader for the individual endophenotypes. Each year the COGS interviewers and testers returned to UCSD for refresher training and quality assurance checks.
Patients and HCS were rated on the Global Assessment of Function Scale (GAF; Hall, 1995). Patients were also administered the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a) and Positive Symptoms (SAPS; Andreasen, 1984b) to assess symptom severity, the University of California San Diego Performance-based Skills Assessment - Brief (UPSA-B; Mausbach et al., 2007) to assess functional capacity, and the Role Functioning Scale (RFS) (Goodman et al., 1993) and the Scale of Functioning (SOF; Rapaport et al., 1996) to assess real-life functioning.
Attention/vigilance was assessed with computerized versions of the DS-CPT (Nuechterlein and Asarnow, 2006; Nuechterlein et al., 1983) and the CPT-IP (Cornblatt et al., 1988b). These PC-based versions were designed to enhance comparability of the tasks across sites. All sites used a NEC Accusync 50 monitor and 70-Hz refresh rates.
The DS-CPT burdens perceptual processes by blurring visual stimuli (Nuechterlein et al., 1986). The PC Windows-compatible DS-CPT program produces a visual vigilance task that involves a quasi-random series of blurred single digits, with 40% random pixel reversal for visual degradation, a “0” target of 0.25 probability, and presentation rate of one digit/sec. Twenty initial examples of the blurred digits are presented using at 290 msec duration, with the tester naming each digit as it appears. Then a practice period of 160 presentations is administered with 29-msec stimulus durations, with the participant clicking the mouse whenever they detect a “0”. Finally, a vigilance test period of 480 presentations is administered using the 29-msec duration. The primary DS-CPT measure is d’, a signal/noise discrimination index from signal detection theory (Green and Swets, 1966; Nuechterlein, 1991) derived from the hit rate and false alarm rate. A response bias measure, ln beta, assesses whether participants tend to respond cautiously or liberally when in doubt about whether a stimulus is a target.
The CPT-IP (Cornblatt et al., 1988a) involves sustained attention in a situation demanding working memory. Subjects are instructed to respond each time the same stimulus occurs twice in a row in a quasi-random sequence within a 3-digit and then a 4-digit condition. Thus, the target is not a fixed number but rather requires temporarily storing each stimulus, comparing it to the next, and then updating working memory for the present stimulus if it does not match the prior one. After 25 practice trials, each condition involves 300 stimuli presented 1/sec with stimulus durations of 50 msec. Participants hold the mouse button down and respond to targets by a brief release of the button, as designed originally by Cornblatt et al. (1988). The d’ index is again the primary variable, with false alarms based only on responses to nearly identical sequential pairs.
Each CPT output file was uploaded to a centralized quality assurance system and checked by an expert (KHN), blind to diagnostic group, to confirm validity. In instances of questionable validity, the QA specialist reviewed the tester's notes and examined the trial-by-trial output file to rate validity.
Statistical analysis
Univariate ANOVAs with site and diagnosis as between-subject factors were used to examine continuous demographic and clinical variables. Chi-square with site and diagnosis factors was used to examine categorical variables. The main analyses of CPT performance were conducted using SPSS GLM ANCOVAs. To examine the patient-HCS group differences on CPT performance while examining the impact of site and sex, we completed ANCOVAs with site, sex, and diagnosis as between-subject factors and age as a covariate. For diagnostic group differences, we also present Cohen's d as a measure of effect size. Relationships between CPT performance and clinical symptom severity, functional capacity, real-life functioning, and psychiatric history variables were examined with bivariate correlations.
RESULTS
Screening for valid CPT results
Of the 2207 participants with uploaded DS-CPT data, only 92 (4.2%) were judged to have unscorable or invalid data. The most common reason was that participants could not discriminate the blurred targets during practice, so the regular DS-CPT trials were not administered (n=63). Of the 2251 participants with uploaded CPT-IP, only 55 (2.4%) of the 3-digit and 102 (4.5%) of the 4-digit condition files were judged unscorable or invalid. The most common reason for the CPT-IP was inability to learn the identical pairs rule or the finger lift response during practice, leading to omission of the regular trials.
Demographic and clinical characteristics of participants across 5 sites
Table 1 summarizes demographic and clinical characteristics of patients and HCS across the 5 sites for subjects with valid DS-CPT data. Significant diagnostic group and site differences were present on key demographic characteristics. Patients were generally somewhat older than HCS (M = 45.9 vs. 38.5, F1,2102=217.06, p<.001) and mean age varied across sites (F4,2102=37.12, p<.001), with a significant diagnosis by site interaction (F4,2102=11.04, p<.001) attributable mainly to significant age differences within each site except Site 2. Patients included more males than HCS (χ2(1)=99.93, p<.001) and sex distribution also varied across sites (χ2(4)=23.64, p<.001). As expected, schizophrenia patients had lower mean years of education (M=12.6 vs.15.0, F1,2102=703.07, p<.001), but in addition a diagnosis by site interaction (F4,2102=10.86, p<.001) indicated that this personal education difference was larger at some sites. Highest parental education, a better indicator of socioeconomic status and intellectual potential, was also somewhat lower for patients than HCS (Ms = 13.4 vs. 14.9, F1,1940=120.96, p<.001) and varied by site (F4,1940=3.38, p<.01), with the patient-HCS difference larger at some sites (F4,1940=4.15, p<.01). Racial distribution for the races with large enough samples for χ2 analyses (Caucasian, African-American, Asian) also differed across diagnosis and sites (χ2(8)=174.42, p<.001).
Table 1.
Demographic and clinical characteristics of schizophrenia patients and healthy comparison subjects (HCS) with valid DS-CPT data
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCS | Patients | HCS | Patients | HCS | Patients | HCS | Patients | HCS | Patients | |
| N* | 206 | 286 | 207 | 233 | 158 | 170 | 189 | 235 | 212 | 216 |
| Age | 40.1 (12.0) | 46.0 (11.3) | 46.4 (8.3) | 48.1 (10.9) | 34.9 (12.3) | 45.6 (10.4) | 32.6 (12.5) | 43.3 (11.5) | 37.0 (14.8) | 46.2 (11.8) |
| % Female | 57.8 | 30.8 | 40.4 | 27.5 | 60.1 | 35.3 | 53.4 | 37.4 | 49.5 | 30.4 |
| Personal Education | 14.9 (2.1) | 12.4 (1.8) | 14.8 (1.7) | 12.9 (1.9) | 15.7 (2.4) | 11.9 (2.2) | 14.8 (2.3) | 12.6 (2.3) | 15.2 (2.4) | 13.2 (1.9) |
| Parental Education (highest) | 14.3 (3.2) | 13.5 (3.0) | 14.4 (2.8) | 13.3 (3.6) | 15.4 (3.5) | 12.6 (3.2) | 15.2 (3.1) | 13.4 (3.2) | 15.4 (3.2) | 13.7 (3.3) |
| % Hispanic | 21.4 | 20.3 | 14.0 | 15.0 | 11.4 | 17.6 | 6.3 | 4.7 | 6.1 | 5.1 |
| Race | ||||||||||
| Native American | 2 | 4 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 |
| Asia | 24 | 7 | 11 | 12 | 20 | 2 | 12 | 7 | 14 | 13 |
| Pacific Islander | 3 | 3 | 2 | 4 | 1 | 1 | 0 | 0 | 7 | 4 |
| African American | 32 | 51 | 53 | 94 | 53 | 102 | 65 | 153 | 17 | 47 |
| Caucasian | 104 | 155 | 127 | 100 | 76 | 52 | 97 | 62 | 151 | 114 |
| More than One | 41 | 66 | 13 | 22 | 7 | 7 | 15 | 12 | 23 | 36 |
| Not Reported | 0 | 0 | 1 | 0 | 1 | 5 | 0 | 0 | 0 | 0 |
| % Right handed | 84.5 | 89.2 | 84.17 | 88.8 | 89.9 | 84.7 | 89.4 | 85.1 | 84.0 | 82.4 |
| Smoking | ||||||||||
| Never:Past:Now | 165:23:18 | 88:38:160 | 172:8:27 | 94:15:124 | 139:1:18 | 61:6:103 | 172:0:17 | 116:1:118 | 186:0:26 | 125:1:90 |
| # cigarettes/day if smoker | 9.4 | 15.2 | 9.5 | 16.5 | 7.3 | 11.8 | 7.3 | 13.7 | 6.9 | 11.1 |
| % Past Mood Dis | 2.9 | 36.6 | 3.9 | 40.3 | 1.3 | 16.9 | 12.6 | 36.8 | 16.0 | 29.2 |
| % Past Sub Dis | 8.3 | 54.2 | 15.9 | 44.2 | 0.6 | 21.8 | 13.8 | 51.1 | 20.3 | 47.2 |
| GAF (last month) | 90.2 (7.4) | 41.0 (5.0) | 81.8 (8.4) | 45.5 (9.1) | 92.0 (5.3) | 48.8 (8.6) | 87.2 (6.1) | 44.2 (9.7) | 82.3 (7.2) | 40.6 (4.3) |
| Age of Onset | 22.3 (7.3) | 21.9 (7.7) | 22.2 (5.7) | 21.7 (6.2) | 23.2 (7.2) | |||||
| # Psych Hosp | 8.2 (12.1) | 9.3 (12.4) | 7.9 (9.7) | 5.7 (8.1) | 5.6 (6.1) | |||||
| Global_SANS | 15.9 (4.0) | 9.1 (4.3) | 4.1 (3.3) | 10.4 (4.1) | 12.7 (3.6) | |||||
| Global_SAPS | 7.8 (4.1) | 6.1 (4.1) | 5.4 (3.0) | 7.4 (4.3) | 6.7 (3.6) | |||||
| MMSE score | 31.3 (3.2) | 30.9 (3.4) | 30.2 (2.9) | 30.8 (3.6) | 32.1 (2.4) | |||||
| UPSA-B Total | 72.4 (14.4) | 73.1 (13.9) | 66.9 (16.9) | 70.9 (14.7) | 74.0 (13.2) | |||||
| SOF Total | 46.5 (5.5) | 48.4 (5.9) | 46.9 (6.6) | 47.8 (5.9) | 43.2 (5.1) | |||||
| RFS Work | 1.6 (1.4) | 2.3 (1.5) | 3.7 (1.1) | 3.7 (1.6) | 3.2 (1.3) | |||||
| RFS INDEP | 5.2 (1.4) | 4.5 (1.4) | 3.9 (1.0) | 5.1 (1.3) | 5.4 (1.2) | |||||
| RFS FAMILY | 4.5 (2.0) | 4.7 (1.9) | 4.0 (1.1) | 5.0 (1.6) | 4.2 (1.5) | |||||
| RFS SOCIALNET | 3.5 (1.9) | 4.0 (1.8) | 3.9 (1.0) | 3.8 (1.8) | 2.8 (1.3) | |||||
For Role Functioning Scale (RFS) for patients: Site 1, N=200; Site 2, N=162; Site 3, N=143; Site 4, N=146; Site 5, N=142
Note: GAF = Global Assessment of Function Scale; SANS = Schedule for Assessment of Negative Symptoms; SAPS = Schedule for Assessment of Positive Symptoms; MMSE = Mini-Mental State Examination; UPSA-B = UCSD Performance-based Skills Assessment; SOF = Scale of Functioning; RFS = Role Functioning Scale
Symptomatic severity and functional level of patients also varied across sites. Negative symptom severity (SANS) differed across sites (F4,1130=267.32, p<.001), as did positive symptom severity (SAPS; F4,1129=14.04, p<.001). GAF scores indicated much lower overall functioning in patients than HCS (M = 43.6 vs. 86.4, F1,2089=17,395.23, p<.001), with the patient-HCS difference varying across sites (F4,2089=44.14, p<.001). Patients differed significantly across sites in functional capacity (UPSA-B: F4,1107 =6.40, p<.001) and in real-life functioning (SOF: F4,1124 =27.01, p<.001; RFS Work: F4,788=81.41, p<.001; RFS Independent Living: F4,788=35.65, p<.001; RFS Family Relationships: F4,785=13.41, p<.001). Number of psychiatric hospitalizations also differed across sites (F4,1130=5.93, p<.001). Age of schizophrenia onset did not differ significantly across sites.
CPT performance: Diagnostic group and site effects
Initial analyses indicated that CPT performance was related to age (DS-CPT d’: r=−.27, p<.001; CPT-IP 3-digit d’: r=−.23, p<.001; CPT-IP 4-digit d’: r=−.24, p<.001) and sex (DS-CPT d’: F1,2108=5.38, p<.03; CPT-IP 3-digit d’: F1,2187=24.75, p<.001; CPT-IP 4-digit d’: F1,2136=15.60, p<.001), so age was included as a covariate and sex as a factor in all ANCOVAs examining CPT variables. Site was included as another between-subjects factor to examine generalization of diagnostic effects across sites.
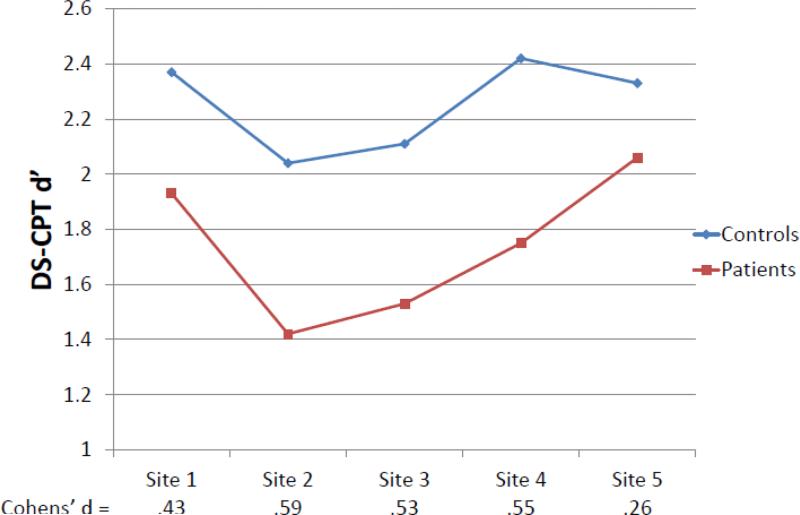
For the primary DS-CPT signal/noise discrimination measure, d’, patients clearly performed worse than HCS (M(SD) = 1.74(1.01) vs. 2.25(1.07), F1,2089=113.09, p<.001), with significant additional effects for the age covariate (F1,2089=72.16, p<.001), site (F4,2089=15.64, p<.001), diagnosis by site (F4,2089=2.44, p<.05), and diagnosis by sex (F1,2089=4.64, p<.05). Among patients, females had poorer signal/noise discrimination than males (M = 1.67 vs. 1.81), but not among HCS (M = 2.28 vs. 2.22). The diagnosis effect size without consideration of age, sex, or site was Cohen's d of 0.57. Diagnosis effect sizes with these factors entered were calculated as Cohen's f but are reported here in Cohen's d equivalents to allow comparison. The diagnosis effect size from the full ANCOVA model was d=0.47, but varied significantly across sites (see Figure 1) from Cohen's d = 0.26 to 0.59. A supplementary analysis added interactions among the demographic factors of age, sex, and racial distribution to the analytic model to determine whether these factors might account for the diagnosis by site interaction. This analysis reduced the diagnosis by site interaction to a clearly nonsignificant level (F4,2072=0.95, p=.44), while revealing significant main effects of diagnosis (F1,2072=8.08, p<.005), age (F1,2072=65.99, p<.001), and race (means for Caucasian, African American, and Other are 2.09, 1.84, and 1.95, F2,2072=10.35, p<.001) and the same diagnosis by sex interaction (F1,2072=5.06, p<.03). Thus, the variation in the diagnosis effect size across sites is apparently attributable to differences in subject age, sex, and race across sites. Analyses of secondary DS-CPT measures are generally consistent with the d’ effects (see Tables 2 and 3).
Figure 1.
Interaction of diagnostic group and site for DS-CPT d’: Age-adjusted means and effect sizes.
Table 2.
CPT performance by site for schizophrenia patients and healthy comparison subjects (HCS) (without age adjustment)
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | All Sites | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCS | Patients | HCS | Patients | HCS | Patients | HCS | Patients | HCS | Patients | HCS | Patients | |
| DS-CPT | ||||||||||||
| N | 206 | 286 | 207 | 233 | 158 | 170 | 189 | 235 | 212 | 216 | 972 | 1140 |
| d’ | 2.41 (1.02) | 1.87 (0.94) | 1.97 (1.08) | 1.37 (0.93) | 2.22 (1.04) | 1.49 (0.99) | 2.57 (1.13) | 1.79 (1.01) | 2.42 (0.97) | 2.02 (1.06) | 2.32 (1.07) | 1.72 (1.01) |
| In beta | 0.84 (0.90) | 0.74 (0.90) | 0.52 (0.76) | 0.48 (0.68) | 0.75 (0.87) | 0.69 (0.74) | 1.22 (1.03) | 0.85 (0.73) | 1.08 (0.96) | 0.77 (0.91) | 0.88 (0.94) | 0.71 (0.81) |
| Hit rate | 0.74 (0.20) | 0.65 (0.21) | 0.70 (0.21) | 0.60 (0.21) | 0.71 (0.22) | 0.58 (0.24) | 0.72 (0.21) | 0.60 (0.23) | 0.72 (0.20) | 0.67 (0.23) | 0.72 (0.21) | 0.62 (0.23) |
| False alarm rate | 0.07 (0.07) | 0.11 (0.09) | 0.13 (0.13) | 0.18 (0.14) | 0.08 (0.06) | 0.14 (0.13) | 0.06 (0.07) | 0.09 (0.07) | 0.07 (0.08) | 0.11 (0.11) | 0.08 (0.09) | 0.12 (0.11) |
| RT hits (msec) | 503 (57) | 553 (80) | 504 (78) | 553 (79) | 517 (71) | 560 (93) | 516 (59) | 567 (82) | 511 (71) | 542 (80) | 510 (68) | 555 (82) |
| RT false alarms (msec) | 493 (82) | 527 (80) | 493 (82) | 534 (79) | 506 (79) | 526 (88) | 503 (92) | 537 (88) | 491 (83) | 506 (89) | 497 (84) | 527 (85) |
| CPT-IP 3-digit | ||||||||||||
| N | 220 | 290 | 202 | 236 | 174 | 207 | 191 | 236 | 212 | 221 | 999 | 1190 |
| d’ | 3.24 (0.81) | 2.23 (0.90) | 3.21 (0.85) | 2.19 (1.00) | 3.13 (1.01) | 1.86 (1.05) | 3.24 (0.80) | 2.14 (0.94) | 3.25 (0.87) | 2.39 (0.90) | 3.22 (0.87) | 2.17 (0.97) |
| Hit rate | 0.87 (0.12) | 0.69 (0.21) | 0.86 (0.14) | 0.69 (0.23) | 0.86 (0.17) | 0.63 (0.24) | 0.89 (0.12) | 0.69 (0.21) | 0.87 (0.14) | 0.73 (0.20) | 0.87 (0.14) | 0.68 (0.22) |
| False alarm rate | 0.04 (0.04) | 0.07 (0.07) | 0.04 (0.05) | 0.08 (0.08) | 0.05 (0.05) | 0.11 (0.10) | 0.05 (0.04) | 0.09 (0.08) | 0.04 (0.04) | 0.07 (0.07) | 0.04 (0.04) | 0.08 (0.08) |
| RT hits (msec) | 490 (65) | 555 (83) | 503 (71) | 563 (96) | 496 (76) | 563 (103) | 493 (72) | 549 (96) | 477 (72) | 532 (83) | 491 (71) | 552 (93) |
| RT false alarms (msec) | 556 (144) | 640 (166) | 581 (164) | 648 (175) | 585 (149) | 610 (168) | 548 (131) | 605 (171) | 562 (134) | 631 (161) | 561 (148) | 621 (168) |
| CPT-IP 4-digit | ||||||||||||
| N | 220 | 283 | 199 | 234 | 167 | 184 | 190 | 233 | 210 | 218 | 986 | 1152 |
| d’ | 2.08 (0.85) | 1.22 (0.69) | 2.17 (0.87) | 1.14 (0.70) | 2.06 (0.86) | 1.07 (0.80) | 2.14 (0.89) | 1.24 (0.71) | 2.09 (0.87) | 1.30 (0.80) | 2.11 (0.87) | 1.19 (0.74) |
| Hit rate | 0.76 (0.16) | 0.58 (0.21) | 0.77 (0.17) | 0.55 (0.20) | 0.76 (0.18) | 0.53 (0.24) | 0.78 (0.15) | 0.60 (0.20) | 0.76 (0.16) | 0.59 (0.22) | 0.77 (0.16) | 0.57 (0.22) |
| False alarm rate | 0.13 (0.09) | 0.19 (0.12) | 0.12 (0.09) | 0.19 (0.13) | 0.14 (0.10) | 0.19 (0.13) | 0.13 (0.10) | 0.20 (0.13) | 0.13 (0.09) | 0.18 (0.12) | 0.13 (0.10) | 0.19 (0.12) |
| RT hits (msec) | 536 (73) | 597 (98) | 541 (79) | 605 (98) | 535 (85) | 586 (114) | 532 (69) | 589 (107) | 524 (76) | 573 (92) | 533 (77) | 590 (102) |
| RT false alarms (msec) | 567 (120) | 611 (113) | 565 (119) | 624 (125) | 563 (119) | 594 (134) | 558 (101) | 615 (131) | 545 (106) | 594 (124) | 560 (113) | 609 (125) |
Note: CPT = Continuous Performance Test; DS-CPT = Degraded Stimulus CPT; CPT-IP = CPT, Identical Pairs; RT = response time. Except for Ns, numbers represent M (SD).
Table 3.
Summary of effects in statistical analyses of secondary CPT variables
| Diagnosis | Age | Sex | Site | Diagnosis X Site | Diagnosis X Sex | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F and p | Direction | F and p | Direction | F and p | Direction | F | F and p | Direction | F and p | Direction | |
| DS-CPT | |||||||||||
| In beta | F1,2089=128.60 p<.001 |
Decrease with age |
F4,2089=11.72 p<.001 |
F4,2089=2.64 p<.05 |
Site 3: Sz<HCS Sites 4&5: Sz>HCS |
||||||
| Hit rate | F1,2089=81.00 p<.001 |
Sz<HCS | F1,2089=9.79 p<.005 |
Decrease with age |
F4,2089=4.90 p<.001 |
F4,2089=2.23 p<.07 |
F1,2089=5.02 p<.05 |
Sz: F<M HCS: F>M |
|||
|
False alarm rate |
F1,2089=45.82 p<.001 |
Sz>HCS | F1,2089=74.54 p<.001 |
Increase with age |
F4,2089=26.86 p<.001 |
||||||
|
RT hits (msec) |
F1,2089=161.74 p<.001 |
Sz>HCS | F1,2089=5.35 p<.05 |
F>M | F4,2089=3.17 p<.05 |
F4,2089=3.17 p<.05 |
Smaller at site 5 |
F1,2089=9.79 p<.005 |
Sz: F>M HCS: F=M |
||
|
CPT-IP: 3-digit |
|||||||||||
| Hit rate | F1,2168=406.62 p<.001 |
Sz<HCS | F1,2168=26.53 p<.001 |
Decrease with age |
F4,2168=5.51 p<.001 |
F4,2168=2.77 p<.03 |
Smaller at site 5 |
||||
|
False alarm rate |
F1,2168=145.16 p<.001 |
Sz>HCS | F1,2168=7.02 p<.01 |
Increase with age |
F4,2168=9.59 p<.001 |
F4,2168=2.21 p<.07 |
Smaller at site 5 |
||||
|
RT hits (msec) |
F1,2167=210.02 p<.001 |
Sz>HCS | F1,2168=47.34 p<.001 |
Increase with age |
F1,2168=15.27 p<.001 |
F>M | F4,2168=5.69 p<.001 |
||||
|
CPT-IP: 4-digit |
|||||||||||
| Hit rate | F1,2117=418.15 p<.001 |
Sz<HCS | F1,2117=15.01 p<.001 |
Decrease with age |
F1,2117=6.25 p<.02 |
F<M | |||||
|
False alarm rate |
F1,2117=91.59 p<.001 |
Sz>HCS | F1,2117=26.53 p<.001 |
Increase with age |
F4,2117=2.79 p<.03 |
||||||
|
RT hits (msec) |
F1,2114=142.93 p<.001 |
Sz>HCS | F1,2114=47.80 p<.001 |
Increase with age |
F1,2114=11.89 p<.001 |
F>M | |||||
Note: CPT = Continuous Performance Test; DS-CPT = Degraded Stimulus CPT; CPT-IP = CPT, Identical Pairs; RT = response time
CPT-IP data are summarized in Table 2. The CPT-IP d’ and DS-CPT d’ were moderately related (for CPT-IP 3-digit, r = .40, .25, and .38 for whole, HCS, and schizophrenia samples; for CPT-IP 4 digit, r = .37, .25, and .35 for whole, HCS, and schizophrenia samples, all p<.001). CPT-IP 3-digit and 4-digit d’ were highly correlated (r = .76, .65, and .73 for whole, HCS, and schizophrenia samples). The primary CPT-IP performance indices, 3-digit d’ and 4-digit d’, showed large patient-HCS differences across sites. For 3-digit d’, patients performed much worse than HCS (M(SD) = 2.19(0.97) vs. 3.17(0.87), F1,2168=520.49, p<.001), with significant additional effects for the age covariate (F1,2168=27.48, p<.001) and site (F4,2168=7.36, p<.001). The diagnosis by site interaction neared significance (F4,2168=2.13, p<.08), as did the diagnosis by sex interaction (F1,2168=3.08, p<.08). The diagnosis effect size without consideration of age, sex, or site was large (Cohen's d = 1.13), as was one that adjusted for age, sex, and site (Cohen's d= 0.98). For 4-digit d’, patients also performed much worse than HCS (M(SD) = 1.21(0.74) vs. 2.07(0.87), F1,2117=509.04, p<.001), with a significant age covariate effect (F1,2117=40.03, p<.001). Site and sex did not yield main effects or interactions with diagnosis (all p>.10). The diagnosis effect size for 4-digit d’ without consideration of age, sex, or site was again large (Cohen's d=1.14), as was one adjusting for age, sex, and site (Cohen's d=0.98). Analyses of secondary CPT-IP variables also generally showed robust patient-HCS differences (Tables 2 and 3).
Correlates of CPT performance within the schizophrenia sample
As shown in Table 4, discrimination (d’) of blurred digits in the DS-CPT was not correlated with visual acuity level, indicating that it did not reflect this more basic visual process. CPT-IP d’ was significantly related to visual acuity, but only weakly. DS-CPT and CPT-IP performance was also generally either unrelated to current positive (SAPS Global Sum) or negative (SANS Global Sum) symptom level or only very weakly related (r<.10). Separation into three symptom dimensions (Andreasen et al., 1995) yields similar correlations, with a weak correlation with Disorganization (r=.10, p<.01) being the strongest symptom relationship. As expected, poorer DS-CPT and CPT-IP performance was significantly related to another indicator of poor cognitive functioning (MMSE Score), low intellectual achievement (years of education), and limited functional capacity (UPSA-B Total). The expected relationship of DS-CPT performance to rated real-world functioning (RFS) was generally absent, while several significant but relatively weak relationships to real-world functioning were present for the CPT-IP (r=.08 to .17).
Table 4.
Associations of CPT performance with clinical, cognitive, and functional variables within schizophrenia sample
| Schizophrenia Patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Degraded Stimulus CPT | CPT Identical Pairs Version | |||||||
| N | d' | Hit Rate | False Alarm Rate | N | 3-digit d' | N | 4-digit d' | |
| Visual Acuity | 1118 | .04 | .00 | −.09** | 1166 | .17*** | 1128 | .17*** |
| SANS Global Sum | 1134 | .04 | .01 | −.06* | 1184 | −.04 | 1147 | −.09** |
| SAPS Global Sum | 1133 | −.03 | −.03 | .00 | 1183 | −.08** | 1145 | −.05 |
| Negative Symptoms | 1134 | .06* | .03 | −.07* | 1184 | .01 | 1147 | −.05 |
| Psychotic Symptoms | 1133 | −.01 | −.02 | −.03 | 1183 | −.04 | 1145 | −.02 |
| Disorganization | 1133 | −.05 | −.04 | .05 | 1183 | −.10** | 1145 | −.07* |
| MMSE Score | 1103 | .19*** | .15*** | .15*** | 1153 | .29*** | 1116 | .28*** |
| Yrs Education | 1139 | .11*** | .11*** | −.02 | 1190 | .19*** | 1152 | .20*** |
| UPSA-B | 1111 | .21*** | .21*** | .10*** | 1161 | .31*** | 1125 | .29*** |
| RFS | ||||||||
| Work | 792 | .05 | .00 | −.08* | 829 | .08* | 796 | .17*** |
| Independent | 792 | .05 | .04 | −.06 | 829 | .15*** | 796 | .16*** |
| Family | 792 | −.02 | .00 | −.01 | 829 | .09* | 796 | .09* |
| Social | 789 | −.05 | .01 | .08* | 826 | .00 | 793 | .04 |
p < .05
p < .01
p < .001
Note: CPT = Continuous Performance Test; SANS = Schedule for Assessment of Negative Symptoms; SAPS = Schedule for Assessment of Positive Symptoms; Negative Symptoms, Psychotic Symptoms, and Disorganization are SANS/SAPS dimensions from Andreasen et al. (1995); MMSE = Mini-Mental State Examination; UPSA-B = UCSD Performance-based Skills Assessment; RFS = Role Functioning Scale.
Examination of the relationship of CPT performance to psychiatric and substance history variables indicated that more prior psychiatric hospitalizations was not related to DS-CPT d’ (r = −.003, NS) and was only very weakly related to lower CPT-IP d’ (r=−.05, p<.10 for 3-digit; r=−.08, p<.01 for 4-digit). Presence of past or current mood disorder was not related to d’ for either CPT. Longer duration of illness was significantly associated with poorer DS-CPT d’ (r=−.19, p<.001) and CPT-IP d’ (r=−.14, p<.001 for 3-digit; r=−.15, p<.001 for 4-digit), but this relationship decreased when age was controlled (DS-CPT: r=−.07, p<.05; CPT-IP 3-digit, r=−.06, p<.05; CPT-IP 4-digit, r=−.04, p=.16), suggesting only a weak relationship of CPT performance to illness duration per se. A past history of substance use disorder was associated with somewhat better DS-CPT d’ (M = 1.77 vs. 1.63, F1,1134=4.70, p<.05), even with age covaried and sex entered as another factor. However, the association with past substance use disorder did not generalize to the CPT-IP.
Smoking status (never, past, current) within the schizophrenia sample did not impact DS-CPT d’ (F2,1136=0.35, NS), but current smoking did slow response time to targets (M = 548, 530, and 563 msec for never, past, and current, respectively, F2,1136=7.14, p<.001). Smoking status did have a significant effect on CPT-IP d’ (3-digit: F2,1187=4.82, p<.01; 4-digit: F2,1149=5.67, p<.01), with current smokers having significantly lower d’ than those who never smoked for 3-digit CPT-IP (M=2.10 vs. 2.27) and both current and past smokers having significantly lower d’ than those who never smoked for 4-digit CPT-IP (M=1.14, 1.06, and 1.28, respectively). Slowing of target response time was also present in the CPT-IP for current smokers relative to those who never smoked (3-digit: F2,1186=8.48, p<.001, M=563 vs. 540 msec; 4-digit: F2,1146=7.32, p<.001, M=601 vs. 578 msec). Thus, current smoking status was associated with both less accurate and slower CPT-IP performance and slower but not less accurate DS-CPT performance. Supplementary analyses including an age covariate and sex and site as factors did not change the pattern of significant associations with smoking status.
Antipsychotic medication status at testing was categorized as none, atypical, typical, or both atypical and typical. With age covaried, CPT-IP d’ was significantly associated with antipsychotic medication status (3-digit: F3,1185=4.74, p<.02; CPT-IP 4-digit: F3,1147=7.58, p<.02) and DS-CPT d’ showed a similar but nonsignificant tendency (F3,1106=1.97, p<.12). Patients on both typical and atypical antpsychotics had significantly poorer CPT-IP d’ than those on no antipsychotic or an atypical antipsychotic. Covarying number of psychiatric hospitalizations and current positive and negative symptom severity did not alter this pattern.
DISCUSSION
This article examined CPT performance in the large COGS-2 samples of schizophrenia patients and healthy subjects across five sites that varied significantly in participant age, sex, education level, parental education level, and race and in patient symptom severity, functional capacity, and real-life functioning. Despite these many significant differences in participant characteristics across sites, CPT performance was significantly impaired across sites in schizophrenia patients compared to HCS. The simple diagnosis effect size was medium for the DS-CPT d’ (Cohen's d=0.57) and large for the CPT-IP d’ (1.13 for 3-digit; 1.14 for 4-digit). With age, sex, and site in the statistical model, the primary DS-CPT d’ score did reveal a larger patient-HCS difference at some sites than others (varying from Cohen's d equivalent of 0.26 to 0.59), but the overall effect size remained at least of medium magnitude (d=.47). Thus, even with the variations in participant characteristics across sites and the challenges of setting up parallel testing conditions across sites, we were able to achieve reasonable diagnostic group effects. Our overall impression is that between-site differences were attributable to varying participant demographic characteristics rather than methodological variance.
In a meta-analysis of cognitive deficits in schizophrenia, Heinrichs and Zakzanis found that the mean CPT effect size between schizophrenia patients and healthy subjects across 15 studies was Cohen's d = 1.18, which is quite large (Cohen, 1988; Heinrichs and Zakzanis, 1998). Our CPT-IP effect sizes are similar, while the DS-CPT effect size is somewhat smaller. The CPT-IP demand for working memory in addition to efficient processing of each brief stimulus may enhance its effect size, as working memory is known to be substantially impaired in schizophrenia (Barch et al., 2002; Kern et al., 2011). The view that the CPT-IP and DS-CPT may partially index separable cognitive functions is also supported by their modest intercorrelations in COGS-2 (r = .25 to .40) and by their loading on separate factors in COGS-1 (Seidman et al., 2015).
Examination of the correlates of CPT performance within the large schizophrenia sample demonstrated that DS-CPT d’ and CPT-IP d’ are either unrelated or only very weakly related to positive, negative, or disorganzation symptom severity, number of prior hospitalizations, and past mood disorder. This relative insulation of deficits from state-related conditions fulfills one endophenotype criterion (Gottesman and Gould, 2003). Poorer DS-CPT and CPT-IP performance was associated with lower MMSE scores, educational achievement, and functional capacity. These results, combined with past evidence that DS-CPT and CPT-IP performance is deficient in groups at genetic risk for schizophrenia and relatively stable across clinical state in schizophrenia (Asarnow et al., 2002; Chen et al., 2004; Chen et al., 1998; Grove et al., 1991; Maier et al., 1992; Nuechterlein et al., 1998; Nuechterlein et al., 1992; Saoud et al., 2000; Snitz et al., 2006), suggests that the deficit in sustained focused attention is a persistent feature that has ongoing implications for educational achievement and functional capacity.
The better DS-CPT performance of patients with a past history of substance abuse, while initially surprising, is actually consistent with literature suggesting that schizophrenia patients who abuse substances have somewhat less severe cognitive deficits than those who do not (Donoghue and Doody, 2012; McCleery et al., 2006; Rabin et al., 2011; Thoma and Daum, 2013). As suggested by McCleery, higher cognitive functioning among schizophrenia patients who previously used substances might reflect higher pre-illness cognitive functioning, which might facilitate social interactions needed for drug-seeking behaviors.
The association of current tobacco smoking with lower signal/noise discrimination (d’) for the CPT-IP and slowing of responses to targets on both CPTs is consistent with studies showing negative effects of chronic smoking on cognition in non-psychiatric samples (Chamberlain et al., 2012; Sabia et al., 2008) and with the findings for working memory in the current COGS-2 schizophrenia sample (Lee et al., this issue).
The association of poorer CPT-IP performance with being on both typical and atypical antipsychotic medication appears likely to be due to greater severity of illness rather than antipsychotic medication impact on CPT performance, given that clinical trials examining effects within-subjects find either no or positive impact of antipsychotics on the CPT (Liu et al., 2000; Nestor et al., 1991; Orzack et al., 1967).
Overall, these results support and strengthen the use of CPT endophenotypes in clinical trials, studies of function, and forthcoming COGS-2 genomic studies. The relationship of CPT performance to functional capacity but not positive symptoms may be very useful in these continuing studies.
Acknowledgements
This study was supported by grants R01-MH065571, R01-MH065588, R01- MH065562, R01-MH065707, R01-MH065554, R01-MH065578, R01-MH065558, R01 MH86135, and K01-MH087889 from the National Institute of Mental Health.
Role of funding sources
Beyond providing funding support, the National Institute of Health does not have any further role in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Nuechterlein completed the CPT quality assurance and all CPT statistical analyses and wrote the initial version of this manuscript. Dr. Green served as PI of the UCLA site and coordinated CPT processing with Dr. Nuechterlein. Joyce Sprock completed quality assurance for all clinical and psychiatric history data entered on the COGS-2 website. All other authors participated in study design and execution, including participant recruitment, diagnosis, endophenotyping, and ongoing quality assurance for the clinical and endophenotype data. All authors were responsible for reviewing and approving the final version of the manuscript.
Conflict of interest
Dr. Nuechterlein has received unrelated research support from Janssen Scientific Affairs, Genentech, and Brain Plasticity, Inc., and has consulted to Genentech, Otsuka, Janssen, and Brain Plasticity, Inc. Dr. Green has been a consultant to AbbVie, Biogen, DSP, EnVivo/Forum and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen. Dr. Lazzeroni is an inventor on a patent application filed by Stanford University on genetic polymorphisms associated with depression. Dr. Light has served as a consultant for Astellas, Forum, and Neuroverse. Dr. Swerdlow has been a consultant for Genco Sciences, Ltd. All other authors declare that they have no conflict of interest.
REFERENCES
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn. Neuropsychiatry. 2013;18(1-2):44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA.: 1984a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) The University of Iowa; Iowa City, IA.: 1984b. [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M. Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch. Gen. Psychiatry. 1995;52(5):341–351. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Appels MC, Sitskoorn MM, Westers P, Lems E, Kahn RS. Cognitive dysfunctions in parents of schizophrenic patients parallel the deficits found in patients. Schizophr. Res. 2003;63(3):285–293. doi: 10.1016/s0920-9964(02)00342-0. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, MacCrimmon DJ. Residual performance deficit in clinically remitted schizophrenics: a marker of schizophrenia? J. Abnorm. Psychol. 1978;87(6):597–608. doi: 10.1037//0021-843x.87.6.597. [DOI] [PubMed] [Google Scholar]
- Asarnow RF, Nuechterlein KH, Subotnik KL, Fogelson DL, Torquato R, Payne D. Neurocognitive impairments in non-psychotic parents of children with schizophrenia and attention deficit hyperactivity disorder: The UCLA Family Study. Arch. Gen. Psychiatry. 2002;59(11):1053–1060. doi: 10.1001/archpsyc.59.11.1053. [DOI] [PubMed] [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: Is there a common prefrontal mechanism? J. Abnorm. Psychol. 2002;111(3):478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Braff D. Information processing and attention dysfunctions in schizophrenia. Schizophr. Bull. 1993;19(2):233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am. J. Addict. 2012;21(Suppl 1):S14–19. doi: 10.1111/j.1521-0391.2012.00290.x. [DOI] [PubMed] [Google Scholar]
- Chen WJ. Taiwan Schizophrenia Linkage Study: lessons learned from endophenotype-based genome-wide linkage scans and perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013;162B(7):636–647. doi: 10.1002/ajmg.b.32166. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Chang CH, Liu SK, Hwang TJ, Hwu HG. Sustained attention deficits in nonpsychotic relatives of schizophrenic patients: a recurrence risk ratio analysis. Biol. Psychiatry. 2004;55(10):995–1000. doi: 10.1016/j.biopsych.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am. J. Psychiatry. 1998;155(9):1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. second ed. Erlbaum; Hillsdale, NJ.: 1988. [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr. Bull. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29(1):65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am. J. Med. Genet. 2001;105(1):11–15. [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs Version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatric Res. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Doody GA. Effect of illegal substance use on cognitive function in individuals with a psychotic disorder: a review and meta-analysis. Neuropsychology. 2012;26(6):785–801. doi: 10.1037/a0029685. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am. J. Psychiatry. 2000;157(8):1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- Finkelstein JRJ, Cannon TD, Gur RE, Gur RC, Moberg PJ. Attentional dysfunctions in neuroleptic-naive and neuroleptic-withdrawn schizophrenic patients and their siblings. J. Abnorm. Psychol. 1997;106(2):203–212. doi: 10.1037//0021-843x.106.2.203. [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hardt J, Hain C, Cornblatt BA. Attentional abilities and measures of schizotypy: their variation and covariation in schizophrenic patients, their siblings, and normal control subjects. Psychiatry Res. 1994;54(3):259–272. doi: 10.1016/0165-1781(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Sewell DR, Cooley EL. Assessing levels of adaptive functioning: The role functioning scale. Community Ment. Health J. 1993;29(2):119–131. doi: 10.1007/BF00756338. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch. Gen. Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Ray A, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Light GA, Braff DL. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2013;170(5):521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove WM, Lebow BS, Clementz BA, Cerri A, Medus C, Iacono WG. Familial prevalence and coaggregation of schizotypy indicators: a multitrait family study. J. Abnorm. Psychol. 1991;100(2):115–121. doi: 10.1037//0021-843x.100.2.115. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The consortium on the genetics of schizophrenia: neurocognitive endophenotypes. Schizophr. Bull. 2007;33(1):49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficits in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Jones LA, Cardno AG, Sanders RD, Owen MJ, Williams J. Sustained and selective attention as measures of genetic liability to schizophrenia. Schizophr. Res. 2001;48(2-3):263–272. doi: 10.1016/s0920-9964(00)00136-5. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb. Exp. Pharmacol. 2012;213:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr. Res. 2011;126(1-3):124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. E. & S. Livingston; Edinburgh: 1919. [Google Scholar]
- Laurent A, Saoud M, Bougerol T, d'Amato T, Anchisi AM, Biloa-Tang M, Dalery J, Rochet T. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89(3):147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Light GA, Nuechterlein KH, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Verbal working memory in schizophrenia: The moderating role of smoking status and antipsychotic medications. Schizophr. Res. 2015 doi: 10.1016/j.schres.2014.08.014. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SK, Chen WJ, Chang CJ, Lin HN. Effects of atypical neuroleptics on sustained attention deficits in schizophrenia: a trial of risperidone versus haloperidol. Neuropsychopharmacology. 2000;22(3):311–319. doi: 10.1016/S0893-133X(99)00137-2. [DOI] [PubMed] [Google Scholar]
- Maier W, Franke P, Hain C, Kopp B, Rist F. Neuropsychological indicators of the vulnerability to schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1992;16(5):703–715. doi: 10.1016/0278-5846(92)90026-b. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr. Bull. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A, Addington J, Addington D. Substance misuse and cognitive functioning in early psychosis: a 2 year follow-up. Schizophr. Res. 2006;88(1-3):187–191. doi: 10.1016/j.schres.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Lochhead SJ, Jones BP, Kugelmass S, Walsh D, Kendler KS. On familial factors in the attentional deficit in schizophrenia: a review and report of two new subject samples. J. Psychiatr. Res. 1992;26(4):383–403. doi: 10.1016/0022-3956(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Sands SF, Horvath TB, Peterson A. Neuroleptics improve sustained attention in schizophrenia. A study using signal detection theory. Neuropsychopharmacology. 1991;4(2):145–149. [PubMed] [Google Scholar]
- Nuechterlein KH. Reaction time and attention in schizophrenia: A critical evaluation of the data and theories. Schizophr. Bull. 1977;3(3):373–428. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. J. Abnorm. Psychol. 1983;92(1):4–28. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of Schizophrenia, Neuropsychology, Psychophysiology and Information Processing. Elsevier Science Publishers; Amsterdam: 1991. pp. 397–433. [Google Scholar]
- Nuechterlein KH, Asarnow RF. Degraded Stimulus Continuous Performance Test (DS-CPT) Program for PCs, Windows Version 1.0 (8/4/06 executable file) Authors; Los Angeles: 2006. [Google Scholar]
- Nuechterlein KH, Asarnow RF, Subotnik KL, Fogelson DL, Ventura J, Torquato RD, Dawson ME. Neurocognitive vulnerability factors for schizophrenia: Convergence across genetic risk studies and longitudinal trait/state studies. In: Lenzenweger MF, Dworkin RH, editors. Origins and Development of Schizophrenia: Advances in Experimental Psychopathology. American Psychological Association; Washington, D.C: 1998. pp. 299–327. [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr. Res. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr. Bull. 1984;10(2):160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental processes in schizophrenic disorders: Longitudinal studies of vulnerability and stress. Schizophr. Bull. 1992;18(3):387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Edell WS, Norris M, Dawson ME. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr. Bull. 1986;12(3):408–426. doi: 10.1093/schbul/12.3.408. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: Image degradation produces rapid sensitivity decrement over time. Science. 1983;220(4594):327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- Orzack MH, Kornetsky C, Freeman H. The effects of daily administration of carphenazine on attention in the schizophrenic patient. Psychopharmacologia. 1967;11(1):31–38. doi: 10.1007/BF00401506. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr. Res. 2011;128(1-3):111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Bazzetta J, McAdams LA, Patterson T, Jeste DV. Validation of the scale of functioning in older outpatients with schizophrenia. Am. J. Geriat. Psychiat. 1996;4(3):218–228. doi: 10.1097/00019442-199622430-00005. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. J. Consult. Psychol. 1956;20(5):343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch. Intern. Med. 2008;168(11):1165–1173. doi: 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoud M, d'Amato T, Gutknecht C, Triboulet P, Bertaud JP, Marie-Cardine M, Dalery J, Rochet T. Neuropsychological deficit in siblings discordant for schizophrenia. Schizophr. Bull. 2000;26(4):893–902. doi: 10.1093/oxfordjournals.schbul.a033503. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O'Craven K, Savoy R, Tsuang MT, Rosen BR. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12(4):505–518. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Hellemann G, Nuechterlein KH, Greenwood TA, Braff DL, Cadenhead KS, Calkins ME, Freedman R, Gur RE, Gur RC, Lazzeroni LC, Light GA, Olincy A, Radant AD, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar C, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Factor structure and heritability of endophenotypes in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenia (COGS-1). Schizophr. Res. 2015 doi: 10.1016/j.schres.2015.01.027. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr. Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Gur RE, Braff DL. Consortium on the Genetics of Schizophrenia (COGS) Assessment of Endophenotypes for Schizophrenia: An Introduction to this Special Issue of Schizophrenia Research. Schizophr. Res. 2015 doi: 10.1016/j.schres.2014.09.047. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Daum I. Comorbid substance use disorder in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin. Neurosci. 2013;67(6):367–383. doi: 10.1111/pcn.12072. [DOI] [PubMed] [Google Scholar]
- Wohlberg GW, Kornetsky C. Sustained attention in remitted schizophrenics. Arch. Gen. Psychiatry. 1973;28(4):533–537. doi: 10.1001/archpsyc.1973.01750340065011. [DOI] [PubMed] [Google Scholar]