Abstract
Increasing evidence supports that early life environmental influences, including nutrition and stress, impact long-term health outcomes and disease susceptibility. The objective of the current study was to determine whether dietary spray-dried plasma (SDP) fed during the first 2 weeks post-weaning (PW) influences subsequent immunological and intestinal injury responses to S. Typhimurium challenge. Thirty two piglets (16–17 d of age) were weaned onto nursery diets containing 0% SDP, 2.5% SDP (fed for 7 d PW), or 5% SDP (for 14 d PW) and were then fed control diets (without SDP), for the remainder of the experiment. At 34 d PW (50 d of age), pigs were challenged with 3×109 cfu S. Typhimurium. A control group (non-challenged) that was fed 0% SDP in the nursery was included. At 2 d post-challenge, distal ileum was harvested for measurement of inflammatory, histological, and intestinal physiological parameters. S. Typhimurium challenge induced elevated ileal histological scores, myeloperoxidase (MPO), IL-8, and TNF, and increased intestinal permeability (indicated by reduced transepithelial voltage (PD) and elevated FD4 flux rates). Compared with S. Typhimurium-challenged controls (0% SDP), pigs fed 5% SDP-14 d exhibited reduced ileal histological scores, MPO, IL-8, and FD4 flux rates. Pigs fed 5% SDP-14 d in the nursery exhibited increased levels of plasma and ileal TNFα in response to challenge, compared with other treatments. These results indicate that inclusion of SDP into PW diets can have influence subsequent immunological responses and intestinal injury induced by later life S. Typhimurium challenge.
Keywords: spray dried plasma, early life nutrition, Salmonella Typhimurium, intestinal inflammation, mucosal immunity, intestinal permeability, weaning
Introduction
The gastrointestinal (GI) tract continues to undergo significant developmental changes in post-natal life. Environmental influences during this critical developmental time period, including diet, stress, and mucosal injury, has been shown to induce long-term changes in intestinal physiology and disease susceptibility in animal models(1–4). Similarly in humans, increasing epidemiological evidence supports the concept that adverse early life environmental factors, such as stress, are associated with subsequent GI diseases such as Irritable Bowel Syndrome (IBS)(5–9). In pigs, early weaning (< 21 d of age) in piglets is a significant, early life stress that has been shown to have deleterious impacts on GI function including increased intestinal permeability(10, 11), inflammation(12), hypersecretion(10), reductions in the activity of brush border digestive enzymes(13), altered nutrient transport mechanisms(14, 15), and marked changes in villus and crypt morphology (reduced villus surface area and increased crypt depth)(16) The mechanisms and factors associated with weaning stress (e.g. maternal and littermate separation, dietary changes, transport stress) are not completely understood, however, it was demonstrated by Moeser et al (2007) that activation of corticotropin releasing factor (CRF) receptor system in the intestine and subsequent activation of mast cells was responsible for increased intestinal permeability and hypersecretion(10) demonstrating the role of stress signaling pathways in the weaned pig intestine. It is now evident that the deleterious effects of early weaning stress on the pig intestinal tract are seen well beyond the immediate PW period. Smith et al., (2011) demonstrated that early weaned pigs (weaned between 15–21 d of age) exhibited greater intestinal permeability at 9 weeks PW, compared with late weaned pigs (weaned between 23–28 d of age)(17). In addition, McLamb et al (2013) showed that early weaned pigs exhibited heightened clinical disease (increased severity of diarrhea and reduced growth rate) and intestinal injury (increased intestinal permeability) in response to a Enterotoxigenic E. coli (ETEC) challenge at approximately 3 weeks PW(1). Overall, results from the aforementioned experiments provide strong evidence that PW intestinal injury can have lasting deleterious impacts on intestinal function. Therefore, therapeutic approaches to ameliorate GI injury during the PW period could positively impact long-term barrier function and defense against subsequent pathogenic challenges.
Dietary inclusion of spray-dried plasma (SDP) proteins into nursery pig diets has proven to have a beneficial effect on PW gastrointestinal health and performance in young pigs.(18, 19) Previous studies demonstrate that SDP not only promotes growth responses in young pigs but also confers protective effects in GI infectious challenges models. Van Dijk et al., (2002) demonstrated that weaned pigs challenged with K88 ETEC and fed a nursery diet containing 8% SDP exhibited reduced diarrhea and increased ADG and ADFI compared with pigs fed control diets containing whey protein(20). In another experiment with weaned pigs, pigs fed diets containing 6% SDP exhibited reduced cytokine responses and intestinal inflammatory cell infiltrates following a challenge with ETEC(21). Similarly, reduced diarrheal disease caused by an experimental rotavirus challenge, was observed in neonatal piglets provided a diet containing 15% SDP, compared with control diets containing soy protein isolate(22). Peace et al (2011) demonstrated that inclusion of SDP at 2.5% and 5% of the diet for two weeks PW, reduced intestinal permeability, intestinal inflammatory cytokines, and diarrhea in early weaned pigs.(18) However, in previous experiments described above, growth responses and intestinal protective effects of SDP described above were measured while SDP was in the diet. Whether inclusion of SDP in early life pig diets retains beneficial effects after its removal from the diet has not been investigated. Given that early weaning stress induces short and long-term deleterious changes in intestinal function and disease susceptibility and that SDP has proven beneficial in reducing early changes in intestinal permeability and inflammatory responses in weaned pigs, we hypothesized that inclusion of SDP in PW pig diets would have sustained, beneficial effects on intestinal responses to a later life pathogenic challenge, after SDP has been removed from the diet. The specific objective of this study was to determine whether inclusion of SDP during the first 2 weeks PW, influenced intestinal epithelial barrier function, immune responses, and clinical disease in response to a later life challenge with S. Typhimurium.
Material and methods
The North Carolina State University Institutional Animal Care and Use Committee approved all studies conducted in these experiments (Protocol# 12-051-A).
Pigs and experimental design
Thirty-two Yorkshire-Large White piglets, weaned between 16–17 d of age and of similar weight (5.49 kg ±0.1 SEM) were used in this experiment. Weaned piglets were housed in four nursery pens (8 pigs/pen; 1.09 m2/pig) and were offered ad libitum access to water and one of three experimental nursery diets containing either 0% SDP (fed to 2 pens, n=16 pigs), 2.5% SDP (fed for 7 d PW; n=8 pigs) or 5% SDP (fed for 14 d PW; n=8 pigs) (Figure 1). Sex and litter origin were distributed equally across experimental groups. The variable dietary levels of 2.5 and 5% SDP along with feeding duration post-weaning (7 d vs. 14 d PW) were selected to mimic the range of dietary level and feeding duration of SDP commonly utilized in commercial swine feeding. Diets were supplied in mash form and were formulated to contain identical levels of metabolizable energy and digestible lysine to meet nutrients requirements of the NRC (1998).(11) At 7 d PW, pigs fed the 2.5% SDP treatment were switched to control (0% SDP) diets. At 14 d PW, all pigs were fed the same diet (0% SDP) and maintained in the nursery for an additional 21 days.
FIGURE 1.
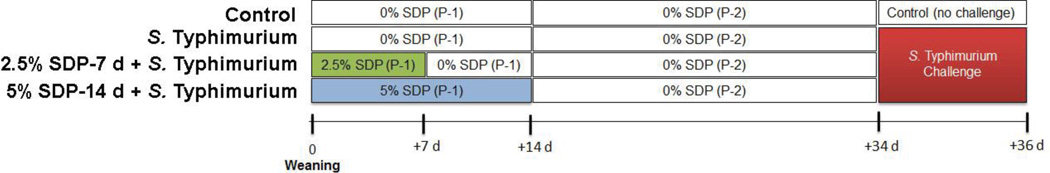
Experimental design. Piglets (n=8/treatment) were weaned from their sow and offered nursery diets containing either 0% SDP, 2.5% Spray dried plasma (SDP) for 1 week post-weaning (PW), or 5% SDP for 2 weeks PW. SDP was removed from experimental diets at indicated times and fed identical diets to controls. At 34 d PW, pigs were challenged with S. Typhimurium. At 2 d post challenge, tissues were harvested for analysis. P-1 = Phase 1 diet; P-2 = Phase 2 diet.
Salmonella Typhimurium challenge
At 34 days PW, all pigs were transferred from the nursery to isolation rooms located in a nearby research facility on the North Carolina State University at the College of Veterinary Medicine campus. Upon arrival, pigs were housed, by treatment, with 8 pigs/pen (0.3 m2/pig). The pens were equipped with tenderfoot flooring and pigs were allowed ad libitum access to feed and water. On the following day, n=8 pigs from each experimental group were inoculated orally with 3 × 109 cfu S. Typhimurium in 4 mL of culture media as described previously.(23) A non-challenged control group was housed in a separate, identical room within the facility and were administered similarly with 4 mL sterile media. The S. Typhimurium DT104 strain used in this study exhibited antimicrobial resistance to ampicillin, chloramphenicol, sulfisoxazole, streptomycin and tetracycline. Salmonella cultures were grown overnight at 37°C on Luria Broth Agar and then added to a sterile 0.7% saline solution to obtain a final concentration of 7.5 × 108 cfu/ml, verified using a NanoDrop 2000c Nanospectrometer (Thermo Fisher Scientific, Waltham, MA). In our study, we chose S. Typhimurium as the challenging agent because it has dual relevance to human and swine diseases(24).
Growth rate and feed intake calculations and fecal scores
Body weight (BW) was recorded at d 0 and d 14 during the PW nursery phases and on d 0 and d 2 of the S. Typhimurium challenge study and ADG was calculated. Given the short (2 d) challenge period, growth data was presented as % BW loss. Pen feed intakes were recorded during the PW and S. Typhimurium challenge periods and estimated feed intake/pig was calculated for each pen. Fecal scores were performed by individuals who were blinded to experimental treatments according to a previously published scoring system by our group (1) using a scale from 1 (no diarrhea) to 4 (severe profuse diarrhea).
Ussing chamber studies
On d 2 post-challenge, pigs were sedated with a TKX cocktail containing Telazol (500 mg), Ketamine (250 mg), and Xylazine (250 mg) administered i.m. at a dose of 0.025mL/kg body weight. Euthanasia was followed by administration of an overdose (86 mg/kg body weight) of sodium pentobarbital solution (Fatal Plus, Virbac Animal Health, Fort Worth, TX) via a catheterized ear vein. Distal small intestine (ileum) was harvested from each pig immediately after euthanasia and opened along the anti-mesenteric border. The intestinal mucosa was stripped from the seromuscular layer in oxygenated (95% O2- 5% CO2) Ringer solution (in mmol/l: 154 Na+, 6.3 K+, 137 Cl−, 0.3 H2PO4, 1.2 Ca2+, 0.7 Mg2+, 24 HCO3−; pH 7.4) and mounted in 1.13 cm2 aperture Ussing chambers (World Precision Instruments, Inc. Sarasota, FL). Ileal mucosa was bathed on the serosal and mucosal sides with 10 ml Ringer solution. The serosal bathing solution contained 10 mM glucose, which was osmotically balanced on the mucosal side with 10 mM mannitol. Bathing solutions were oxygenated (95% O2- 5% CO2) and circulated in water-jacketed reservoirs maintained at 37°C. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Tissues were maintained in the short-circuited state, except for brief intervals to record the open-circuit PD. Transepithelial electrical resistance (TER, measured as Ω·cm2) was calculated from the spontaneous PD and short-circuit current (Isc), as previously described(25). After a 30-min equilibration period on Ussing chambers, TER and Isc was recorded at 15-minute intervals over a 1-hour period and then averaged to derive the basal TER and Isc values for a given animal.
Paracellular Permeability to 4 kDa FITC Dextran (FD4)
After a 30-min equilibration period on Ussing chambers, FD4 (Sigma, 100mg/mL) was added to the mucosal bathing reservoir of Ussing chambers. 15-minute after the addition of FD4, standards were taken from the serosal side of each chamber and a 60 minute flux period was established by taking 0.5 ml samples in triplicate from the mucosal compartment. The quantity of FD4 was established by measuring the fluorescence in mucosal reservoir fluid samples in a fluorescence plate reader at 540 nm. Data was presented as the rate of FD4 flux in mg FD4 flux.min.cm2
Histologic Analyses of Intestinal Tissues
Ileum was fixed in 10% neutral buffered formalin and processed for paraffin-embedding. Paraffin blocks were sectioned (5µm thick) and stained with hematoxylin and eosin for histological analysis. A histological scoring system was applied to the tissue sections and was performed by a board-certified veterinary pathologist (L.B.B) who was blinded to experimental treatments. The intestinal scoring system used was based on villus morphology and blunting (villi height and crypt depth), villi fusion, reduced lymphoid recruitment, and neutrophil numbers. The detailed scoring criteria were designated as follows, villus blunting: 0 = crypt to tip ratio of at least 1:4; 1 = crypt to tip ratio of 1:3; 2 = 1:2; 3 = 1:1 and; 4 complete tip loss; lymphoid depletion and villus fusion: 0 = normal, 1 = mild, 2 = moderate, and 3 = severe; neutrophils: 0 = none to 10 neutrophils/40× field; 1 = 11–20 neutrophils/40× field; 2 = 21–30 neutrophils/40× field; and 3 = 31–40 neutrophils/ 40× field. Neutrophils were identified based on nuclear and cytoplasmic morphology.(26) Measurements for crypt depth and villous height were taken utilizing the calibrated measurement caliper option and the villus measurements were taken from three well-oriented villi in five different fields/slide, such that 15 villi per slide/pig were measured. Villi chosen for measurement were based on the criteria that 1) the entire crypt and villi were captured in cross section and 2) the central lacteal was present. Villi overlying gut associated lymphoid tissue was excluded from measurement. Photomicrographs were acquired with 20× and 40× magnifications at a resolution using imaging software (Infinity Analyze Software, Lumenera, Ottawa, Ontario, Canada) running a high resolution digital camera (Lumenera) equipped to a clinical light microscope (Meiji Microscope Solutions, Model OMFL400, San Jose, CA).
Ileal cytokine analysis
Ileal mucosa was homogenized in PBS containing protease inhibitors and the supernatant was collected and analyzed for protein content using a BCA assay(18). Samples were then diluted 1:10 in PBS and assayed for TNF, IL-8, and IL-6 using commercial porcine ELISA assays (R&D Systems, Minneapolis, MN). Concentrations of each cytokine were expressed on a per mg protein basis.
Myeloperoxidase (MPO) assay
The distal ileum was obtained from each pig, opened lengthwise, and rinsed in cold Ringer's solution. The epithelium and lamina propria were scraped from the seromuscular layers over ice using a glass slide and then frozen in liquid nitrogen and stored at −80°C. The ileal mucosal scrapings were thawed and homogenized in 0.5% hexadecyltrimethylammonium bromide buffer (50 mM phosphate buffer, pH 6) to release myeloperoxidase (MPO) from the primary granules of neutrophils. The homogenate was subjected to three cycles of freezing at −80°C, thawed, and sonicated on ice. Samples were centrifuged at 21,000 × g at 4°C for 15 min and the supernatant assayed for MPO activity. An aliquot of the supernatant was allowed to react with a solution of tetramethylbenzidine in N-dimethylformamide and H2O2. Absorbance (655 nm) readings were taken at 30 second intervals over 15 minutes. MPO activity was determined based on a MPO standard curve and was expressed in units per gram (wet weight) mucosa (for ileum) or per mL of plasma(27).
Western blot analysis of CRF receptors in porcine ileum
Ileal mucosal protein was extracted from mucosal scrapes using Mammalian Protein Extraction Reagent containing protease and phosphatase inhibitors (Fisher Scientific, Waltham, MA, USA). Samples were sonicated and centrifuged at 14000 rpm for 15 min at 4°C. The protein concentration was determined using Pierce BCA protein assay kit (Fisher Scientific). Total protein was resolved by SDSPAGE and transferred to polyvinylidene difluoride membrane. The membranes were blocked with 5% w/v nonfat milk in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) for 1 h at room temperature, washed in TBS-T and incubated with CRF-RI/II antibody that detects both receptors (Santa Cruz Biotechnology, Dallas, TX, USA). Subsequently, the membranes were washed and incubated with an appropriate secondary antibody for 1 hour at room temperature followed by washing with TBS-T and incubation in SuperSignal West Pico Chemiluminescent Substrate (Fisher Scientific). As an internal loading control, the antibody was stripped from the membrane with RestoreTM Western blot stripping buffer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and the membrane was re-probed with a β - actin antibody (Cell Signaling Technology, Danvers, MA). Bands were visualized with ChemiDoc MP Imaging System (Biorad, Hercules, CA, USA), densitometric analysis was performed using the Biorad Image Lab software (version 4.1) and the CRF receptor band intensities were normalized to β - actin.
Statistics
Data were reported as means±SE based on the experimental number (n). With the exception of histological and fecal score data, all data were analyzed using a standard 1-way ANOVA (Sigmastat, Jandel Scientific, San Rafael, CA). A post-hoc Tukey's test was used to determine differences between treatments following ANOVA. Statistical significance was set at a level of p<0.05. Histological and fecal scores were analyzed using the non-parametric Kruskal-Wallis test (GraphPad Prism) with a Dunns post-test.
Results
Effects of early life dietary SDP on clinical responses to subsequent S. Typhimurium challenge
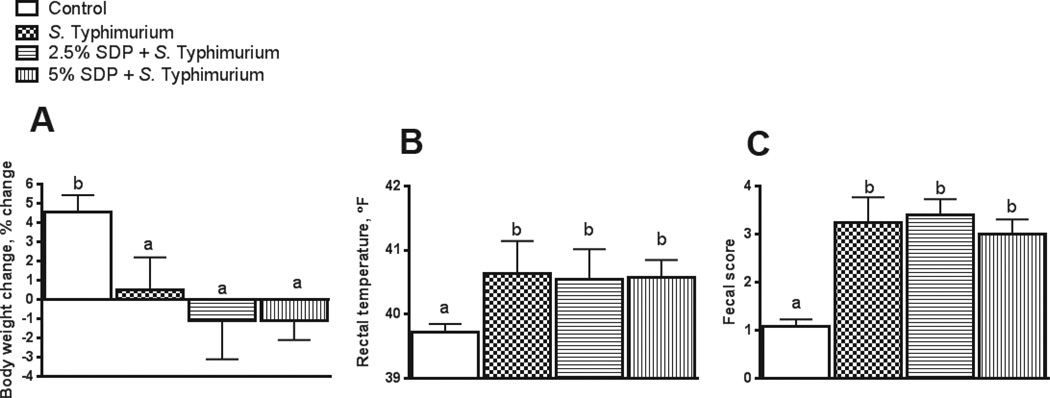
In the first two weeks PW, estimated feed intake for pigs receiving 0% SDP, 2.5% SDP (for 7 d), and 5% SDP (for 14 d) was 0.221 kg/d, 0.231 kg/d, and 0.238 kg, respectively. Average daily gain (kg/d), during the first two weeks PW, for pigs receiving 0% SDP, 2.5% SDP, and 5% SDP diets was 0.121±.015, 0.119±.021, and 0.142±.025, respectively. All pigs remained clinically normal throughout the nursery phase. During the 2-d S. Typhimurium challenge study, at 34 d post-weaning, the control (non-challenged, 0% SDP) pigs gained 5% of their body weight whereas growth responses in pigs challenged with S. Typhimurium were significantly reduced and ranged between 0.5 to −1% body weight gain (Figure 2A). Compare with non-challenged control pigs, estimated feed intake in S. Typhimurium-challenged pigs over the 2 d challenge period was 1.54, 1.20, 1.22, and 1.17 kg in pens from control, 0% SDP, 2.5% SDP, and 5% SDP challenged groups, respectively. All pigs challenged with S. Typhimurium challenge exhibited diarrhea indicated by higher (P<0.05) fecal scores compared with unchallenged controls (Figure 2C). Rectal body temperatures were also significantly elevated in challenged pigs compared with controls (P<0.05; Figure 2B). Dietary inclusion of SDP (2.5% or 5% SDP) during the PW period had no significant effect on body weight loss, fecal score, or body temperature responses to S. Typhimurium challenge in this study.
FIGURE 2.
Body weight, body temperature, and fecal scores in pigs challenged with S. Typhimurium. Body-weight loss was calculated from body weights recorded on d 0 and d 2 post-challenge (A). Rectal temperature (B) and fecal scores (C) were recorded on d 2 post-challenge. SDP: Spray-Dried Plasma. Values are means + SEMs; n=8. Labeled means without a common letter differ (p<0.05), 1-way ANOVA.
Effects of early life dietary SDP on histological injury responses to S. Typhimurium challenge
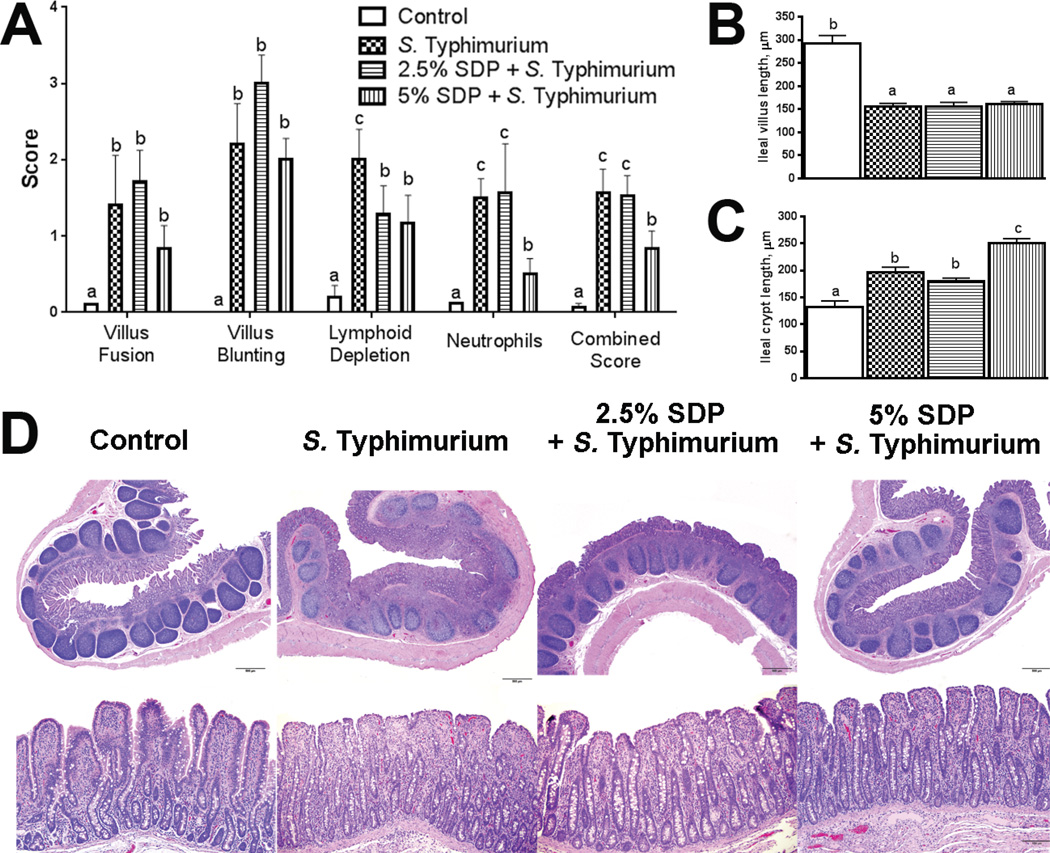
Compared with the non-challenged control group, ileum from S. Typhimurium-challenged pigs exhibited greater histological injury scores (Figure 3A). Histological scores from pigs fed 5% SDP-14 d during the nursery period were lower compared with challenged controls. Marked lymphoid depletion, an index of an overwhelming immune response, was observed in all S. Typhimurium-challenged pigs, but was less severe in pigs fed the 2.5%–7 d and 5% SDP-14 d PW diets. Extensive villus blunting (Figure 3A and B) and fusion (adhesion) was observed in all pigs challenged with S. Typhimurium; however, there were no effects of PW SDP treatments on these parameters. Crypt depth was increased (P<0.05) in ileum from S. Typhimurium-challenged pigs compared with controls (Figure 3C). Ileum from pigs fed the 5% SDP-14 PW diet had increased (P<0.05) crypt depth compared to all other treatments. Increased numbers of ileal neutrophils (Figure 3A) were observed in response to S. Typhimurium challenge which corresponded with higher ileal myeloperoxidase (M PO), a marker of neutrophil activation (Figure 4A). MPO and neutrophil numbers were lower in ileum from pigs fed 5% SDP-14 d in the PW period.
FIGURE 3.
Impact of early life dietary SDP on histological damage caused by subsequent challenge with S. Typhimurium. Histological scores (A) villus height (B), and crypt length (C) and histological appearance (D) from pig ileal tissues, at 2 d post-S. Typhimurium challenge. Values are means + SEMs; n=8. Labeled means without a common letter differ (p<0.05), 1-way ANOVA. Representative histological sections were taken at 4× and 20× magnification.
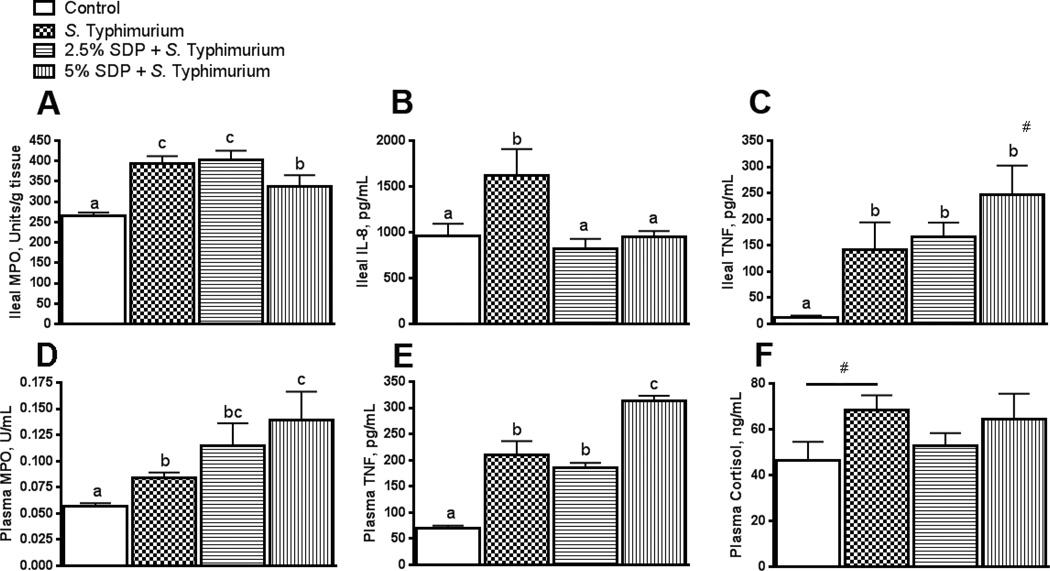
FIGURE 4.
Impact of early life dietary SDP on ileal and plasma immunological responses to subsequent S. Typhimurium challenge. Ileal MPO (A), ileal IL-8 (B) ileal TNF (C), plasma MPO (D), plasma TNF (E) and plasma cortisol (F) were measured at 2 d post-S. Typhimurium challenge. MPO: myeloperoxidase. SDP: Spray-Dried Plasma. Values are means + SEMs; n=8. Labeled means without a common letter differ (p<0.05), 1-way ANOVA. #Indicates at trend (P=0.07 for TNF and P=0.09 for plasma cortisol) between 5% SDP and S. Typhimurium control.
Effects of SDP on Ileal and plasma cytokines in response to later life S. Typhimurium challenge
TNF concentrations were elevated in the ileum from all S. Typhimurium-challenged groups compared with the non-challenged control group (Figure 4C). There was a trend (P=0.06) for elevated TNF levels in response to S. Typhimurium challenge in pigs fed 5% SDP-14 d, compared with other challenged treatments. Ileal IL-8 was increased in S. Typhimurium-challenged in controls (Figure 4B); however, IL-8 levels were lower in the ileum from challenged pigs fed 2.5%–7 d or 5% SDP-14 d in the PW period (Figure 4B).
To assess the effects of S. Typhimurium infection and SDP nursery feeding on systemic inflammatory responses, plasma levels of MPO (Figure 4D), TNF (4E), and cortisol (4F) were assessed 2 d post-challenge. Plasma TNF was elevated in all pigs challenged with S. Typhimurium. In line with responses observed in the ileum, pigs fed 5% SDP-14 d in the nursery diet exhibited the greatest levels of plasma TNF in response to S. Typhimurium challenge. Plasma cortisol levels tended (p=0.09) to be elevated in control S. Typhimurium-challenged but was not different from other experimental treatments.
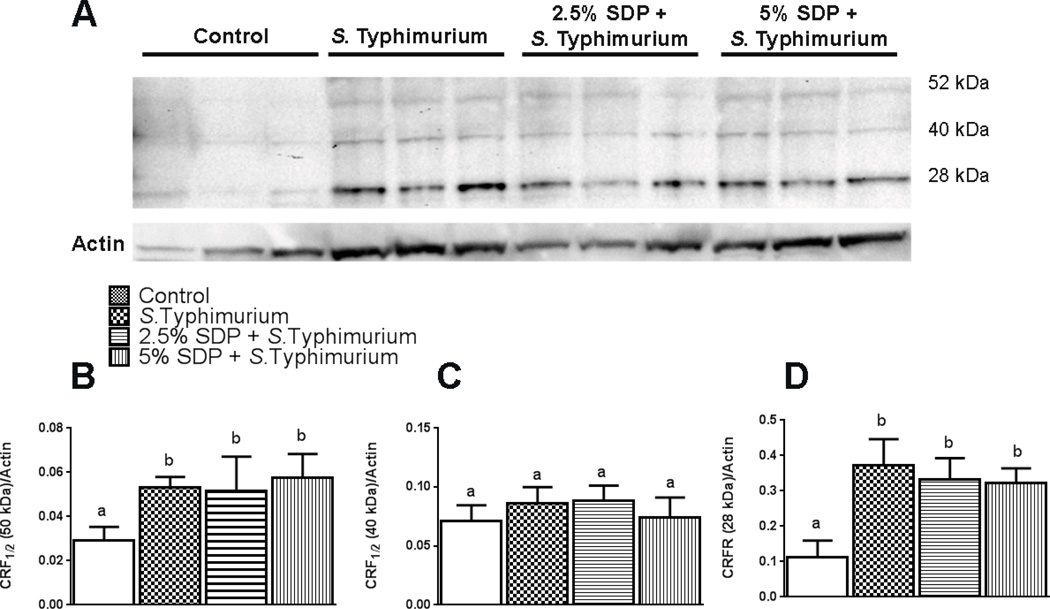
Utilizing an antibody that recognizes both CRF receptor subtypes (CRF1 and CRF2), we found that the CRF1/2 antibody recognized three major protein bands in porcine ileal protein extracts at ~ 55 kDa, 37 kDa, and 28 kDa (Figure 4). These protein bands are consistent with the unprocessed form (55 kDa), the deglycosylated form (37 kDa) and the soluble CRF receptor forms (28kDa)(28) and have been reported previously in the rodent intestine(29, 30). Based upon densinotometric analysis, intestinal CRF receptor1/2 proteins (50 kDa and 28 kDa bands) were markedly up-regulated (p<0.05) in response to S. Typhimurium challenge. The nursery SDP treatment did not, however, appear to influence the level of ileal CRF receptor expression in challenged pigs.
Effects of SDP on intestinal permeability in response to later life S. Typhimurium challenge
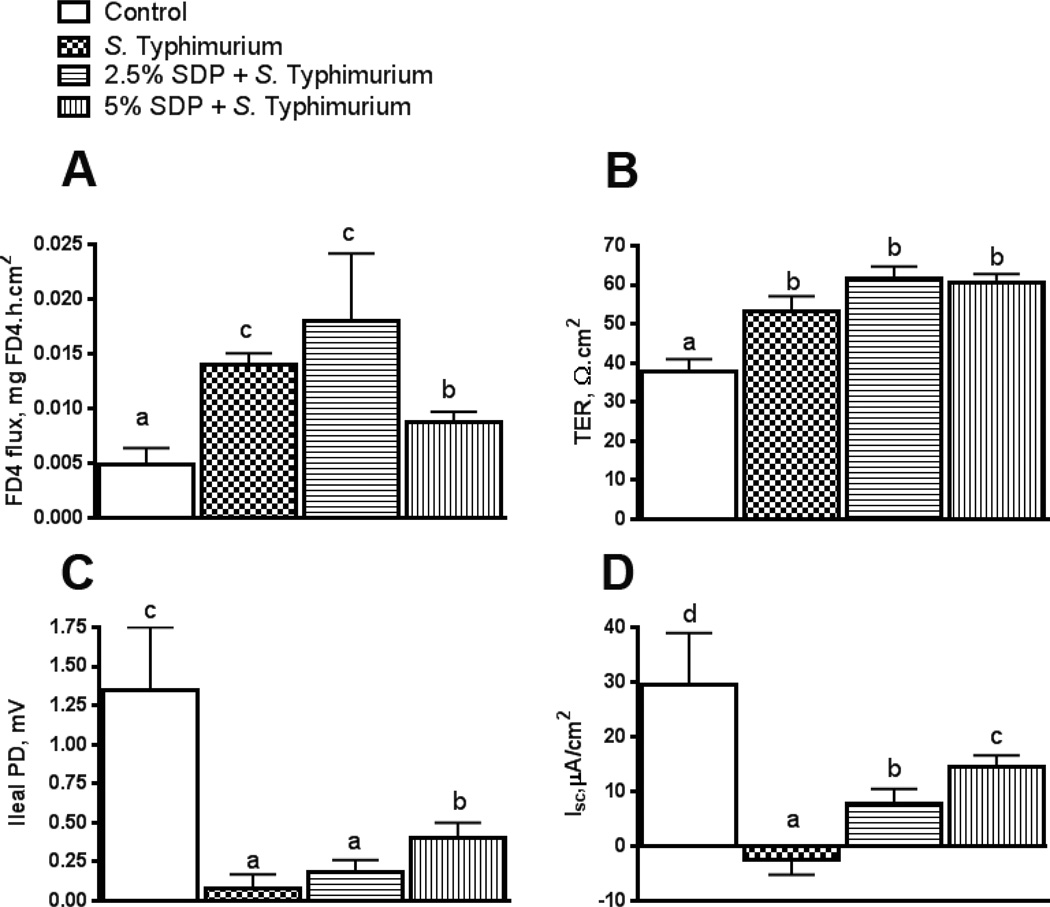
At 2 d post-challenge, FD4 flux rates, an index of paracellular permeability, were elevated (P<0.05) in ileum from pigs challenged with S. Typhimurium (P<0.05) (Figure 6A). Pigs fed 5% SDP-14 d in the PW diet exhibited lower FD4 flux rates at 2 d post-challenge, compared with other challenged treatment groups. Ileal TER was higher in challenged pigs compared with non-challenged controls (P < 0.05; Figure 6B). There was a trend (P = 0.06) for increased ileal TER in pigs fed 2.5%–7 d and 5% SDP-14 d in post-weaning diets compared with challenged control pigs. Transepithelial potential difference (PD) and short circuit current (Isc) were reduced in S. Typhimurium- challenged pigs compared with unchallenged controls (Figure 6C and D, respectively). Pigs fed 5% SDP-14 d exhibited greater ileal PD, compared with challenged controls (p<0.05). Ileal Isc was greater in pigs fed 2.5% SDP-7d and 5% SDP-14d compared with unchallenged controls.
FIGURE 6.
Impact of early life dietary SDP on intestinal permeability and transepithelial short circuit current (Isc) following subsequent S. Typhimurium challenged in pigs. FD4 flux rates (A), ileal TER (B), ileal transepithelial PD (C), and Isc were measured using ileum mounted on Ussing chambers on 2 d post-S. Typhimurium challenge. PD: potential difference, SDP: Spray-Dried Plasma, TER: transepithelial electrical resistance. Values are means + SEMs; n=8. Labeled means without a common letter differ (p<0.05), 1-way ANOVA.
Discussion
Inclusion of SDP into animal diets has been shown to promote growth responses (31–34) and lessen inflammatory processes and clinical disease pathogen challenge models(20–22, 35). Previously, we demonstrated that dietary SDP, at 2.5% and 5% of weaned pigs diets, was beneficial in reducing intestinal permeability and inflammation induced in early weaned pigs(18). While the aforementioned studies in rodents and pigs demonstrated the beneficial effects of SDP on growth and intestinal inflammatory responses to stress and pathogenic challenges, the response variables were measured when SDP was currently being supplied in the diet. Whether SDP confers beneficial effects on the intestine after its removal from the diet has not been investigated. Here, we showed that dietary inclusion of SDP during the first 2 weeks PW can modify subsequent intestinal and immunological responses to a pathogenic challenge with S. Typhimurium in pigs.
In the present study, pigs fed diets containing SDP during the first two weeks PW exhibited differential intestinal and systemic immune responses following a S. Typhimurium at 50 d of age. Compared with challenged controls, pigs fed PW diets containing 2.5%–7 d and 5% SDP-14 d exhibited reduced ileal IL-8 levels following a challenge with S. Typhimurium. IL-8 is major inflammatory cytokine produced by intestinal epithelial cells during S. Typhimurium infection and acts as a chemoattractant for the recruitment of circulating neutrophils into the intestine resulting in the classic intestinal inflammatory lesions associated with S. Typhimurium enteritis(36, 37) In line with this role, pigs fed the 5% SDP-14 d nursery dietary treatment had reduced neutrophil infiltration, MPO levels, and histological injury in response to S. Typhimurium challenge. In agreement with our findings, Bosi et al (2004) demonstrated that piglets fed 6% SDP exhibited reduced ileal IL-8 concentrations caused by ETEC challenge; however, unlike our study, IL-8 levels were measured while SDP was in the diet at the time of the challenge(21). Interestingly, despite the dampened histopathological and inflammatory responses exhibited by challenged pigs fed 5% SDP-14 d in the nursery, ileal and plasma TNF levels with greater compared with challenged controls. As mentioned above, S. Typhimurium induces an intestinal inflammatory response mediated via the production of pro-inflammatory cytokines including TNF and IL-8 and subsequent neutrophil recruitment and activation(38). While TNF is best recognized as a pro-inflammatory cytokine that is central to the pathogenesis of a number of inflammatory disorders and in stress-induced intestinal permeability(39, 40), TNF is also recognized as a critical and beneficial modulator of immune function and pathogen defense. For example, Nauciel and Espinasse-Maes (1992) demonstrated that administration of anti-TNF antibodies to mice exacerbated bacterial proliferation and mortality following a sub-lethal dose of S. Typhimurium (41). In similar studies by Gulig et al. (1997)(42) and Tite et al. (1991)(43) anti-TNF antibodies increased the numbers of splenic CFU of S. Typhimurium following challenge. Overall, these studied suggest that elevated TNF responses are critical for the control of infections. Similar to the present study, Touchette et al. (2002) showed that early weaned pigs fed a diet containing 7% SDP for 7 d PW exhibited a 2-fold higher increase in serum TNF in response to systemic LPS challenge compared with pigs that did not received SDP(44). The authors also demonstrated a marked (110-Fold) increase in (IFNα) in pigs fed the diet with SDP compared to a 16-fold increase for pigs fed the diet without SDP. Overall, the present studies, along with previous investigations, demonstrate that dietary SDP can modulate local and systemic immune responses to weaning and pathogen challenges, however, the current study provides the first evidence that the effects of SDP on immune responses can be retained after the removal of SDP from the diet.
In addition to investigating the effects of early dietary SDP and subsequent S. Typhimurium challenge on inflammatory signals, we also investigated stress signaling pathways. Specifically, we showed that plasma cortisol and intestinal expression of CRF receptors were increased 2 d post-challenge; however, there were no differences between pigs fed SDP. While elevations in plasma cortisol, following S. Typhimurium challenge, have been shown previously in pigs(23), the current findings demonstrating the marked up-regulation of intestinal CRF receptors1/2 during an acute challenge with S. Typhimurium is novel. Given the increasingly recognized role of the intestinal CRF system in inflammatory and stress-induced functional GI disorders, in people and laboratory research animal models(17, 45, 46)these findings warrant further investigation into the role of CRF in infectious inflammatory diseases.
In the present study, S. Typhimurium challenge induced impairment in intestinal barrier function, indicated by increased ileal permeability to the paracellular probe FD4. The increase in ileal permeability in S. Typhimurium-challenged pigs was attenuated in pigs fed the 5% SDP-14 d nursery diet, suggesting either an intestinal barrier protective or reparative influence of early SDP dietary inclusion. It is not understood how early SDP feeding resulted in lasting barrier protective effects during S. Typhimurium infection in the present study. However, it is known that intestinal neutrophil infiltration in response to S. Typhimurium infection is a central process contributing to the breakdown of intestinal barrier function. Neutrophil-mediated disruption of intestinal barrier function involves a multi-step mechanism including increased phosphorylation of myosin light chain (MLC) and increased MLC kinase, up-regulation of tight junction phosphotyrosine and phopshoserine residues(47), and activation of epithelial protease activated receptors (PAR)(48). Given that the 5% SDP-14 d inclusion to nursery diets resulted in reduced neutrophil infiltration in response to S. Typhimurium infection, it is plausible that this may represent an important mechanism by which SDP conveyed a protective effect on the intestinal barrier in the present study.
We observed unexpected results with regards to ileal TER in the present study. Despite the elevated FD4 permeability in the ileum at 2 d post-challenge, ileal TER was significantly elevated in all challenged groups. FD4 flux rates and TER measure two different aspects of intestinal epithelial barrier function: TER reflects changes in ion (predominantly Na+) permeability across the tight junction pores while FD4 flux reflects large molecule fluxes across leaky tight junctions. Another difference between the two measurements is that TER is calculated, based from measured values of transepithelial voltage (PD) and current (Isc) according to Ohm’s law (V=IR), and expressed based on surface area of the tissue chamber aperture. Therefore, significant alterations in either PD or Isc could significantly impact calculated TER values. Further analysis of the PD across S. Typhimurium-infected ileum tissues revealed a significant reduction in PD, which indicates a compromised ability of the intestinal epithelium to resist ion flow through the paracellular space, and thus is in line with the elevated FD4 flux. However, in contrast to the FD4 flux data, PD was not significantly influenced by the early nursery 5% SDP-14 d dietary treatment. S. Typhimurium challenge also resulted in significant reductions in ileal Isc which, in turn, likely contributed to the increased calculated TER values. Furthermore, Isc were greater in pigs fed 2.5%–7 d and 5% SDP-14 d in nursery diets which explained the increased TER pigs fed the SDP treatments. The basis for increased Isc observed in challenged pigs fed SDP in the nursery is not understood. However, the suppressive influence of S. Typhimurium on Isc has been demonstrated in previous investigations in pigs and mice. (49, 50) The mechanisms for reduced Isc in the ileum from S. Tyhphimurium-challenged pigs could be due to reduced anion (Cl− or HCO3−) secretion or electrogenic cation (e.g. Na+) absorption. In a recent study, it was demonstrated that mice challenged with S. Typhimurium exhibited reduced basal- and adenosine 3′,5′-cyclic monophosphate–mediated electrogenic Isc, an effect associated with reduced expression and(or) localization of colonic epithelial ion transporters including the Cl−/HCO3− exchanger down-regulated in adenoma and the cystic fibrosis transmembrane regulator (CFTR).(51) These suppressive effects were in part mediated by secreted S. Typhimurium effector proteins. Therefore, in light of these findings, it is plausible that the influence of early SDP on subsequent Isc responses to S. Typhimurium challenge could be directly related to the effects of SDP treatments on subsequent S. Typhimurium pathogenicity in the porcine intestine. The precise host intestinal pathways modulated by early SDP feeding in pigs that contribute to the Isc response remain to be elucidated.
Despite marked changes in immune and epithelial barrier responses, SDP had little influence on S. Typhimurium-induced morphology of the intestinal villi (villus blunting or villus fusion) and epithelium (denuded epithelium). Interestingly, increased crypt depths were observed in pig fed 5% SDP-14 d in the nursery. Increased crypt depth (crypt expansion) is a hallmark of intestinal injury, but at the same time is an index of epithelial repair processes as increased proliferation of immature crypt enterocytes will migrate up the villus to replace damage or denuded villus tip epithelium. Therefore, the increased crypt depths in pigs fed the 5% SDP nursery diet could indicate increased epithelial renewal that might potentially prove beneficial in later stages of recovery from S. Typhimurium.
As mentioned previously, there are a number of studies in the literature that describe the beneficial impact of dietary SDP on growth responses. However, in the present study, there were no significant differences in growth and (or) clinical responses in pigs observed either during the PW period or during the subsequent S. Typhimurium post-challenge period. Despite the lack of measurable growth response to SDP in the present study, significant effects on immunological and intestinal responses were demonstrated. There are several reasons that could explain the lack of SDP growth responses in the current study. First, the primary objective of the current study was not to measure growth performance, but to determine whether early dietary SDP influenced subsequent immunological and intestinal physiology responses to a later life S. Typhimurium challenge. Therefore, sufficient animal numbers needed to achieve the statistical power required to appropriately evaluate growth responses were not included in the experimental design. Second, the experimental environment in which the pigs were raised in the current study may not have been ideal to demonstrate a SDP-dependent growth response. It was shown previously that the effects of SDP on pig growth were observed in commercial farm environment, but not an experimental university research setting.(34). A third reason for the lack of SDP growth response, specifically observed in the post-challenge phase of the experiment, is the short time period (2 d) in which BW changes were measured, which may have been insufficient to assess post-challenge growth responses during the peak challenge response. Given the beneficial effects of early SDP observed on intestinal physiology and immunological responses following S. Typhimurium challenge, growth measurements over a longer post-challenge period (e.g. 7–14 d) could have influence the effects of SDP on growth responses in challenged pigs.
Collectively, data from this study demonstrate that early dietary inclusion of SDP impacts intestinal immune and epithelial pathophysiologic responses to S. Typhimurium challenge, after SDP has been removed from the diet. Given that stress and diet are increasingly recognized as key early life factors that determine long-term health outcomes in humans and animals, a more fundamental understanding of biological mechanisms and optimal nutritional intervention strategies, such as dietary SDP, have potential to positively impact long-term intestinal health.
FIGURE 5.
Western blot (A) and densitometric analysis (B) of CRF receptors1/2 in porcine ileal mucosal scrapes from control and S. Typhimurium-challenged pigs. Graph (B–D) shows the mean, densitometry values +/− SEMs for each protein band, normalized to β-actin. n=3. *p<0.05.
Acknowledgements
We would like to thank the North Carolina State University Laboratory Animal Resources for their excellent technical assistance and advice during this study
Financial Support
The present study was supported by funded by American Proteins Company (APC), Inc. (to A.J.M) and The National Institutes of Health (A.J.M, K08 DK084313, R01 HD072968).
Footnotes
Conflicts of Interest
None
Authorship
The authors contributions are as follows: J.M. (APC Inc.), J.M.C (APC Inc.), and A.J.M. designed research; A.J.M., L.L.E., P.B., E.S., S.T., M.M, S.D. and L.B.B conducted research; A.J.M., L.L.E., S.D. and L.B.B analyzed data; P.B., S.D. and A.J.M wrote the paper. A.J.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Literature Cited
- 1.McLamb BL, Gibson AJ, Overman EL, et al. Early Weaning Stress in Pigs Impairs Innate Mucosal Immune Responses to Enterotoxigenic E. coli Challenge and Exacerbates Intestinal Injury and Clinical Disease. Plos One. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennon EM, Maharshak N, Elloumi H, et al. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflammatory bowel diseases. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gareau MG, Jury J, Yang PC, et al. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–88. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- 4.Boudry G, Douard V, Mourot J, et al. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast cell regulation of the ileal barrier in piglets. J Nutr. 2009;139:1110–1117. doi: 10.3945/jn.108.102640. [DOI] [PubMed] [Google Scholar]
- 5.Agostini A, Rizzello F, Ravegnani G, et al. Adult attachment and early parental experiences in patients with Crohn's disease. Psychosomatics. 2010;51:208–215. doi: 10.1176/appi.psy.51.3.208. [DOI] [PubMed] [Google Scholar]
- 6.Chitkara DK, van Tilburg MA, Blois-Martin N, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100:2071–2078. doi: 10.1111/j.1572-0241.2005.41753.x. [DOI] [PubMed] [Google Scholar]
- 9.Apley J, Hale B. Children with recurrent abdominal pain: how do they grow up? Br Med J. 1973;3:7–9. doi: 10.1136/bmj.3.5870.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moeser AJ, Klok CV, Ryan KA, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 11.Moeser AJ, Ryan KA, Nighot PK, et al. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 12.Pie SLJ, Blazy F, Laffitte J, et al. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- 13.Makkink CA, Negulescu GP, Qin G, et al. Effect of dietary protein source on feed intake, growth, pancreatic enzyme activities and jejunal morphology in newly-weaned piglets. Brit J Nutr. 1994;72:353–368. doi: 10.1079/bjn19940039. [DOI] [PubMed] [Google Scholar]
- 14.Boudry G, Lalles JP, Malbert CH, et al. Diet-related adaptation of the small intestine at weaning in pigs is functional rather than structural. J Pediatr Gastroenterol Nutr. 2002;34:180–187. doi: 10.1097/00005176-200202000-00014. 2002. [DOI] [PubMed] [Google Scholar]
- 15.Boudry G, Peron V, Le Huerou-Luron I, et al. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- 16.Nabuurs MJ, Hoogendoorn A, van der Molen EJ, et al. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res Vet Sci. 1993;55:78–84. doi: 10.1016/0034-5288(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 17.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peace RM, Campbell J, Polo J, et al. Spray-Dried Porcine Plasma Influences Intestinal Barrier Function, Inflammation, and Diarrhea in Weaned Pigs. J Nutr. 2011;141:1312–1317. doi: 10.3945/jn.110.136796. [DOI] [PubMed] [Google Scholar]
- 19.Van Dijk AJ, Margry RJ, Van Der Lee AG, et al. Growth performance and health status in weanling piglets fed spray-dried porcine plasma under typical Northern European conditions. J Anim Physiol Anim Nutr (Berl) 2002;86:17–25. doi: 10.1046/j.1439-0396.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Dijk AJ, Enthoven PM, Van den Hoven SG, et al. The effect of dietary spray-dried porcine plasma on clinical response in weaned piglets challenged with a pathogenic Escherichia coli. Vet Microbiol. 2002;84:207–218. doi: 10.1016/s0378-1135(01)00463-1. [DOI] [PubMed] [Google Scholar]
- 21.Bosi P, Casini L, Finamore A, et al. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J Anim Sci. 2004;82:1764–1772. doi: 10.2527/2004.8261764x. [DOI] [PubMed] [Google Scholar]
- 22.Corl BA, Harrell RJ, Moon HK, et al. Effect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirus. J Nutr Biochem. 2007;18:778–784. doi: 10.1016/j.jnutbio.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Balaji R, Wright KJ, Hill CM, et al. Acute phase responses of pigs challenged orally with Salmonella typhimurium. J Anim Sci. 2000;78:1885–1891. doi: 10.2527/2000.7871885x. [DOI] [PubMed] [Google Scholar]
- 24.Bahnson PB, Kim JY, Weigel RM, et al. Associations between on-farm and slaughter plant detection of Salmonella in market-weight pigs. J Food Potect. 2005;68:246–250. doi: 10.4315/0362-028x-68.2.246. [DOI] [PubMed] [Google Scholar]
- 25.Argenzio RA, Liacos JA. Endogenous prostanoids control ion transport across neonatal porcine ileum in vitro. Am J Vet Res. 1990;51:747–751. [PubMed] [Google Scholar]
- 26.Wickramasinghe SN. Bone Marrow. In: Mills SE, editor. Histology for Pathologists. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 799–836. [Google Scholar]
- 27.Zadrozny LM, Stauffer SH, Armstrong MU, et al. Neutrophils do not mediate the pathophysiological sequelae of Cryptosporidium parvum infection in neonatal piglets. Infect Immun. 2006;74:5497–5505. doi: 10.1128/IAI.00153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slominski A, Zbytek B, Pisarchik A, et al. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakshmanan J, Magee TR, Richard JD, et al. Localization and gestation-dependent pattern of corticotrophin-releasing factor receptor subtypes in ovine fetal distal colon. Neurogastroenterol Motil. 2008;20:1328–1339. doi: 10.1111/j.1365-2982.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Malley D, Dinan TG, Cryan JF. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides. 2010;31:662–670. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Pierce JL, Cromwell GL, Lindemann MD, et al. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J Anim Sci. 2005;83:2876–1285. doi: 10.2527/2005.83122876x. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk AJ. Spray-dried plasma in diets for weaned piglets: influence on growth and underlying mechanisms. Tijdschr Diergeneeskd. 2002;127:520–523. [PubMed] [Google Scholar]
- 33.Zhao J, Harper AF, Estienne MJ, et al. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J Anim Sci. 2007;85:1302–1310. doi: 10.2527/jas.2006-434. [DOI] [PubMed] [Google Scholar]
- 34.Coffey RD, Cromwell GL. The impact of environment and antimicrobial agents on the growth response of early-weaned pigs to spray-dried porcine plasma. J Anim Sci. 1995;73:2532–2539. doi: 10.2527/1995.7392532x. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Bosque A, Amat C, Polo J, et al. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J Nutr. 2006;11:2838–2843. doi: 10.1093/jn/136.11.2838. [DOI] [PubMed] [Google Scholar]
- 36.McCormick BA, Colgan SP, Delp-Archer C, et al. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. The J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick BA, Parkos CA, Colgan SP, et al. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 38.Moeser AJ, Blikslager AT. Mechanisms of porcine diarrheal disease. J Am Vet Med Assoc. 2007;231:56–67. doi: 10.2460/javma.231.1.56. [DOI] [PubMed] [Google Scholar]
- 39.Soderholm JD, Streutker C, Yang PC, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–1824. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. Plos One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulig PA, Doyle TJ, Clare-Salzler MJ, et al. Systemic infection of mice by wild-type but not Spv- Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1997;65:5191–5197. doi: 10.1128/iai.65.12.5191-5197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tite JP, Dougan G, Chatfield SN. The involvement of tumor necrosis factor in immunity to Salmonella infection. Journal of Immunology. 1991;147:3161–3164. [PubMed] [Google Scholar]
- 44.Touchette KJ, Carroll JA, Allee GL, et al. Effect of spray-dried plasma and lipopolysaccharide exposure on weaned pigs: I. Effects on the immune axis of weaned pigs. J Anim Sci. 2002;80:494–501. doi: 10.2527/2002.802494x. [DOI] [PubMed] [Google Scholar]
- 45.Gourcerol G, Wu SV, Yuan PQ, et al. Activation of Corticotropin-Releasing Factor Receptor 2 Mediates the Colonic Motor Coping Response to Acute Stress in Rodents. Gastroenterology. 2011;140:1586–1596. doi: 10.1053/j.gastro.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatzaki E, Anton PA, Million M, et al. Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J Gastroenterol. 2013;19:1416–1423. doi: 10.3748/wjg.v19.i9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edens HA, Parkos CA, Liang TW, et al. Non-serum-dependent chemotactic factors produced by Candida albicans stimulate chemotaxis by binding to the formyl peptide receptor on neutrophils and to an unknown receptor on macrophages. Infect Immun. 1999;67:1063–1071. doi: 10.1128/iai.67.3.1063-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin AC, Lee WY, Nusrat A, et al. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh MC, Rostagno MH, Gardiner GE, et al. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids: Part II. Effects on intestinal histology and active nutrient transport. J Anim Sci. 2012;90:2599–2608. doi: 10.2527/jas.2010-3599. [DOI] [PubMed] [Google Scholar]
- 50.Aschenbach JR, Ahrens F, Schwelberger HG, et al. Functional characteristics of the porcine colonic epithelium following transportation stress and Salmonella infection. Scand J Gastroenterol. 2007;42:708–716. doi: 10.1080/00365520601053297. [DOI] [PubMed] [Google Scholar]
- 51.Marchelletta RR, Gareau MG, McCole DF, et al. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonella-induced enteritis. Gastroenterology. 2013;145:1358–1368. doi: 10.1053/j.gastro.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]