Abstract
We report a case of metastatic papillary thyroid carcinoma and undifferentiated nonkeratinizing nasopharyngeal carcinoma to the same cervical lymph node following chemotherapy for mantle cell lymphoma. Total thyroidectomy, right cervical nodal dissection, radioactive iodine-131 therapy and radiotherapy to the nasopharynx and the neck resulted in remission of both tumors. No recurrence was noted in follow-up for 48 months.
Keywords: Mantle cell lymphoma, Lymph node, Papillary thyroid cancer, Nasopharyngeal undifferentiated carcinoma
As far as we know, concurrent occurrence of carcinomas in thyroid and nasopharynx with metastases has not been reported. Here we report a case of a 36-year-old male with synchronous thyroid papillary carcinoma and undifferentiated nonkeratinizing nasopharyngeal carcinoma with metastases to the same lymph node, 39 months following chemotherapy for mantle cell lymphoma.
Case Presentation
A 36-year-old male Chinese patient first presented with a 1.0 × 1.0 cm left cervical mass, 38.5 °C, WBC11.8 × 109/L, antibiotic treatment, 3 days after clinical signs and symptoms return to normal. 12 months later, bilateral cervical lymphadenopathy was noted, the largest on the left side of 5.0 × 4.0 cm. Initial left cervical lymph node biopsy showed extensive infiltration of small to medium-size lymphocytes (Fig. 1) with the following immunohistochemical staining pattern usually observed in mantle cell lymphoma: LCA (+), CD79a (+), CD20 (+), CD43 (+), Bcl-2 (+), Cyclin D1 (weak +), CD5 (+), CD10 (−), CD3 (−), CD45RO (−), tdt (−), ALK (−), and Bcl-6 (−). Clinically the lymphoma was staged as 1A. Six cycles of chemotherapy with CHOP regimen (Cyclophosphamide 750 mg/m2, Adriamycin 50 mg/m2, Vincristine 1.4 mg/m2 and Prednisone 60 mg/m2 daily for 5 days) were given with good response. 39 months later, however, masses were noted in the midline of the anterior neck, most likely located in the right lobe of the thyroid, together with symptoms of tinnitus, hoarseness of voice and epistaxis. Cervical CT scan revealed an 8.0 × 7.5 cm thyroid mass with metastases in the right cervical lymph nodes. Total thyroidectomy and right cervical lymph node dissection were performed. Histologic examination of the thyroid carcinoma (PTC) revealed follicular and papillary structures, psammoma bodies, ground-glass like nuclei, nuclear grooves, intra-nuclear pseudo inclusion bodies, and overlapping nuclei, together with focal squamous metaplasia (Fig. 2). 18 out of 22 right cervical lymph nodes had metastases from PTC. One of the lymph nodes showed both PTC (Figs. 3, 4, large arrow) and undifferentiated nonkeratinizing carcinoma (UNC) (Figs. 3, 4, small arrow). The lymph node capsule was intact, PTC and UNC were present in some of the region, and the fibers with a processing cleft artifact could be seen in both of them. The PTC and most of the metastases in the right cervical lymph nodes were positive for CK7, CK19, GST, thyroglobulin, TTF-1, ret oncoprotein and Galectin-3. The metastatic UNC was negative for these antibodies and calretinin, but positive for CK5/6, CK8/18 and EMA immunostains. PTC with metastases in cervical lymph nodes was diagnosed on histologic examination. In addition, one of the lymph nodes showed both metastatic PTC and UNC. Radioactive thyroid I131 imaging revealed no thyroid tissue in other parts of the body. Magnetic resonance imaging (MRI) of the nasopharynx was recommended. MRI scan showed thickening of the right nasopharyngeal mucosa with a 1.7 × 1.5 cm soft tissue mass and small lymph nodes in parapharyngeal spaces and posterior cervical triangle. There was no invasion of the base of the skull (Fig. 5). Biopsy of the right nasopharynx showed UNC (Fig. 6), similar in morphology to the metastatic carcinoma in the lymph node and positive for CK5/6, CK8/18, and EMA immunostains. The tumor cells were negative for EBV small RNA (EBER1) and EBV oncogene latent membrane protein-1 (EBV LMP-1, PNA ISH Detection Kit, Dako Cytomation, Denmark). The thyroid carcinoma was staged as T3N1M0, Stage 1 (by AJCC in 2002). Postoperatively, radioactive iodine-131 therapy was administered twice, followed by oral thyroxine therapy. For the nasopharyngeal carcinoma (T1N2M0, stage 3, AJCC), 6 MV-X ray radiotherapy with dosage of 70 Gy/30 sessions over 7 weeks to nasopharynx, and 60 Gy/30 sessions over 6 weeks to metastatic cervical lymph nodes was initiated. Complete remission was noted after radiotherapy. Follow-up examination every 3 months showed no recurrence or metastases 48 months after diagnosis of nasopharyngeal carcinoma.
Fig. 1.
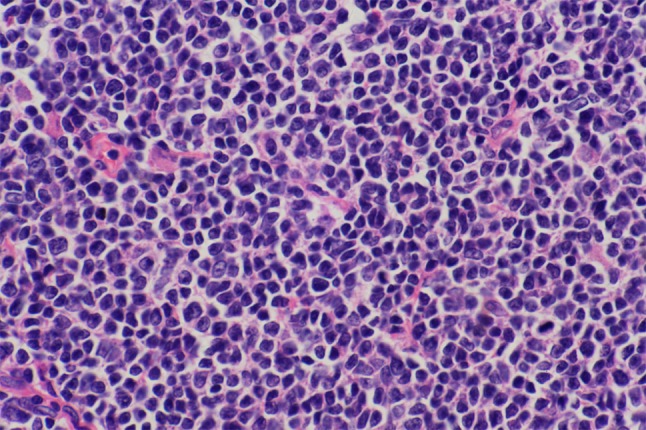
Diffusely distributed cells of mantle cell lymphoma. H&E × 400
Fig. 2.
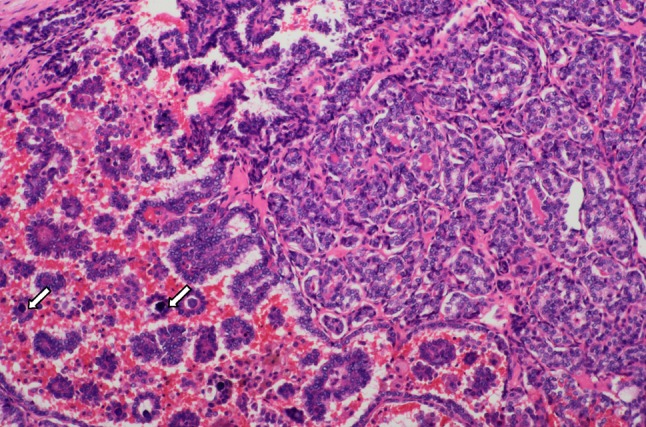
Thyroid carcinoma with follicular and papillary structures, psammoma bodies (arrow), ground-glass like nuclei, nuclear grooves, intra-nuclear pseudo inclusion bodies and overlapping nuclei. H&E × 100
Fig. 3.
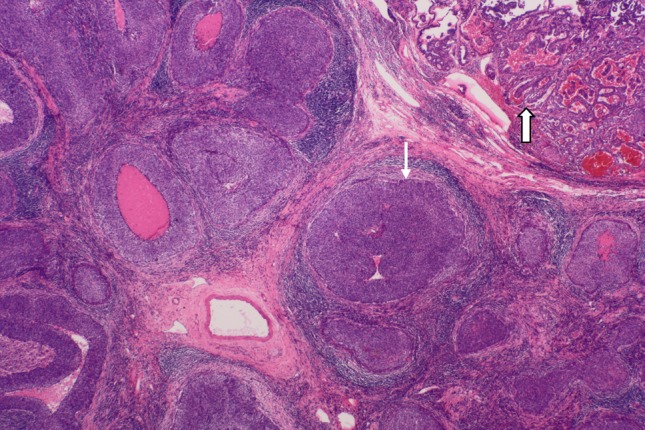
Mantle cell lymphoma after chemotherapy cured, lymphoid follicles collapse, lymphatic sinus atrophy, fibrosis. The remnants of Lymphoid tissue and reactive hyperplasia of fibrous tissue appear around UNC (small arrow) and PTC (large arrow). H&E × 20
Fig. 4.
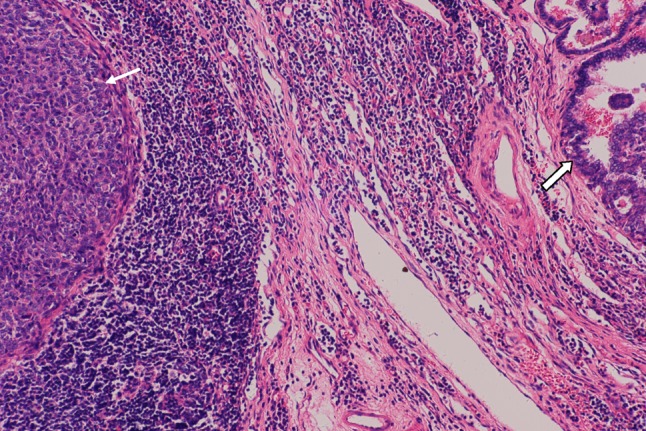
The remnants of lymphocytes and a small amount of fibrous tissue hyperplasia present between UNC (small arrow) and PTC (large arrow). There is collapse of lymphoid follicles beside the undifferentiated carcinoma. H&E × 100
Fig. 5.
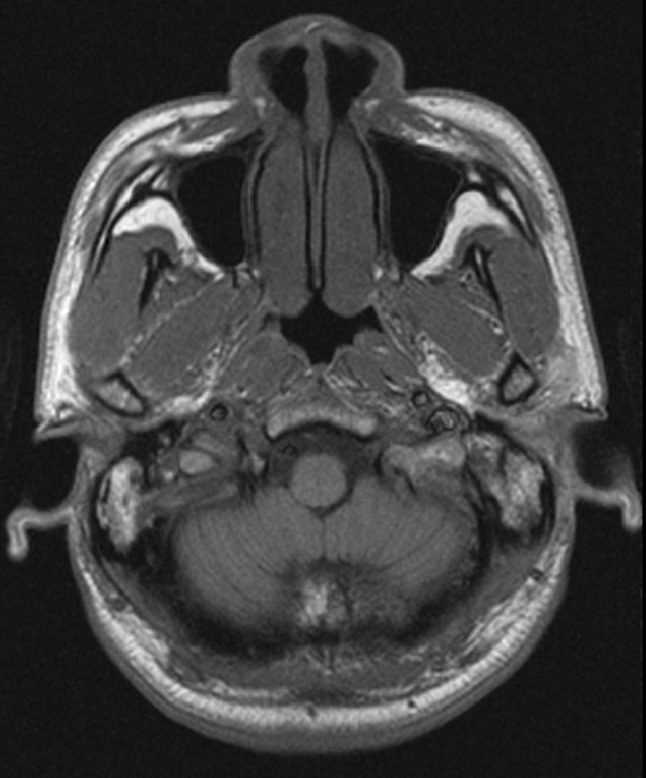
MRI image of right nasopharyngeal area with 17 × 15 mm opacity in the right posterior nasopharyngeal wall, bulging towards posterior nasal cavity and no visualization of opening of the Eustachian tube
Fig. 6.
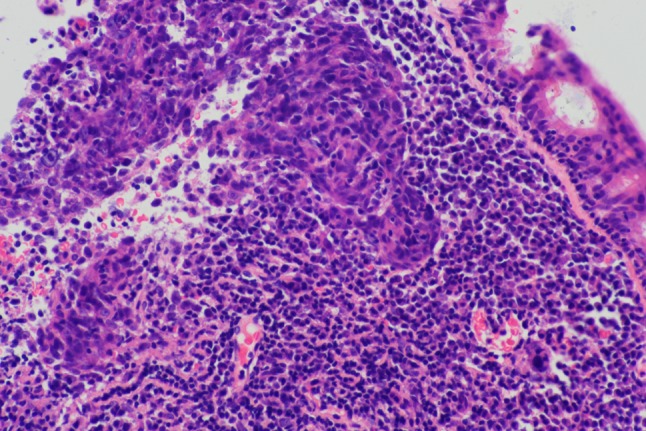
Nasopharyngeal biopsy with undifferentiated nonkeratinizing squamous carcinoma. H&E × 100
Discussion
Mantle cell lymphoma is a relatively rare lymphoma [1] and is generally associated with poor prognosis. Thyroid carcinoma is the most common endocrine malignancy and accounts for about 1 % of all cancers [2]. Nasopharyngeal carcinoma is a fairly common malignant tumor in South China, particularly in Guangdong province [3], with an incidence of 10–20 cases per 100,000 in the male population, and presenting mostly with enlarged cervical nodules [3–5]. Among all metastatic cancers in cervical lymph nodes, 1 % to 9 % are of unknown origin [6, 7]. When both adenocarcinoma and squamous carcinoma appear in the same lymph node, it is very difficult to determine their histological origins. The squamoid focus in the lymph node in question could potentially represent (1) mesothelial nests in the lymph node, (2) squamous metaplasia accompanying metastatic papillary carcinoma, or (3) separate synchronous metastasis from nasopharyngeal carcinoma. As these cells were negative for calretinin immunostain and as the nasopharyngeal carcinoma and the metastatic undifferentiated carcinoma in the lymph node had similar morphology and immunohistochemical staining pattern, we concluded that the metastatic focus in the lymph node was of nasopharyngeal origin. Synchronous occurrence of thyroid carcinoma and nasopharyngeal carcinoma with metastases in the same lymph node following chemotherapy for mantle cell lymphoma was extremely rare. Search of available literature showed one case report of nasopharyngeal carcinoma metastasizing to normal thyroid [8], but no reported case of synchronous thyroid and nasopharyngeal carcinomas metastasizing to lymph node. Since there was no family history of malignancy in this patient, we surmised that chemotherapy to the mantle cell lymphoma may have altered the immunologic response in this patient to allow subsequent development of two different carcinomas.
Acknowledgments
We want to thank Fred Bogott, M.D. and Ph.D., at Austin Medical Center, Austin of Minnesota, USA, for his excellent English editing of this manuscript.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 229–323. [Google Scholar]
- 2.Tang K-T, Lee C-H. RRAF mutation in papillary thyroid carcinoma: pathogenetic role and clinical implications. J Chin Med Assoc. 2010;73(3):113–128. doi: 10.1016/S1726-4901(10)70025-3. [DOI] [PubMed] [Google Scholar]
- 3.Chang ET, Adami H. The enigmatic epidemiology of Nasopharyngeal carcinoma. Cancer Epidermiol Biomakers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 4.Sham JST, Choy D, Wei WI. Nasopharyngeal carcinoma: orderly neck node spread. Int J Radiat Oncol Biol Phys. 1990;19:929–933. doi: 10.1016/0360-3016(90)90014-B. [DOI] [PubMed] [Google Scholar]
- 5.Lee AWM, Pooh YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- 6.Lefevre JL, Coche-Dequeant B, Van Ton J. Cervical lymph nodes from unknown primary tumor in 190 patients. Am J Surg. 1990;160:443–446. doi: 10.1016/S0002-9610(05)80562-8. [DOI] [PubMed] [Google Scholar]
- 7.Medini E, Medini A, Lee CKK, et al. The management of metastatic squamous cell carcinoma in cervical lymph nodes from an unknown primary. Am J Clin Oncol. 1998;21(2):121–125. doi: 10.1097/00000421-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Jalaludin MA, Rajadurai P, Va R, Prasad U. Thyroid metastasis from nasopharyngeal carcinoma: a case report. J Laryngol Otol. 1994;108(10):886–888. doi: 10.1017/S0022215100128415. [DOI] [PubMed] [Google Scholar]


