Abstract
Pregnancy is a unique and well-choreographed physiological process that involves intricate interplay of inflammatory and anti-inflammatory milieu, hormonal changes, and cellular and molecular events at the maternal-fetal interface. IL-10 is a pregnancy compatible cytokine that plays a vital role in maintaining immune tolerance. A wide array of cell types including both immune and non-immune cells secret IL-10 in an autocrine and paracrine manner. IL-10 binds to a specific receptor complex and activates JAK-STAT and PI3K-Akt signaling pathways while inhibiting NF-κB signaling pathway. IL-10 exerts its anti-inflammatory effects mainly by decreasing pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and TNF-α, by inducing heme oxygenase-1, and by inhibiting antigen presentation via blocking major histocompatibility complex (MHC) class II expression. Prior studies from our group and others have shown that IL-10 also functions as a potent protector against vascular dysfunction, and enhancement of IL-10 may serve as an immunotherapeutic intervention to treat adverse pregnancy outcomes. This review seeks to critically evaluate the archetypal functions of IL-10 as an immune suppressive factor as well as its novel functions as a vascular protector and modulator of endoplasmic reticulum (ER) stress and autophagy in the context of normal and adverse pregnancy outcomes.
Keywords: Adverse pregnancy outcomes, Angiogenesis, Preeclampsia, Immune tolerance, Endoplasmic reticulum stress, Autophagy
Introduction
Pregnancy involves immunological modulation in a spatio-temporal manner at the maternal-fetal interface. Survival of the allogeneic fetus in the uterine microenvironment depends on the maintenance of maternal immune tolerance. In fact, the utero-placental tissue produces an array of anti-inflammatory cytokines and other factors that limit immunological aggression towards the fetus.1–4 Thus, the balance of locally produced pro-inflammatory and anti-inflammatory effectors is essential to a successful pregnancy outcome.5,6 Among these locally produced factors, interleukin-10 (IL-10) seems to be the most temporal immunosuppressive and anti-inflammatory molecule.1
The IL-10 family of cytokines belongs to the Class II cytokine family and consists of nine members: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26 and the more distantly related IL-28, IL-28B and IL-29 based on their similarities with regard to the structure and location of their encoding genes and their protein structures and receptor complexes (reviewed in Ref 7). IL-10 was originally identified in T helper 2 (Th2) cell clones as a “cytokine synthesis inhibitory factor” that has the ability to inhibit the activity of inflammatory T helper 1 (Th1) type cells.8 Although originally defined as a product of Th2 cells, this cytokine is now known to be produced by a wide array of cell types, including both immune and non-immune cells.2,7
IL-10 works in either autocrine or paracrine manner in response to the inflammatory limb of the immune system to inhibit over-activation of inflammatory signals mainly by inhibiting activities of pro-inflammatory cytokines such as IL-1, IL-6, IL-12, and tumor necrosis factor (TNF)-α.9–11 IL-10 also inhibits antigen presentation by blocking major histocompatibility complex (MHC) class II expression and co-stimulatory molecules such as CD80/CD86.3,11 Additionally, IL-10 regulates differentiation and proliferation of several immune cells including T cells, B cells, natural killer (NK) cells, antigen-presenting cells, mast cells and granulocytes.11,12 Importantly, we and others have identified novel roles for IL-10 as a potent protector against vascular dysfunction that is associated with hypertension and inflammation during pregnancy IL-10.13–20
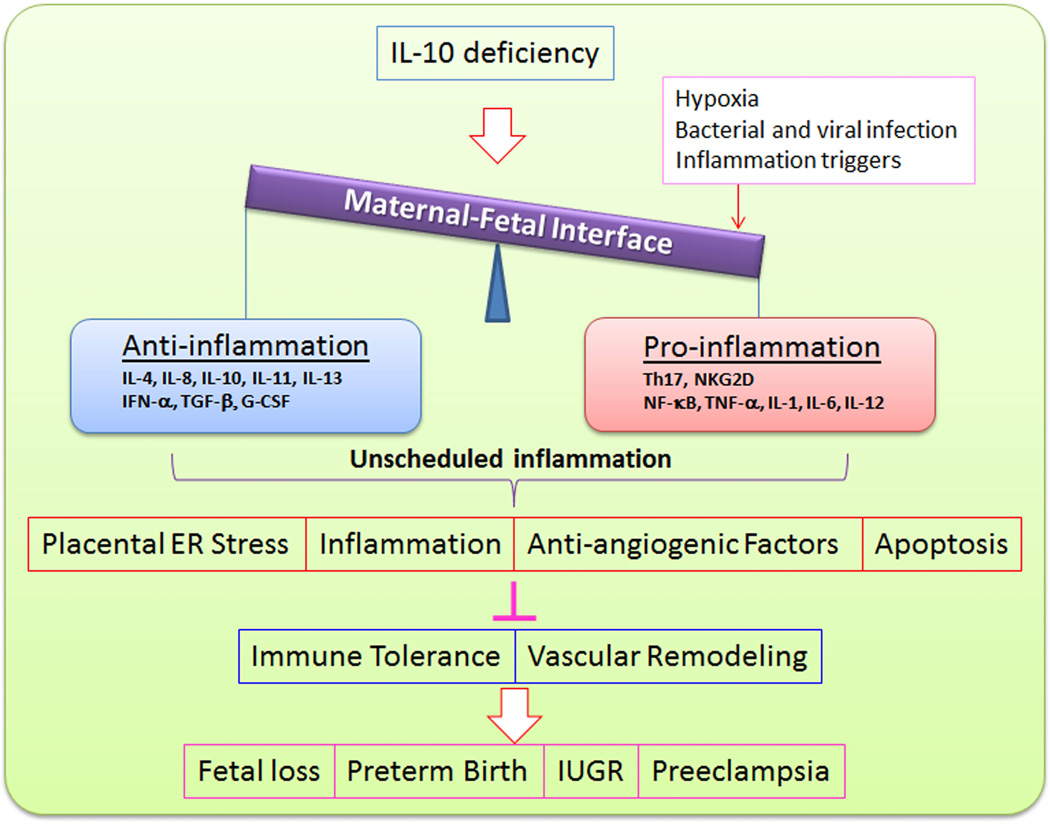
Dysregulation of IL-10 has been shown to be associated not only with cancer, autoimmune and inflammatory diseases, atopic diseases (reviewed in Ref 12), but also with adverse pregnancy complications such as preterm birth, miscarriage, fetal growth restriction and preeclampsia.3,21–24 Recombinant human IL-10 is currently being tested in clinical trials for treating many diseases including rheumatoid arthritis, inflammatory bowel disease, psoriasis, organ transplantation, and chronic hepatitis C.12 Interestingly, experimental studies from our laboratory and others have shown that administration of recombinant IL-10 reverses or alleviates symptoms of many adverse pregnancy outcomes in animal models.20,25–30 Thus, the IL-10/IL-10 receptor (IL-10R) axis may become a new target for therapeutic intervention and treatment of these adverse pregnancy outcomes. In this review, we focus on the archetypal function of IL-10 as an immune suppressive factor as well as its novel functions as a vascular protector and modulator of endoplasmic reticulum (ER) stress and autophagy in the context of normal and adverse pregnancy outcomes (Figure 1).
Figure 1.
Role of IL-10 in adverse pregnancy outcomes. IL-10 deficiency coupled with other insults including hypoxia, bacterial and viral infection as well as inflammatory triggers disturbs the balance between anti-inflammation and pro-inflammation at the maternal-fetal interface and consequently results in placental ER stress, inflammation and apoptosis as well as release of anti-angiogenic factors. As a result, maternal immune tolerance and vascular remodeling are perturbed, leading to many adverse pregnancy outcomes.
IL-10 Gene and Expression
Human IL-10 is an acid-sensitive homodimeric protein with a monomeric molecular weight of 18.5 kDa that is encoded on chromosome 1 in both mice and humans. Mouse IL-10 and human IL-10 are fairly conserved in their amino acid sequences sharing ~73% homology and mainly differ in N-glycosylation sites with human IL-10 lacking of this site.11,12 The gene promoter polymorphisms have been described that influence transcriptional, phenotypic, and functional characteristics of a spectrum of genes.31,32 Recent reports provide evidence for genetic and epigenetic regulation of IL-10 production.33–38 The IL-10 gene promoter contains several transcription factor-responsive elements which can be modulated by endotoxin, TNF-α, catecholamines, and cAMP-elevating drugs.12 Three linked single nucleotide polymorphisms (SNPs) in the IL-10 gene promoter have been found at −1082(G/A), −819(C/T), and −592(C/A) base pairs upstream from the transcriptional start site.1,31 GCC, ACC, and ATA, the major haplotypes of the −1082, −819, and −592 SNPs, have been shown to have effect on the expression of IL-10 with the GCC haplotype being associated with high in vitro IL-10 production and the ACC and ATA haplotypes with low IL-10 expression.31 Polymorphic changes in the human IL-10 gene promoter at three well characterized sites, −1082, −819, and −592, are thought to contribute to dysregulated IL-10 production and to the onset and severity of autoimmune, neoplastic, and infectious disorders such as systemic lupus erythematosus and Alzheimer’s disease.1,31,33–37,39,40 Importantly, evidence has also been found for the association of the IL-10 gene promoter polymorphisms with adverse pregnancy outcomes.31,41 Our laboratory prepared reporter gene promoter constructs containing GCC, ACC, and ATA haplotypes using DNA from recurrent spontaneous abortion patients harboring polymorphic changes at −1082 (G-A), −819 (C-T), and −592 (C-A) sites in the IL-10 promoter.31 These individual luciferase reporter constructs were transiently transfected into either primary term trophoblasts or THP1monocytic cells. Our results revealed that GCC promoter construct was significantly activated in lipopolysaccharide (LPS)-treated trophoblast cells, but not in monocytic cells, whereas the ACC promoter construct showed weaker activation in both cell types. Importantly, LPS inhibited constitutive activation of ATA promoter in THP1 cells, but not in trophoblasts.31 Similarly, a more recent study also suggested that the IL-10 T-819 C, but not G-1082A gene promoter polymorphism, can be a major genetic regulator in the pathogenesis of preeclampsia (PE).41
Many transcriptional factors have been shown to regulate IL-10 gene expression. A prior study demonstrated that c-Maf is an essential transcription factor for IL-10 gene expression in macrophages activated with LPS.42 Furthermore, Sharabi et al showed that Twist2 deficient mice when challenged with LPS exhibit a decrease in IL-10 secretion and c-Maf mRNA.43 It was further suggested that Twist2 could bind to the c-Maf promoter and activate c-Maf transcription, leading to IL-10 expression. In addition, programmed cell death protein 4 (PDCD4), a tumor suppressor, inhibits cap-dependent translation of mRNA via interacting with the eukaryotic translation-initiation factors elF4a and elF4G, which leads to inhibition of translation of many target mRNAs including that encoding IL-10. More recently, van den Bosch et al reported that PDCD4 also interacts with Twist2 and inhibits c-Maf induction via sequestering Twist2, which leads to inhibition of IL-10 expression.44 This study showed that LPS promotes PDCD4 degradation and subsequently results in dissociation of Twist2 from PDCD4, which induces IL-10 expression via binding of dissociated Twist2 to c-Maf.44 Additionally, the transcription factor Blimp-1, encoded by Prdm1, has been reported to be an important regulator of IL-10 production as IL-10 production in Th1 cells was strictly dependent on Blimp-1 but was further increased by the synergistic function of c-Maf.45
IL-10 is expressed by a broad range of cell types in the adaptive immune system, including Th1, Th2, Th17, regulatory T cells (Tregs), CD8+ T cells and B cells, as well as in the innate immune system, including monocytes, macrophages, dendritic cells, natural killer (NK) cells, granulocytes, neutrophils, esinophils, and mast cells. Additionally, IL-10 is produced by non-immune cells, such as keratinocytes and tumor cells.9 Numerous studies have indicated that IL-10 expression is regulated by these cell types at different stages of an immune response (reviewed in Ref 9). The maternal-fetal interface is composed of trophoblast cells of fetal origin intermingled with specialized maternal lymphocytes, stromal cells, and endothelial cells that comprise the decidua.3 IL-10 was detected in the media from human pre-implantation embryo culture, suggesting that pre-implantation embryos secret this cytokine.46 IL-10 expression has been found in placental villous trophoblasts, uterine NK cells (uNK cells), monocytes, and Tregs in the decidua.47 IL-10 receptors are localized to placental trophoblasts, decidual stromal cells, macrophages, and uNK cells.48 Modulation of IL-10 is tightly controlled and programmed at the maternal-fetal interface at different stages of normal pregnancy. In mice, IL-10 is expressed in all three trimesters of gestation and peaks at gestational day 12.49 The kinetics of IL-10 expression in normal human placental tissue suggests higher levels of IL-10 during first and second trimesters compared to third trimester of pregnancy.6 IL-10 also undergoes labor-associated changes as its production locally decreases prior to labor and delivery of the fetus and placenta, but increases post labor.6, 50 We and others have demonstrated in both human and mouse pregnancy models that IL-10 is a critical molecule for a successful pregnancy outcome.6,13,22–24,31,48,51,52 Moreover, the placental expression of IL-10 is reduced in many adverse pregnancy complications such as spontaneous abortion, preterm birth, and PE with minimum effects in circulating peripheral blood mononuclear cells (PBMCs).52–54
Signaling Pathways and Biological Functions
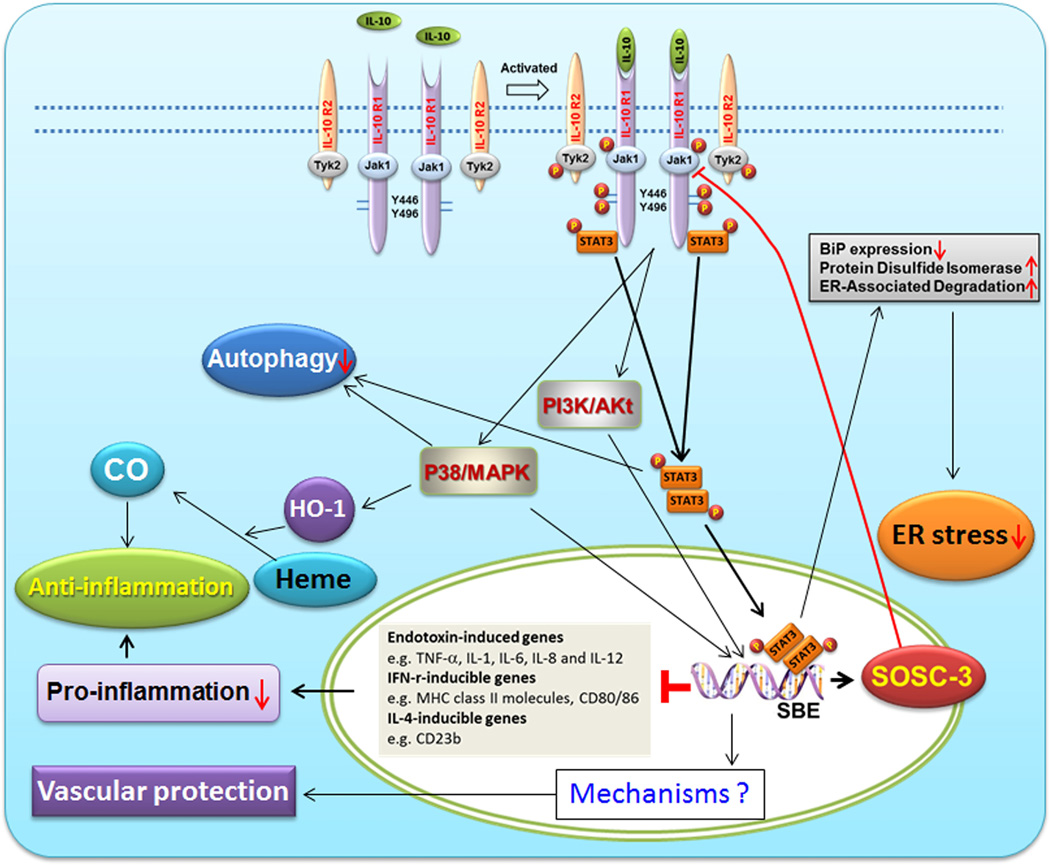
IL-10 exerts its biological effects through binding to IL-10 receptor (IL-10R). Functional IL-10R is a tetramer consisting of two ligand-binding subunits (IL-10R1 or IL-10Rα) and two accessory signaling subunits (IL-10R2 or IL-10Rβ). Expression of IL-10R is reported on hematopoietic as well as nonhematopoietic cells.11 IL-10R1 is constitutively expressed on placental cytotrophoblasts.55 IL-10R2−/− mice behave like IL-10−/− mice indicating that the second subunit of the receptor is essential for IL-10 signaling. The most well described IL-10-mediated signaling pathway is the Jak/STAT pathway (reviewed in Ref 56) (Figure 2). Briefly, IL-10R1 and IL-10R2 constitutively associate with Janus kinase 1 (Jak1) and Tyrosine kinase 2 (Tyk2), respectively. Binding of IL-10 to the extracellular domain of IL-10R1 elicits the phosphorylation of the receptor-associated Jak1and Tyk2, both of which, in turn, phosphorylate specific residues (Y446 and Y496) on the cytoplasmic tail of the IL-10R1. Upon phosphorylation, these tyrosine residues and their flanking peptide sequences serve as temporary docking sites for the latent transcription factor, signal transducer and activator of transcription-3 (STAT3).56 Once binding to these sites, STAT3 is phosphorylated by the receptor-associated Jak1 and then forms homodimers and translocates to the nucleus where it binds to STAT-binding elements (SBE) in the promoters of various IL-10-responsive genes. Notably, as one of these IL-10-responsive genes, suppressor cytokine signaling-3 (SOCS)-3, after induced by the STAT3 complex, blocks phosphorylation of Jak1and subsequently inhibits JAK/STAT-dependent signaling.56 Through rapidly inducing de novo synthesis of SOCS-3, IL-10 has the ability to inhibit the expression of many genes, including endotoxin (e.g., LPS)-inducible cytokine genes (e.g., TNF-α, IL-1, IL-6, IL-8, and IL-12), IFN-γ-inducible genes (e.g., MHC class II molecules, CD80/CD86, intercellular adhesion molecule-1 and inducible nitric oxide synthase), as well as IL-4-inducible genes (e.g., CD23b and the type-I and type-II IL-1 receptors).56 Thus, IL-10 exerts anti-inflammatory effects through inhibiting the productions of pro-anti-inflammatory cytokines and preventing antigen presentation via blocking major histocompatibility complex (MHC) class II expression and co-stimulatory molecules such as CD80/CD86 (Figure 2).
Figure 2.
IL-10 signaling pathways in normal and abnormal conditions. Binding of IL-10 to its specific receptor complex (IL-10R1 and IL-10R2) leads to activation of several signaling pathways, including well-established Jak/STAT pathway, PI3K/Akt- and P38/MAPK-mediated pathways, as well as CO-mediated pathway. Through its signal transduction, IL-10 plays a role in anti-inflammation, vascular protection and inhibition of ER stress and autophagy.
Prior studies have suggested the existence of JAK/STAT-independent signaling pathways that mediate anti-inflammatory effects of IL-10.57 IL-10 induces the expression of heme oxygenase-1 (HO-1), a stress-inducible protein with potential anti-inflammatory effects, through a p38 mitogen-activated protein kinase-dependent pathway (Figure 2). HO-1 is the rate-limiting enzyme involved in the catabolism of heme, which leads to the generation of biliverdin, free iron and carbon monoxide (CO). CO inhibits the expression of LPS-induced pro-inflammatory cytokines and enhances LPS-induced expression of IL-10 in macrophages.58 Co-treatment of a HO-1 inhibitor or a CO scavenger significantly decreases the inhibitory effects of IL-10 on LPS-induced TNF-α and NO production, as well as the expression of matrix metalloproteinase-9 in macrophages.57 Collectively, HO-1 and CO play critical roles in mediating the anti-inflammatory effect of IL-10 both in vitro and in vivo.
In addition to its suppressive effect on inflammation, IL-10 can also activate a major survival pathway consisting of phosphoinositide-3K kinase (PI3K) and its downstream substrates p70 S6-kinase and Akt/protein kinase-B. Through activation of this pathway, IL-10 promotes survival of astrocytes or induces proliferation of mast cells.59
IL-10-mediated Modulation at the Maternal-Fetal Interface
Immune tolerance
Local immune tolerance, angiogenesis, cytokine and hormonal balance, cellular and molecular mimicry, genetic and epigenetic as well as environmental cues influence pregnancy outcomes.4 Successful pregnancy relies on limited inflammation during implantation, immune tolerance (anti-inflammation) during mid-pregnancy, and inflammation again during parturition.4 Thus, programming of inflammation and anti-inflammation during pregnancy in a spatio-temporal manner is critical to maintenance of maternal immune tolerance for a successful pregnancy (Figure 1). Although the mechanisms underlying the immune tolerance are poorly understood, accumulating data indicate that both maternal innate and adaptive immune responses cross-talk with the fetal-placental unit to maintain a balance between anti- and pro-inflammatory responses. IL-10, a potent immunosuppressive cytokine, has been considered as a pivotal modulator of immune tolerance at the maternal-fetal interface. The generation of mice with IL-10 gene deficiency provided an opportunity for novel approaches to investigate the role of IL-10 in regulating the immune tolerance at the maternal-fetal interface. Pregnant IL-10−/− mice exhibit increases in placental size and maternal blood sinuses60 and feature susceptibility to low doses of LPS, CpG and polyinosinic:polycytidylic acid (polyI:C) as compared to WT counterparts.22,48,61 In humans, IL-10 deficiency has been found to be associated with many adverse pregnancy outcomes such as recurrent spontaneous abortion (RSA), preterm birth, and pre-eclampsia.53,62–66 Collectively, these data suggest that IL-10 functions as an important protective agent contributing to the regulation of maternal immune tolerance during pregnancy.
Uterine NK (uNK) cells are the major lymphocytes present in the decidua during pregnancy and have the ability to produce and be regulated by IL-10.22,67–69 Importantly, unlike the primordial immune functions of NK cells, the specialized uNK cells under the influence of pregnancy milieu function to support pregnancy with their unique ability to aid in regulating trophablast invasion and vascular remodeling.53,70 Fu et al recently reported that decidual NK (dNK) cells posses a unique ability to maintain immune tolerance and successful pregnancy by suppressing inflammatory Th17 cells.71 Th17 cells are strong inducers of tissue inflammation and represent a lineage of proimflammatory T-helper cells that are involved in autoimmune diseases.71–76 There is only a small population of Th17 cells that exists in the decidua during the first trimester of normal human pregnancy.71 Redundant Th17 cells prevent induction of tolerance in transplantation and pregnancy77,78 and cause fetal loss71 as well as participate in inflammatory infiltration in women with recurrent spontaneous abortion (RSA). On the other hand, an inflammatory microenvironment induced by IL-1β and IL-6 in the decidua of women with RSA may also promote the expansion and recruitment of Th17 cells. Importantly, observations by Fu et al further showed that the expression levels of IL-10, IL-1 receptor antagonist (IL-1RA) and IFN-γ were significantly decreased in dNK cells from women with RSA. IL-10 has been reported to be the key cytokine responsible for suppressing Th17 cells.79 These data suggest that IFN-γ, IL-10 and IL-1RA may be synergistically involved in regulating the inhibition of Th17 cells by dNK cells, which contributes to dNK cell-mediated immune tolerance.71
Additionally, Treg and DC cells and M2 microphages were also found to exert an immunosuppressive phenotype and mediate the maternal immune tolerance through IL-10.80–84 Further studies on these intricate interactions are strongly warranted.
IL-10 and Vascular Mediation
While anti-inflammatory role of IL-10 has been well established, impact of IL-10 on vascular biology has only begun to emerge. Increasing evidence has challenged the perception of IL-10 solely as an immunosuppressive factor. The IL-10−/− mice provide a powerful biological approach to investigate the role of this cytokine in the endothelial function and adverse pregnancy outcomes. Prior studies from our group and others have demonstrated that IL-10 also functions as a protector against vascular dysfunction.13,14–20,84,85 IL-10−/− pregnant mice when challenged with environmental toxicants such as polychlorinated biphenyls (PCBs) exhibit preterm birth, intrauterine growth restriction, increase in amniotic fluid as well as impaired spiral artery remodeling and placental angiogenesis at the maternal-placental interface.13 Mechanistically, these perturbations were associated with reduced placental protein levels of aquaporin-1 in IL-10−/− mice.13 Intriguingly, recombinant IL-10 rescued PCBs-induced pregnancy perturbations and restored aquaporin-1 expression to normal pregnancy levels.13 We have developed a novel in vitro model of endovascular activity that recapitulates the interaction between first trimester extravillous trophoblast lacking IL-10 production and endothelial cells and closely mimics decidual spiral artery remodeling in response to serum from normal pregnancy.14 Using this model, we have shown that PCBs disrupt the endovascular activity and this disruption can be reversed by exogenous IL-10.13 Moreover, we have shown that IL-10 deficiency coupled with hypoxia leads to in vivo endothelial dysfunction including hypertension and extensive capillary occlusion with swollen cytoplasm in renal glomerulus as well as elevation of anti-angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1), all of which can be reversed by administration of recombinant IL-10.20 These results suggest a protective role of IL-10 in maintenance of normal pregnancy and placental angiogenesis. The role of IL-10 as a vascular protector has also been observed in non-pregnant animals. For instance, IL-10 has been shown to function as a key mediator of vascular protection in LPS- or angiotensin II-induced and aging-related endothelial dysfunction and many diseases such as hypertension, diabetes and atherosclerosis.15–19,84,85
IL-10 Dysregulation in Adverse Pregnancy Outcomes
Preeclampsia
Preeclampsia (PE) is a multisystem disorder of pregnancy that is characterized by hypertension, proteinurine, edema, and intrauterine growth restriction. It is a leading cause of maternal and fetal morbidity and mortality and affects 5–8% of all pregnancies worldwide. Although the etiology of PE remains poorly understood, it is generally accepted that deficiency in placental perfusion caused by improper trophoblast invasion and poor spiral artery remodeling is the major pathological axis at the maternal-fetal interface.87–92 As a consequence, placenta-derived flux of inflammatory molecules and anti-angiogenic factors occurs in maternal systemic circulation that leads to maternal endothelial dysfunction and symptoms of hypertension, proteinuria and kidney injury.87,93 IL-10, as a potent anti-inflammatory factor, has attracted extensive attentions for its roles in normal pregnancy versus PE. We have developed a “humanized” PE model in IL-10−/− mice that faithfully recapitulates most of the features of the human disease. In this model, administration of sera from PE patients into pregnant IL-10−/− mice results in the indication of the key features of PE including development of proteinuria, hypertension, glomerular endotheliosis and fetal growth restriction, as well as elevated levels of anti-angiogenic factors such as sFlt-1 and soluble endoglin (sEng). In contrast, the same serum sample(s) induced a partial PE phenotype in wild type mice. Mechanistically, in the absence of IL-10, these serum samples impaired spiral artery transformation and caused hypoxic injury in uteroplacental tissue. In addition, our laboratory has also generated a hypoxia-induced PE model in IL-10−/− mice. In this model, exposure of IL-10−/− mice to 9.5% oxygen triggered PE-like symptoms and induced trophoblast-specific apoptosis in utero-placental tissue through activation of the proapoptotic cascade of caspase 3. Notably, recombinant IL-10 reversed hypoxia-induced features in pregnant IL-10−/− mice.20 Similar observations have also been reported suggesting that exogenous IL-10 can normalize blood pressure and endothelial function in pregnancy-induced hypertensive rats.25 In these studies, the beneficial effects of IL-10 in pregnant deoxycorticosterone/saline-treated rats were associated with decreased plasma levels of endothelin-1, decreased levels of circulating and placental IFN-γ, as well as decreased aortic and placental expression of platelet-endothelial cell adhesion molecule (PECAM) although the effect on placental angiogenesis and spiral artery remodeling effects remain unclear.25 Similarly, IL-10 deficiency exacerbates Toll-like receptor 3 (TLR3)-induced PE-like symptoms in mice and exogenous IL-10 treatment confers beneficial effects on endothelial function.27 Collectively, these experimental studies suggest that IL-10 deficiency may contribute to poor placentation and induction of vasoactive anti-angiogenic factors. This notion is further supported by data from human studies showing reduced production of IL-10 in placental tissues and serum samples from PE women.61,94–98
Preterm Birth and Recurrent Spontaneous Abortion
Normal term labor involves an increase in the production of various inflammatory mediators by the fetal membranes and myometrium such as IL-1β, IL-6, IL-8, TNF-α, and PGE2, as well as a decrease in local IL-10 expression.1,6,99 Recurrent spontaneous abortion (RSA), affecting about 1–3% of women, is defined as the occurrence of three or more spontaneous abortion of clinically diagnosed pregnancies during early weeks of gestation.1,52 About 50% of RSA cases are of unexplained variety and their etiology remains enigmatic. A major cause of preterm labor is considered to be infection, especially local infection at the maternal-fetal interface. Intrauterine infection may lead to the induction of an overwhelming inflammatory cascade similar to that occurs in normal labor.1 Likewise, immunological factors have been thought to be responsible for the remaining 50% unexplained RSA. Clinical observations have highlighted IL-10 as a pivotal player in the pathological processes of unexplained RSA and preterm birth as numerous studies showed reduced levels of IL-10 production, IL-10-producing cells, and Th1cytokine/IL-10 ratios in PBMCs, decidual and placental tissues and sera from women with RSA.53,100–108 All these data support the notion that perturbation in the balance of pro- and anti-inflammatory cytokine expression as well as deregulation of angiogenesis-associated cytokines are associated with RSA and preterm birth (Figure 1).
Using IL-10−/− mice, we developed pre-clinical models for fetal resorption and preterm birth and found that pregnant IL-10−/− mice, when challenged with low doses of LPS or CpG, ligands for TLR4 and TLR9, respectively, experienced fetal resorption or preterm birth depending on the gestational age–dependent exposure.22,23,48 These adverse pregnancy outcomes were directly associated with activation and amplification of TNF-α–producing uNK cells or macrophages.22,23,48 In these models, IL-10 had a critical role as a vascular protector and anti-inflammatory cytokine for maintaining pregnancy in response to mild or moderate levels of inflammation.2,3 Similar observations were also reported in rat models that administration of IL-10 attenuated LPS-mediated fetal dearth rate and fetal growth restriction.29,30 A recent study provided a supporting evidence for the role of IL-10 in preventing preterm birth as increased IL-10 production occurring via augmented cAMP accumulation contributes to resistance to LPS-induced preterm birth in mice with deficiency in G-protein coupled receptor CB2.109
Our prior studies indicated that preterm labor and delivery in IL-10−/− mice were associated with placental infiltration of cytotoxic uNK cells and placental cell death. Depletion of NK cells or TNF-α neutralization in these mice restored term delivery. Furthermore, TNF-alpha neutralization prevented uNK cell infiltration and placental cell apoptosis. These results suggest that the uNK cell-TNF-α-IL-10 axis plays an important role in the genesis of infection/inflammation-induced preterm labor/delivery.23 Similarly, injection of TLR9 ligand, CpG on gd 6 or gd 14 resulted in fetal resorption or preterm birth in IL-10−/− mice, but not in its wild type counterparts. Thus, our results clearly indicate that IL-10 deficiency coupled with TLR4 or TLR9 activation induces fetal loss and preterm birth.48 Consistent with these observations, nonobese diabetic (NOD) mice, known to be deficient in both Treg cells and other IL-10+ cells, was found to be sensitive to CpG for induction of pregnancy loss.120,111 Adaptive transfer of in vitro 2-amino-2-[2– (4-octylphenyl)ethyl]propane-1,3-diol-induced Treg cells to NOD mice rescued CpG-mediated pregnancy loss via increasing the numbers of decidual Foxp3+ Treg cells and other IL-10-producing cells.26 However, our recent study suggests that although IL-10 functions as a pregnancy-compatible cytokine,48 TLR3-mediated induction of inflammation at the maternal-fetal interface may alter the anti-inflammatory characteristics of this cytokine.61 In this study, we provided evidence for immune programming of fetal loss in response to polyinosinic:polycytidylic acid (polyI:C), a viral mimic and an inducer of inflammatory milieu. In wild type (WT) mice, poly(I:C) treatment induced expansion of NKG2D+ uNK cells and expression of Rae-1 (an NKG2D ligand) on uterine macrophages and led to fetal resorption. In IL-10−/− mice, NKG2D− T cells instead became the source of fetal resorption during the same gestation period. Interestingly, both uterine NK and T cells produced TNF-α as the key cytotoxic factor contributing to fetal loss. Interestingly, polyI:C-treated IL-10−/− mice when supplemented with recombinant IL-10 experienced fetal loss through NKG2D+ uNK cells, similar to the response in WT mice. These results indicate that pregnancy-disrupting inflammatory events mimicked by poly(I:C) are regulated by IL-10 and other inflammatory signals.61
IL-10, Endoplasmic Reticulum (ER) Stress, and Pregnancy
ER is an essential organelle for protein synthesis, folding, post-translational modification (N-linked glycosylation, disulfide bond formation and oligomerization) and secretion. These processes are intricately programmed and rely on the optimum levels of ATP, Ca2+ and an oxidizing environment. Pathological stimuli can interrupt the protein folding process through disturbing metabolic and energy balance, leading to accumulation of misfolded proteins in the ER, a condition termed “ER stress”. These pathological stimuli include factors that cause ER calcium depletion, altered glycocylation, nutrient deprivation, oxidative stress, DNA damage, or energy perturbation.112,113 In order to remove these misfolded proteins, cells evolve a series of evolutionarily conserved signal transduction pathways, collectively referred to as the unfolded protein response (UPR). Upon aggregation of misfolded proteins, binding immunoglobulin protein (BiP) or glucose-regulated protein 78 (GRP78) binds to these misfolded proteins and dissociates from three membrane-bound ER stress sensors, protein kinase R-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). Dissociation of BiP from these stress sensors results in sequential activation of PERK-, ATF6- and IRE1-initiated pathways: i) activated PERK blocks protein translation by phosphorylation of eIF2a (eukaryotic translation initiation factor 2a), ii) activated ATF6 acts as a transciption factor to induce expression of ER-resident chaperones such as BiP, and iii) activated IRE1 splices X-box binding protein 1 (XBP1) mRNA. The spliced XBP1 gene product induces the transcription of different genes involved in the ER-associated degradation pathway. Thus, activation of the UPR machinery leads to restoration of protein homeostasis within ER lumen by: i) inhibiting protein translation to reduce the burden on the folding machinery, ii) generating more ER-resident chaperones to increase the capacity of the UPR machinery, and iii) activating the ER-associated ubiquitin proteasomal degradation pathway to remove accumulated misfolded proteins.112–117 However, if ER stress is persistent and excessive, and ER homeostasis cannot be re-established, the UPR will initiate the mitochondrial cytochrome c-independent apoptotic pathway to eliminate the stressed cells.114,115 Thus, the UPR functions as a cell-protective mechanism as well as a cell-destructive terminator. Notably, there is evidence suggesting that chronic UPR activation leads to an activation of NF-κB and the inflammasome, which promote local inflammation.118
Deficient placental perfusion induces oxidative stress and triggers release of inflammatory cytokines, anti-angiogenetic factors and placental debris, which lead to placental ER stress.119,120 Accumulating evidence suggests that placental ER stress is an important contributor to the pathology of many adverse pregnancy outcomes including early pregnancy loss, impaired placental development, intrauterine growth restriction and early-onset form of PE.121–127 Burton and coworkers recently reported that increased expression levels of many components in the UPR such as phosphorylated IRE1a, ATF6, XBP1 and BiP were observed only in the placenta from the early-onset PE, but not from both the late-onset PE and normotensive controls, suggesting a higher level of the ER stress and UPR activation in the placenta from early-onset PE.126
Intriguingly, IL-10 is emerging as a novel modulator of the ER stress (Figure 2).118,128, Shkoda et al found that intestinal epithelial cells isolated from IL-10−/− mice exhibit increased expression levels of BiP, a prototypic marker for ER stress under conditions of chronic inflammation, suggestive of an increased ER stress in the absence of IL-10.128 ER stress response protein, BiP, like all heat shock protein 70 family members, depletes cellular ATP via tightly binding ATP, leading to inhibition of protein folding in ER and thereby contributing to ER stress.128 In addition, accumulating evidence suggests that BiP independently contributes to the initiation and/or perpetuation of chronic inflammatory processes.128 Further observations revealed that IL-10 inhibited BiP and its miRNA expression via blocking ATF6 nuclear translocation and subsequently preventing ATF6 from binding to the BiP gene promoter.128 Similar observations have been reported in a recent study suggesting that IL-10 attenuates tunicamycin-induced ER stress through suppression of BiP expression and induction of ER proteins that promote correct protein folding as well as enhancement of ER-associated degradation, which are entirely dependent on the activation of STAT1 and STAT3 signaling pathways, partially dependent on the inhibition of ROS, but independent of NF-κB inhibition.118 These studies consistently suggest a novel role for IL-10 in suppressing ER stress in addition to the roles as an anti-inflammatory and vascular factor. Accordingly, placental ER stress induced by IL-10 deficiency may provide an additional mechanism by which IL-10 deficiency coupled with other insults such as hypoxia induces PE-like features as reported in our prior studies.20 Similarly, the mechanism by which exogenous IL-10 rescues LPS- or hypoxia-induced symptoms of adverse pregnancy outcomes observed in our laboratory and others may also be associated with IL-10-mediated alleviation of placental ER stress.20,29,30 Further experiments, however, are warranted to support these speculations.
IL-10 as a Modulator of Autophagy
Autophagy is a process that involves engulfing of large damaged organelles and protein aggregates into a double membrane vesicle termed the autophagosome, fusion of the autophagosome with the lysosome, and finally degradation by lysosomal resident hydrolases.129,130 Autophagy serves as a major cell survival mechanism for orchestrating the sequestration and degradation of damaged organelles and regulating energy and nutrient homostasis.131 A body of evidence has shown that autophagy plays pivotal roles in innate and adaptive immunity both in the direct elimination of intracellular bacteria and in the presentation of endogenously expressed antigens via MHC.131 Autophagy can be induced by numerous stimuli including nutrient deprivation/amino acid starvation, growth factor withdrawal, ER stress, mitochondrial damage as well as by various immune factors including both host- and pathogen-derived molecules such as toll-like receptor ligands and inflammatory cytokines such as IL-1, IL-2, IL-6, TNF-α and IFN-γ.131 In contrast, anti-inflammatory cytokines including IL-4, IL-10 and IL-13 inhibit autophagy. In turn, autophagy itself can regulate the production and function of cytokines, including IL-1, IL-18, TNF-α, and Type I IFN.131 IL-10 has been shown to inhibit autophagy through the PI3K/Akt pathway132 or through the PI3K/Akt and STAT3 pathways in murine macrophages infected with HIV-1.133 A more recent study demonstrates that IL-10 inhibits the autophagic flux in the MRC5 lung fibroblast cells via PI3K/Akt signaling pathway that is independent of starvation.134 Collectively, IL-10 functions as an important negative modulator of autophagy in the context of infection.
Deregulated autophagy has been shown to be associated with brain and cardiac ischemia.135,136 Interestingly, deregulated autophagy is also implicated in fetal growth restriction and PE.137–141 However, the claims about whether autophagy is deficient or enhanced in these diseases are highly controversial. Previous studies indicate that autophagy plays a role in extravillous trophoblast cell invasion and vascular remodeling under physiological hypoxia conditions, and that sEng, an anti-angiogenic factor, inhibits activation of autophagy, suggesting that impaired autophagy is involved in poor placentation in PE.139,140 Incubation of the PBMCs with sera from women with PE or normal pregnancy reveals that autophagy induction increases with gestational age in the cells treated with sera from normotensive women, but not from PE women.141 These results imply that the autophagy activity is increased in normal pregnancy but decreased in PE. In contrast, an independent study showed that increased expression of microtube-associated protein light chain 3 (LC3, a marker for autophagy) was observed in the placenta from severe PE, but not from normal pregnancy, suggesting that increased autophagic activity in the placenta may be implicated in the pathophysiology of PE.138 The mechanisms for this controversy remain to be investigated. However, negative effect of IL-10 on the production of pro-inflammatory cytokines that are capable of inducing autophagy should be considered. While the role of IL-10 as a regulator of autophagy has been studied in lymphocytes and fibroblasts, it is not known whether IL-10 plays a role in the regulation of autophagy at the maternal-fetal interface in the context of adverse pregnancy outcomes.
Conclusions
IL-10 functions not only as a potent immunosuppressive agent but also serves as a vascular protector and modulator of ER stress and autophagy at the maternal-fetal interface. IL-10 is a pivotal player involved in the maternal immune tolerance for survival of an allogeneic fetus. Our work using IL-10−/− mice reveals that IL-10 deficiency coupled with other insults such as hypoxia and inflammatory triggers (i.e. LPS, CpG and polyI:C) contributes to the pathologies of adverse pregnancy outcomes including pregnancy loss, preterm birth and preeclampsia, suggesting a link of IL-10 dysregulation to the anomalies of pregnancy. It is possible that uterine immune cells acquire an inflammatory phenotype and produce inflammatory cytokines in the absence of IL-10. Uterine regulatory T cells, NK cells and macrophages are the primary targets for such a dysregulation. These concepts should be pursued to better understand the causative mechanisms for adverse pregnancy outcomes in humans.
Acknowledgements
This work was supported in part by a grant from NIH P20RR018728.
References
- 1.Murphy SP, Sharma S. IL-10 and pregnancy. In: Gil Mor., editor. Immunology of Pregnancy. NY: Springer Science; 2004. pp. 26–36. [Google Scholar]
- 2.Kalkunte S, Nevers T, Norris WE, Sharma S. Vascular IL-10: a protective role in preeclampsia. J Reprod Immunol. 2011;88(2):165–169. doi: 10.1016/j.jri.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63(6):482–9110. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol. 2014;58(2–3–4):219–229. doi: 10.1387/ijdb.140109ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dealtry GB, O'Farrell MK, Fernandez N. The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol. 2000;123(2):107–119. doi: 10.1159/000024441. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–8110. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the Anti-Inflammatory cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 12.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55(2):241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 13.Tewari N, Kalkunte S, Murray DW, Sharma S. The water channel aquaporin 1 is a novel molecular target of polychlorinated biphenyls for in utero anomalies. J Biol Chem. 2009;284(22):15224–15232. doi: 10.1074/jbc.M808892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta. 2008;29(10):871–878. doi: 10.1016/j.placenta.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunnett CA, Berg DJ, Faraci FM, Feuerstein G. Vascular effects of lipopolysaccharide are enhanced in interleukin-10-deficient mice. Stroke. 1999;30(10):2191–2195. doi: 10.1161/01.str.30.10.2191. [DOI] [PubMed] [Google Scholar]
- 16.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol. 2000;279(4):H1555–H1562. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 17.Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: role of superoxide. Diabetes. 2002;51(6):1931–1937. doi: 10.2337/diabetes.51.6.1931. [DOI] [PubMed] [Google Scholar]
- 18.Gunnett CA, Lund DD, Faraci FM, Heistad DD. Vascular interleukin-10 protects against LPS-induced vasomotor dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(2):H624–H630. doi: 10.1152/ajpheart.01234.2004. [DOI] [PubMed] [Google Scholar]
- 19.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54(3):619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57(3):505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkunte S, Boij R, Norris W, Friedman J, Lai Z, Kurtis J, Lim KH, Padbury JF, Matthiesen L, Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177(5):2387–2398. doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175(6):4084–9010. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200(3):308.e1–308.e9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76(5):738–4810. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- 25.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R713–R719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Liu X, Shan B, Wu J, Sharma S, Sun Y. Prevention of CpG-induced pregnancy disruption by adoptive transfer of in vitro-induced regulatory T cells. PLoS One. 2014;9(4):e94702. doi: 10.1371/journal.pone.0094702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;58(3):489–496. doi: 10.1161/HYPERTENSIONAHA.111.172114. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, Mitchell BM. Cotreatment With Interleukin 4 and Interleukin 10 Modulates Immune Cells and Prevents Hypertension in Pregnant Mice. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu100. in press. [DOI] [PubMed] [Google Scholar]
- 29.Rivera DL, Olister SM, Liu X, Thompson JH, Zhang XJ, Pennline K, Azuero R, Clark DA, Miller MJ. Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 1998;12(2):189–197. doi: 10.1096/fasebj.12.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN, Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol. 2001;98(3):476–480. doi: 10.1016/s0029-7844(01)01424-7. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Stabila J, Pietras L, Singh AR, McGonnigal B, Ernerudh J, Matthiesen L, Padbury JF. Haplotype-dependent differential activation of the human IL-10 gene promoter in macrophages and trophoblasts: implications for placental IL-10 deficiency and pregnancy complications. Am J Reprod Immunol. 2010;64(3):179–187. doi: 10.1111/j.1600-0897.2010.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicinschi LA, Lopez-Carrillo L, Camargo MC, Correa P, Sierra RA, Henry RR, Chen J, Zabaleta J, Piazuelo MB, Schneider BG. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and-10 genes. Int J Cancer. 2006;118(3):649–657. doi: 10.1002/ijc.21364. [DOI] [PubMed] [Google Scholar]
- 33.Lim S, Crawley E, Woo P, Barnes PJ. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- 34.Shin HD, Winkler C, Stephens JL, Bream J, Young H, Goedert JJ, O’Brien T, Vlahov D, Buchbinder S, Giorgi J, Rinaldo C, Donfield S, Willoughby A, O’Brien SJ, Smith MW. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL-10. Proc Natl Acad Sci USA. 2000;97:14467–14472. doi: 10.1073/pnas.97.26.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin MT, Storer B, Martin PJ, Tseng LH, Gooley T, Chen PJ, Hansen JA. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 36.Steinke JW, BarekZi E, Hagman J, Borish L. Functional analysis of-571 IL-10 promoter polymorphism reveals a repressor element controlled by Sp1. J Immunol. 2004;173:3215–3222. doi: 10.4049/jimmunol.173.5.3215. [DOI] [PubMed] [Google Scholar]
- 37.Costa GC, da Costa ROCHA MO, Moreira PR, Menezes CA, Silva MR, Gollob KJ, Dutra WO. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis. 2009;199:451–454. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 38.Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, Nguyen D, Wright KL, Atadja PW, Bhalla K, Pinilla-Ibarz J, Seto E, Sotomayor EM. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10(1):92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gezen-Ak D, Dursun E, Hanağası H, Bilgiç B, Lohman E, Araz ÖS, Atasoy IL, Alaylıoğlu M, Önal B, Gürvit H, Yılmazer S. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer's disease or mild cognitive impairment. J Alzheimers Dis. 2013;37(1):185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 40.Di Bona D, Rizzo C, Bonaventura G, Candore G, Caruso C. Association between interleukin-10 polymorphisms and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2012;29(4):751–759. doi: 10.3233/JAD-2012-111838. [DOI] [PubMed] [Google Scholar]
- 41.Sowmya S, Ramaiah A, Sunitha T, Nallari P, Jyothy A, Venkateshwari A. Role of IL-10 -819(t/c) promoter polymorphism in preeclampsia. Inflammation. 2014;37(4):1022–1027. doi: 10.1007/s10753-014-9824-2. [DOI] [PubMed] [Google Scholar]
- 42.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol. 2005;174(6):3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharabi AB, Aldrich M, Sosic D, Olson EN, Friedman AD, Lee SH, Chen SY. Twist-2 controls myeloid lineage development and function. PLoS Biol. 2008;6(12):e316. doi: 10.1371/journal.pbio.0060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Bosch MW, Palsson-Mcdermott E, Johnson DS, O'Neill LA. LPS induces the degradation of PDCD4 to release Twist2, activating c-Maf transcription to promote IL-10 production. J Biol Chem. 2014 doi: 10.1074/jbc.M114.573089. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann C, Heinrich F, Neumann Katrin, Junghans V, Mashreghi MF, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kühl AA, Heimesaat MM, Esser C, Im SH, Radbruch A, Rutz S, Scheffold A. Role of Blimp-1 in programing Th effector cells into IL-10 producers. JEM. 2014 doi: 10.1084/jem.20131548. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184(2):539–4810. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozörnek MH, Bielfeld P, Krüssel JS, Moustafa M, Mikat-Drozdzynski B, Koldovsky U, Kuhn U. Interferon gamma and interleukin 10 levels in preimplantation embryo culture media. J Assist Reprod Genet. 1995;12(9):590–593. doi: 10.1007/BF02212580. [DOI] [PubMed] [Google Scholar]
- 48.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183(2):1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 50.Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immunomodulators in human choriodecidua. J Clin Endocrinol Metab. 1998;83:4332–4337. doi: 10.1210/jcem.83.12.5335. [DOI] [PubMed] [Google Scholar]
- 51.Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol. 1999;205(1):194–204. doi: 10.1006/dbio.1998.9122. [DOI] [PubMed] [Google Scholar]
- 52.Plevyak M, Hanna N, Mayer S, Murphy S, Pinar H, Fast L, Ekerfelt C, Ernerudh J, Berg G, Matthiesen L, Sharma S. Deficiency of decidual IL-10 in first trimester missed abortion: a lack of correlation with the decidual immune cell profile. Am J Reprod Immunol. 2002;47:242–250. doi: 10.1034/j.1600-0897.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- 53.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, Sharma S. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 54.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27(4–5):445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Szony BJ, Bata-Csörgo Z, Bártfai G, Kemény L, Dobozy A, Kovács L. Interleukin-10 receptors are expressed by basement membrane anchored, alpha(6) integrin(+) cytotrophoblast cells in early human placenta. Mol Hum Reprod. 1999;5:1059–1065. doi: 10.1093/molehr/5.11.1059. [DOI] [PubMed] [Google Scholar]
- 56.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19(6):563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 57.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 58.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 59.Strle K, Zhou JH, Broussard SR, Venters HD, Johnson RW, Freund GG, Dantzer R, Kelley KW. IL-10 promotes survival of microglia without activating Akt. J Neuroimmunol. 2002;122(1–2):9–19. doi: 10.1016/s0165-5728(01)00444-1. [DOI] [PubMed] [Google Scholar]
- 60.Roberts CT, White CA, Wiemer NG, Ramsay A, Roberston SA. Altered placental development in interleukin-10 null mutant mice. Placenta. 2003;17:S94–S99. doi: 10.1053/plac.2002.0949. [DOI] [PubMed] [Google Scholar]
- 61.Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10−/− mice. J Immunol. 2013;190(7):3639–4710. doi: 10.4049/jimmunol.1203488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–1936. [PubMed] [Google Scholar]
- 63.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A Deficiency of Placental IL-10 in Preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 64.Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196:122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 65.Rezaei A, Dabbagh A. T-helper (1) cytokines increase during early pregnancy in women with a history of recurrent spontaneous abortion. Med Sci Monit. 2002;8:CR607–CR610. [PubMed] [Google Scholar]
- 66.Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MM. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49:308–318. doi: 10.1034/j.1600-0897.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 67.Chaouat G, Tranchot Diallo J, Volumenie JL, Menu E, Gras G, Delage G, Mognetti B. Immune suppression and Th1/Th2 balance in pregnancy revisited: a (very) personal tribute to Tom Wegmann. Am. J. Reprod. Immunol. 1997;37:427–434. doi: 10.1111/j.1600-0897.1997.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 68.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003;50:444–452. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 69.Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol. 2008;59(5):425–432. doi: 10.1111/j.1600-0897.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182(7):4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, Wei H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A. 2013;110(3):E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23(6):702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 74.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, Lin QD. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod. 2010;25(10):2591–2596. doi: 10.1093/humrep/deq198. [DOI] [PubMed] [Google Scholar]
- 76.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 77.D'Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, Yagita H, Azuma M, Sayegh MH, Guleria I. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187(9):4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 81.Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernández N, Arck PC. Dendritic cells: key to fetal tolerance? Biol Reprod. 2007;77(4):590–598. doi: 10.1095/biolreprod.107.060632. [DOI] [PubMed] [Google Scholar]
- 82.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17(3):209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 83.Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front Immunol. 2014;28:5, 125. doi: 10.3389/fimmu.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsu P, Santner-Nanan B, Joung S, Peek MJ, Nanan R. Expansion of CD4(+) HLA-G(+) T Cell in human pregnancy is impaired in pre-eclampsia. Am J Reprod Immunol. 2014;71(3):217–228. doi: 10.1111/aji.12195. [DOI] [PubMed] [Google Scholar]
- 85.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31(11):2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kinzenbaw DA, Chu Y, Peña Silva RA, Didion SP, Faraci FM. Interleukin-10 protects against aging-induced endothelial dysfunction. Physiol Rep. 2013;1(6):e00149. doi: 10.1002/phy2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat. Med. 2008;14:855–862. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 88.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br. J. Obstet. Gynaecol. 1977;84(9):656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 89.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 90.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 91.Kalkunte S, Lai Z, Norris WE, Pietras LA, Tewari N, Boij R, Neubeck S, Markert UR, Sharma S. Novel approaches for mechanistic understanding and predicting preeclampsia. J Reprod Immunol. 2009;83(1–2):134–138. doi: 10.1016/j.jri.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalkunte SS, Neubeck S, Norris WE, Cheng SB, Kostadinov S, Vu Hoang D, Ahmed A, von Eggeling F, Shaikh Z, Padbury J, Berg G, Olofsson A, Markert UR, Sharma S. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol. 2013;183(5):1425–1436. doi: 10.1016/j.ajpath.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 94.Wilczyn´ ski JR, Tchorzewski H, Głowacka E, Banasik M, Lewkowicz P, Szpakowski M, Zeman K, Wilczyn´ ski J. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm. 2002;11:105–111. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalantar F, Rajaei S, Heidari AB, Mansouri R, Rashidi N, Izad MH, Mirahmadian M. Serum levels of tumor necrosis factor-α, interleukin-15 and interleukin-10 in patients with pre-eclampsia in comparison with normotensive pregnant women. Iran J Nurs Midwifery Res. 2013;18(6):463–466. [PMC free article] [PubMed] [Google Scholar]
- 96.Peraçoli JC, Bannwart-Castro CF, Romao M, Weel IC, Ribeiro VR, Borges VT, Rudge MV, Witkin SS, Peraçoli MT. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol. 2013;100(2):129–134. doi: 10.1016/j.jri.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL-10, TNF-α & IFN-γ: potential early biomarkers for preeclampsia. Cell Immunol. 2013;283(1–2):70–74. doi: 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Pinheiro MB, Martins-Filho OA, Mota AP, Alpoim PN, Godoi LC, Silveira AC, Teixeira-Carvalho A, Gomes KB, Dusse LM. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine. 2013;62(1):165–173. doi: 10.1016/j.cyto.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 99.Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. 2003;1:122. doi: 10.1186/1477-7827-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127–3310. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin LP, Fan DX, Zhang T, Guo PF, Li DJ. The costimulatory signal upregulation is associated with Th1 bias at the maternal-fetal interface in human miscarriage. Am J Reprod Immunol. 2011;66(4):270–810. doi: 10.1111/j.1600-0897.2011.00997.x. [DOI] [PubMed] [Google Scholar]
- 102.Hanzlikova J, Ulcova-Gallova Z, Malkusova I, Sefrna F, Panzner P. TH1-TH2 response and the atopy risk in patients with reproduction failure. Am J Reprod Immunol. 2009;61(3):213–2010. doi: 10.1111/j.1600-0897.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 103.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4(9):1020–1410. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 104.Fukui A, Kwak-Kim J, Ntrivalas E, Gilman-Sachs A, Lee SK, Beaman K. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil Steril. 2008;89(1):157–6510. doi: 10.1016/j.fertnstert.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Rolle L, Memarzadeh Tehran M, Morell-Garcia A, Raeva Y, Schumacher A, Hartig R, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. 2013;70(6):448–5310. doi: 10.1111/aji.12157. [DOI] [PubMed] [Google Scholar]
- 106.Wang WJ, Hao CF, Lin QD. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol. 2011;92(1–2):97–10210. doi: 10.1016/j.jri.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196(2):122–3010. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 108.Banerjee P, Ghosh S, Dutta M, Subramani E, Khalpada J, Roychoudhury S, Chakravarty B, Chaudhury K. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS One. 2013;8(11):e80940. doi: 10.1371/journal.pone.0080940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun X, Cappelletti M, Li Y, Karp CL, Divanovic S, Dey SK. Cnr2 deficiency confers resistance to inflammation-induced preterm birth in mice. Endocrinology. 2014 doi: 10.1210/en.2014-1387. en20141387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun Y, Qin X, Shan B, Wang W, Zhu Q, Sharma S, Wu J, Lin Y. Differential effects of the CpG-Toll-like receptor 9 axis on pregnancy outcome in nonobese diabetic mice and wild-type controls. Fertil Steril. 2013;99(6):1759–1767. doi: 10.1016/j.fertnstert.2013.01.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang W, Lin Y, Zeng S, Li DJ. Improvement of fertility with adoptive CD25+ natural killer cell transfer in subfertile non-obese diabetic mice. Reprod Biomed Online. 2009;18(1):95–103. doi: 10.1016/s1472-6483(10)60430-0. [DOI] [PubMed] [Google Scholar]
- 112.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cuanalo-Contreras K, Mukherjee A, Soto C. Role of Protein Misfolding and Proteostasis Deficiency in Protein Misfolding Diseases and Aging. Int J Cell Biol. 2013;2013:638083. doi: 10.1155/2013/638083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014;217(1):137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scheper W, Hoozemans JJ. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem. 2009;16(5):615–626. doi: 10.2174/092986709787458506. [DOI] [PubMed] [Google Scholar]
- 116.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 117.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 118.Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, McDonald V, Florin TH, McGuckin MA. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144(2):357–368. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 119.Burton GJ, Yung HW. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. 2011;1(1–2):72–78. doi: 10.1016/j.preghy.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao HJ, Zhu YM, He WH, Liu AX, Dong MY, Jin M, Sheng JZ, Huang HF. Endoplasmic reticulum stress induced by oxidative stress in decidual cells: a possible mechanism of early pregnancy loss. Mol Biol Rep. 2012;39(9):9179–9186. doi: 10.1007/s11033-012-1790-x. [DOI] [PubMed] [Google Scholar]
- 122.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1(1):61–76. doi: 10.1016/s0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- 123.Kawakami T, Yoshimi M, Kadota Y, Inoue M, Sato M, Suzuki S. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.12.008. pii: S0041-008X(13)00561-9. [DOI] [PubMed] [Google Scholar]
- 124.Lian IA, Løset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP, Bjørge L, Freed KA, Moses EK, Austgulen R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32(11):823–829. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173(2):451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yung HW, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones DS, Burton GJ. Differential Activation of Placental Unfolded Protein Response Pathways Implies Heterogeneity in Causation of Early- and Late-onset Pre-eclampsia. J Pathol. 2014 doi: 10.1002/path.4394. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z, Wang H, Xu ZM, Ji YL, Chen YH, Zhang ZH, Zhang C, Meng XH, Zhao M, Xu DX. Cadmium-induced teratogenicity: association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol Appl Pharmacol. 2012;259(2):236–247. doi: 10.1016/j.taap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 128.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132(1):190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 129.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hung TH, Hsieh TT, Chen SF, Li MJ, Yeh YL. Autophagy in the Human Placenta throughout Gestation. PLoS One. 2013;8(12):e83475. doi: 10.1371/journal.pone.0083475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harris J. Autophagy and cytokines. Cytokine. 2011;56(2):140–144. doi: 10.1016/j.cyto.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 132.Park HJ, Lee SJ, Kim SH, Han J, Bae J, Kim SJ, Park CG, Chun T. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol Immunol. 2011;48(4):720–727. doi: 10.1016/j.molimm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 133.Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One. 2010;5(7):e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang L, Zhang H, Qian J, Wang K, Zhu J. Interleukin-10 blocks in vitro replication of human cytomegalovirus by inhibiting the virus-induced autophagy in MRC5 cells. Biochem Biophys Res Commun. 2014;448(4):448–453. doi: 10.1016/j.bbrc.2014.04.120. [DOI] [PubMed] [Google Scholar]
- 135.Liu C, Gao Y, Barrett J, Hu B. Autophagy and protein aggregation after brain ischemia. J Neurochem. 2010;115(1):68–78. doi: 10.1111/j.1471-4159.2010.06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo T, Park Y, Sun X, Liu C, Hu B. Protein misfolding, aggregation, and autophagy after brain ischemia. Transl Stroke Res. 2013;4(6):581–588. doi: 10.1007/s12975-013-0299-5. [DOI] [PubMed] [Google Scholar]
- 137.Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PLoS One. 2012;7(7):e40957. doi: 10.1371/journal.pone.0040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(9):912–920. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- 139.Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 2013;S0165–0378(13) doi: 10.1016/j.jri.2013.06.002. 00078-8. [DOI] [PubMed] [Google Scholar]
- 140.Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, Nikaido T, Okamoto A, Yoshimori T, Saito S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9(3):303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kanninen T, Jayaram A, Jaffe Lifshitz S, Witkin S. Altered autophagy induction by sera from pregnant women with pre-eclampsia: a case-control study. BJOG. 2014;121(8):958–964. doi: 10.1111/1471-0528.12755. [DOI] [PubMed] [Google Scholar]