Abstract
Metastatic carcinoma to cervical lymph nodes presenting as an unknown primary is quite common. In most cases, the primary site is ultimately identified. Carcinomas that remain of unknown primary after a thorough search are uncommon. This review will focus on those cases that initially present as unknown primaries, since this is the setting in which pathologists first encounter these cases and in which they play an important role in guiding patient management. Most are squamous cell carcinomas, the majority of which are human papillomavirus (HPV)-related and originate in the palatine tonsils and base of tongue. HPV-related oropharyngeal squamous cell carcinomas are increasing in incidence and have unique clinical and pathologic features that make them particularly likely to present as an unknown primary. Understanding these features has led to improved detection of the primary tumors. Further, even when the primary tumor is not found, prognosis is very dependent on characterization of the tumor HPV status. Papillary thyroid carcinomas may also initially present without a known or clinically detectable primary, either as a neck mass or incidentally in a neck dissection performed for another indication. The latter is a very indolent disease. Finally, primary salivary gland carcinomas may mimic an unknown primary and need to be distinguished from cutaneous metastases to the parotid gland, which may present without a recognized skin tumor. Here, we review the clinical and pathologic features of these entities and provide a systematic approach to their diagnosis.
Keywords: Carcinoma of unknown primary, Squamous cell carcinoma, Head and neck, Human papillomavirus, Thyroid, Salivary
Introduction
It is not uncommon for patients to present with a neck mass in the absence of any other known primary tumor. The clinical differential diagnosis may include both benign lesions and malignant neoplasms, the latter including either primary tumors or metastases. A subset of these patients will, in fact, have metastatic carcinoma to a neck lymph node even in the absence of any known primary malignancy. This is often referred to as carcinoma of unknown primary (CUP). Cases that initially present as CUP account for 5–10 % of head and neck cancers and approximately 75 % or more of these are squamous cell carcinomas (SCCs) [1, 2]. When strictly defined, “true” CUP refers to metastases for which the primary tumor cannot be located after an exhaustive clinical, radiographic and surgical evaluation has been performed, the latter often including biopsies and/or resection of suspected primary sites. Fortunately, for the majority of patients who initially present with metastasis of an unknown or occult primary site, the primary tumor can eventually be located. “True” CUP accounts for only 1–2 % of head and neck cancers and may be decreasing due to improved detection methods [2].
Here, we will more broadly consider cases that initially present as CUP, rather than “true” CUP, because this is the scenario in which the pathologist often plays an important role in guiding patient management. When faced with such a case, it is helpful to have a consistent approach to arrive at the correct diagnosis and to report clinically relevant information. The aim is to review the clinical and pathologic features of head and neck CUP and consider the various entities that enter into the differential diagnosis. While the majority of CUPs are SCCs, many of which are human papillomavirus (HPV)-related, other tumor types including thyroid and salivary carcinomas will also be discussed.
General Approach to Carcinoma of Unknown Primary (CUP)
When examining a potential CUP case, it is useful to ask several questions: Is the lesion truly malignant? If so, is it a carcinoma (i.e. what non-epithelial entities are in the differential diagnosis)? What kind of carcinoma is it? Is it possible that it is not a metastasis but rather that the neck is the primary site? If a metastasis is favored, what is the most likely primary site? What histologic or ancillary studies (immunohistochemistry or molecular testing) are prognostic? These questions will be addressed more specifically in the sections below.
Once a potential CUP case is identified, the lymph node group involved can help point to the most likely site of tumor origin. Common patterns of lymph node metastases in the head and neck are summarized in Table 1. Oropharyngeal SCCs, including those that are HPV-related, usually metastasize to levels II and III, and sometimes also level IV, but tumors from several other head and neck sites (oral cavity, larynx, hypopharynx and nasopharynx) may also spread to these nodes. Retropharyngeal or level V lymph node metastases should raise concern for a nasopharyngeal primary. It is important to remember that level IIB is close to the tail of the parotid gland from which salivary gland tumors can arise. Cutaneous metastases to parotid lymph nodes are also not uncommon. Thyroid carcinomas most frequently spread to the central compartment (level VI) but may also involve lateral neck, supraclavicular and upper mediastinal nodes. Metastases in supraclavicular lymph nodes should raise suspicion for a primary site outside the head and neck (such as lung, breast, esophagus, and genitourinary tract). Those in the left supraclavicular region (so-called “Virchow’s node”), in particular, should raise suspicion for a primary site below the diaphragm [3]. These non-head and neck metastases will not be considered in detail here.
Table 1.
Common patterns of lymph node metastasis in the head and neck
| Nodal group | Primary tumor sites |
|---|---|
| Level IA (submental) | Anterior oral cavity, lower lip |
| Level IB (submandibular) | Oral cavity, anterior nasal cavity, submandibular gland, midfacial face skin |
| Level II (upper jugular) | Oropharynx, oral cavity, nasopharynx, nasal cavity, larynx, hypopharynx |
| Level III (mid jugular) | Oropharynx, oral cavity, nasopharynx, larynx, hypopharynx |
| Level IV (lower jugular) | Oropharynx, larynx, hypopharynx, upper esophagus, thyroid |
| Level V (posterior triangle) | Nasopharynx, posterior scalp skin, thyroid |
| Level VI (anterior compartment) | Thyroid, larynx, hypopharynx, upper esophagus |
| Supraclavicular | Non-head and neck, thyroid |
| Retropharyngeal | Nasopharynx, posterior pharynx |
| Parotid | Lateral/upper facial and scalp skin, parotid gland |
Squamous Cell Carcinoma of Unknown Primary in the Era of Human Papillomavirus
By far the most common site of origin of head and neck SCCs that initially present as an unknown primary is the oropharynx (specifically the crypt epithelium of the palatine tonsils and base of tongue or lingual tonsil), where approximately 90 % of detectable primary tumors are ultimately found [4]. The nasopharynx and hypopharynx (pyriform sinus) are other less common ‘hot spots’ for occult primary tumors. High rates of transcriptionally-active HPV (~80 %) have been detected in those tumors for which an occult primary is eventually discovered in the oropharynx [5]. In addition, up to one-third of metastatic head and neck SCCs where the primary site remained unknown after a thorough diagnostic workup (“true” CUP) are HPV-related [6, 7]. It is presumed that many HPV-related “true” CUP cases also originated in the palatine tonsils or base of tongue, where the majority of HPV-related SCCs arise, but were either such small tumors that they were not sampled or detected histologically on biopsy or resection of tissue from these sites, or that the primary tumors simply regressed.
The relatively high prevalence of transcriptionally-active HPV among SCC CUP cases is multifactorial. One contributing factor is the rising incidence of HPV-related SCC in general. The vast majority of head and neck HPV-related SCCs arise in the oropharynx and the incidence of HPV-related oropharyngeal cancer has increased dramatically (225 %) over the past several decades, while HPV-negative oropharyngeal cancers have been on the decline [8]. In addition, HPV-related oropharyngeal SCCs frequently metastasize early in the course of disease when the primary tumor is still quite small (Fig. 1) [9]. In some cases, the primary tumor may be so small as to have an ‘in situ’ appearance along the tonsillar crypt epithelium without classical features of stromal invasion. The small size of the primary tumor combined with their predilection for the tonsillar crypts, which are deep invaginations of the surface epithelium that are not visible on clinical exam, make these primary tumors especially difficult to detect. Therefore, it is not uncommon for a patient to present with a neck metastasis and a small, occult primary tumor that is HPV-related and located deep in the oropharyngeal tonsillar crypts. In fact, Weiss et al. [10] found that 34 % of HPV-related oropharyngeal SCCs initially present as CUP, which is a much rate higher than for head and neck cancers overall (5–10 %) [1].
Fig. 1.
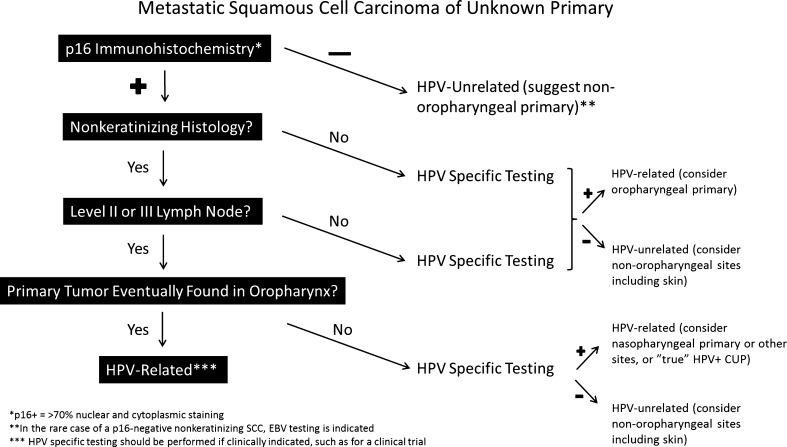
Small, occult HPV-related NK SCC arising in a tonsillar crypt. On low power, the tumor is difficult to appreciate (circled in red) and does not involve the surface epithelium (lower right) (a). On higher magnification, nests of tumor cells with virtually no stromal desmoplasia extend from a tonsillar crypt and blend in with the surrounding lymphoid tissue (b)
Even though early metastases are common, the prognosis of HPV-related oropharyngeal SCC is generally favorable [11]. The reason for this unique tumor biology (early metastasis, yet favorable prognosis) is not entirely clear. One theory is that because the specialized reticulated epithelium of the tonsillar crypts has a discontinuous basement membrane and contains small, intraepithelial blood vessels, carcinomas that arise from this epithelium are capable of metastasizing very early on without actually acquiring genetic alterations necessary to invade the submucosa [12]. For this reason, we do not diagnosis ‘carcinoma in situ’in the tonsillar crypts as even early, ‘in situ’-appearing lesions may still have metastatic potential. Rather, these are simply diagnosed as “squamous cell carcinoma” without commenting on the presence or absence of invasion.
Human papillomavirus (HPV)-related oropharyngeal SCCs and their metastases have unique morphologic features that are important to recognize, especially in the setting of an unknown primary, as they can help point to the most likely primary sites (specifically the palatine tonsil and base of tongue) (Fig. 2a). Most are nonkeratinizing (NK) and typically have a ‘ribbon-like’ growth pattern with large nests that have smooth edges and minimal stromal desmoplasia. The individual tumor cells are usually oval to spindled with indistinct cell borders, high nuclear to cytoplasmic ratios and indistinct nucleoli. Mitotic activity is brisk. Limited keratinization may be present, either at the center or periphery of tumor nests. In some cases, the tumors are truly basaloid with a ‘jigsaw puzzle’ pattern (where the tumor nests mold to each other) and abrupt keratinization, features indistinguishable from non-HPV-related basaloid SCC of other head and neck sites [13] (Fig. 2b). Less commonly, the histology may be undifferentiated or of another characterized variant of SCC. A minority of HPV-related SCCs (~5 %) are of the keratinizing (K) type, but overall most K SCCs (85 %) are not HPV-related [9]. Another histologic feature commonly seen in metastases from HPV-related SCCs is cystic degeneration. An extensively cystic lymph node metastasis is a strong predictor of a tonsil or base of tongue primary SCC with up to 85 % originating at these sites and a similar number being HPV-positive [14].
Fig. 2.

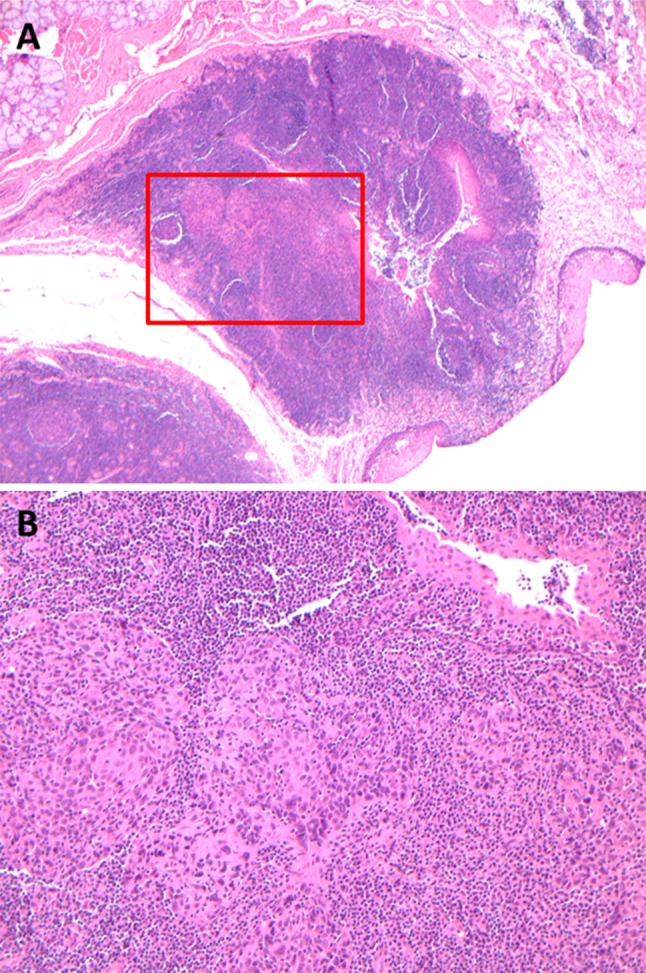
HPV-related oropharyngeal SCC is usually NK (a) but in some cases may be basaloid (b), or have mixed histologic features. NK SCC has broad nests of tumor cells that have oval to spindled nuclei, high N:C ratios and indistinct cell borders (a). Squamous maturation is minimal. Mitotic activity is brisk. Basaloid SCC has a ‘jigsaw puzzle’ pattern, stromal hyalinization and more round nuclei but also has high N:C ratios and frequent mitoses, squamous maturation is typically focal and abrupt (b)
It is important to remember that HPV-related SCC can occur, although much less commonly, at other head and neck sites. When faced with an HPV-related unknown primary, the nasopharynx in particular should also be considered as a site of origin, if the primary is not found in the oropharynx. A subset of nasopharyngeal SCC is HPV-related with similar NK or undifferentiated histology and may also present with an occult primary [15].
The differential diagnosis for metastatic HPV-related SCC is relatively broad. Epstein-Barr virus (EBV)-related nasopharyngeal carcinoma can appear similar, as NK or undifferentiated histology can be seen in both HPV and EBV-related tumors [16]. This can be resolved with viral testing (see below). Salivary gland tumors such as basal cell adenocarcinoma and adenoid cystic carcinoma can enter the differential diagnosis of an HPV-related NK or basaloid SCC. However, metastatic HPV-related SCCs are usually not in close proximity to salivary glands and have a higher mitotic rate. Further, adenoid cystic carcinoma and basal cell adenocarcinoma essentially never present as CUP in the neck lymph nodes. Benign branchial cleft cysts often have a similar clinical presentation to HPV-related CUP, namely as a cystic lateral neck mass. The histology also overlaps at least in the sense that branchial cleft cysts are squamous-lined and can be located within lymph nodes [17]. Likewise, metastatic HPV-related SCC can also be cystic and deceptively bland. However, SCC has areas along the cyst lining that show loss of maturation, nuclear atypia and mitotic activity—features not seen in a branchial cleft cyst (Fig. 3). p16 immunohistochemistry (IHC) can be useful to differentiate the two, as branchial cleft cysts show patchy and weaker staining rather than the strong and diffuse staining pattern seen in HPV-related SCC [18]. Non-epithelial tumors with spindle cell morphology, such as melanoma and follicular dendritic cell sarcoma, should also be considered in the differential diagnosis of HPV-related NK SCC, which often has spindle cells and little evidence of squamous maturation. Although rare, follicular dendritic cell sarcoma most commonly arises in neck lymph nodes and can be mistaken for a metastasis even though it is a primary tumor. However, follicular dendritic cell sarcoma usually has a fascicular or whorled growth pattern that is uncommon in SCC, and much less mitotic activity. Further, NK SCC will usually have a nested growth pattern that is usually lacking in follicular dendritic cell sarcoma and spindle cell melanoma. Epithelioid angiosarcoma and lymphoma may enter the differential diagnosis of poorly-differentiated K SCC and undifferentiated SCC, respectively.
Fig. 3.
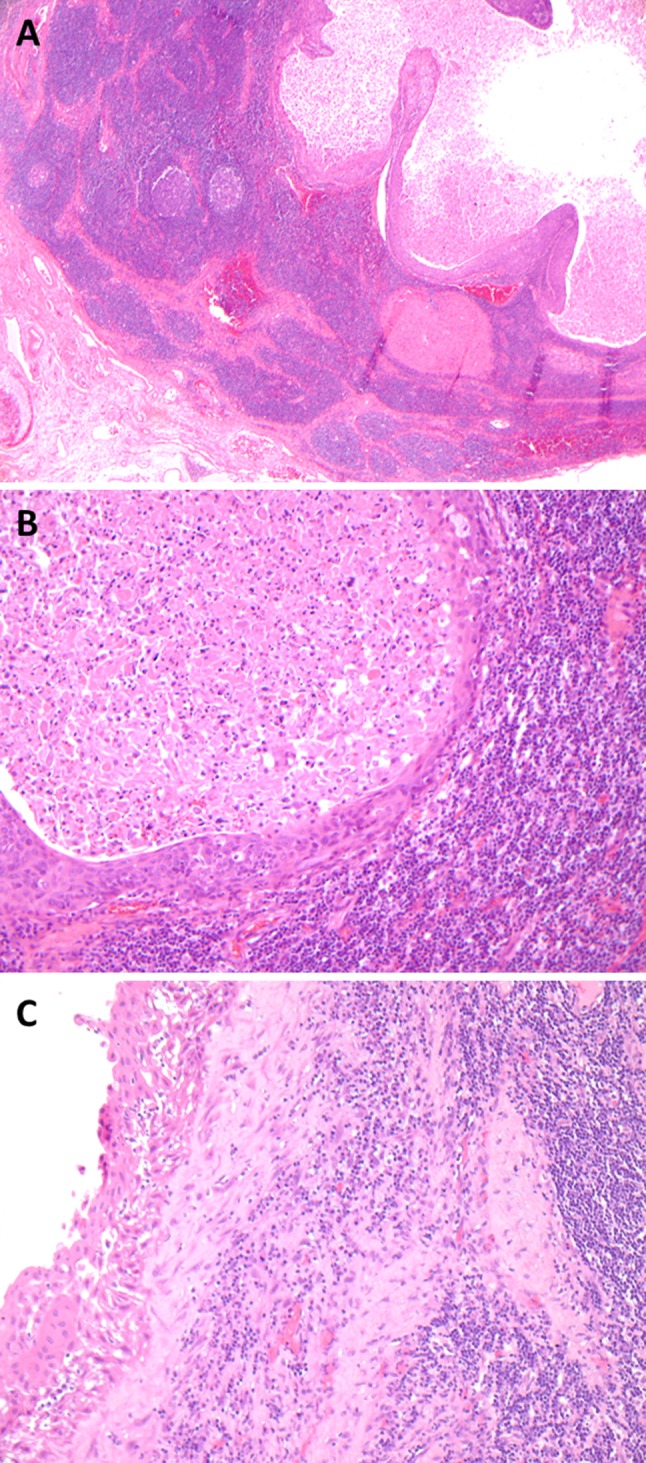
Metastatic HPV-related NK SCC versus a benign branchial cleft cyst. On low power, a cystic metastasis consists of a simple intranodal cyst with a slightly thickened lining (a). On higher power, a lack of maturation towards the surface and nuclear atypia are helpful features to diagnose NK SCC (b). Within the center of the cystic metastasis are numerous maturing and degenerating squamous cells that may mimic K SCC and be p16 negative on FNA. Branchial cleft cysts, like cystic metastases, have a squamous lining and are surrounded by lymphoid tissue that in some cases is a lymph node. The squamous lining may show reactive atypia, (c) but still matures towards the surface
Human papillomavirus (HPV) specific and/or surrogate marker (p16) testing is useful in metastatic SCC of unknown primary. Positivity for transcriptionally-active high risk HPV (but not simply HPV DNA alone) favors an oropharyngeal primary, indicates a better prognosis and may be necessary to stratify patients for clinical trials [19]. Strong and diffuse, nuclear and cytoplasmic p16 staining by IHC is an excellent surrogate marker for SCCs harboring transcriptionally-active virus (“HPV-related” SCC) when the primary site is oropharyngeal and is a well-established standalone prognostic biomarker in oropharyngeal SCC [20]. It is also easy to perform and interpret. However, outside of the oropharynx, p16 overexpression loses specificity for transcriptionally-active HPV among head and neck SCCs [21]. In addition, approximately 20 % of aggressive cutaneous head and neck SCCs may show diffuse p16 positivity that is unrelated to high-risk HPV, including in their lymph node metastases [22]. Currently, there is no universally accepted strategy for HPV testing of unknown primaries. However, we recommend an approach detailed in Fig. 4, in which p16 IHC is performed on all SCC CUP cases with a cutoff of 70 % nuclear and cytoplasmic positivity. A positive p16 IHC result should at least be followed by HPV specific testing (ISH—in situ hybridization or PCR) if: (1) features are not typical of an oropharyngeal primary (i.e. metastasis in lymph node group outside levels II and III or histology other than NK), (2) a primary is subsequently found outside the tonsils/base of tongue or is not found at all (“true” CUP), or (3) HPV-specific testing is otherwise clinically indicated (such as for entry into a clinical trial). Furthermore, if the histology is NK or undifferentiated and p16/HPV testing is negative, EBER ISH for EBV should also be performed, since EBV-related nasopharyngeal carcinoma can have similar morphology and rarely presents as CUP in the neck.
Fig. 4.
Recommended algorithm for p16/HPV testing of SCC CUP on surgical specimens
It is important to note that metastatic SCC of unknown primary is often initially encountered on fine needle aspiration (FNA) biopsy of a neck mass. NK features typical of HPV-related oropharyngeal SCC have been described on FNA [23]. These include cohesive clusters of cells that are relatively monomorphic and have high nuclear to cytoplasmic ratios. Densely eosinophilic cytoplasm characteristic of K SCC is absent or sparse. However, FNA classification as NK or K does not always correlate with histology [23]. This may be due to a subset of poorly differentiated K SCCs lacking overtly keratinized cells and thus mimicking an NK SCC. In addition, the central component of a cystic metastasis usually contains maturing and degenerating squamous cells even if the overall histology is NK (Fig. 3b). Therefore, if the center of a cystic lesion is sampled on FNA, the sampled cells may have a keratinizing appearance, even in an HPV-related NK SCC. In general, we recommend avoiding use of the classifiers of K or NK in FNAs of SCC. p16 IHC may also be problematic on FNA material and should not be relied upon for determining HPV status in an SCC. p16 staining is generally more focal and patchy when performed on cell blocks of aspirated material and a cutoff for determining positivity has not been established. We have also encountered a few cases of HPV-related oropharyngeal SCCs that were entirely p16 negative on FNA but were p16 positive on resection. These discrepant cases are likely due to inadequate cellularity or sampling of the central portion of cystic metastases such that the aspirates consisted mainly of maturing and/or degenerating squamous cells, which are often p16 negative even when the majority of the tumor is p16 positive. HPV-specific testing such as ISH on cell blocks or ethanol-fixed smears may be a better approach. One newer technique that appears promising is the use of liquid phase HPV assays on aspirated fluid from lymph nodes that are currently routinely used in cervical cytology [24]. Liquid phase assays are already widely available and avoid the need for a cell block as the aspirate is placed directly into liquid media. Alternatively, material can also be obtained by scraping cells from stored slides. When combined with cytologic evaluation to confirm the presence of tumor cells, accuracy was 100 % for high-risk HPV detection by the hybrid-capture 2 liquid phase assay in one study, using p16 IHC and DNA ISH on corresponding resected specimens as controls [24].
Once the diagnosis of metastatic SCC of unknown primary is established, usually by FNA and after clinical examination of the skin and head and neck mucosal surfaces, a more thorough search for the primary is undertaken. Unfortunately, imaging lacks sensitivity and specificity for small occult primary tumors. PET/CT scans are more sensitive than other imaging modalities but are associated with a high false positive rate due to physiologic uptake of FDG in lymphoid tissue of the tonsils and base of tongue that is frequently asymmetric [25, 26]. Therefore, surgical evaluation of the most likely primary sites is important. Although HPV status may be taken into consideration, the location of the metastasis is the main factor used to determine the most likely primary sites.
The traditional clinical approach is to perform an exam under anesthesia with panendoscopy, biopsy and frozen section evaluation of suspicious lesions or, if no lesions are found, random biopsies from the most likely primary sites. If a primary tumor is not found by this initial approach, for patients with level II or III lymph node metastases, ipsilateral tonsillectomy and base of tongue resection, by either transoral robotic surgery (TORS) or transoral laser surgery (TLM), is associated with a much higher rate of primary tumor detection and is now standard at many institutions. Reported primary tumor detection rates are up to 94 % for TLM (compared to 25 % for a traditional panendoscopic approach) and 77.3 % for TORS [26, 27]. Some advocate bilateral tonsillectomy, since occult primary SCC may be found in the contralateral tonsil in a minority of cases. The true incidence of contralateral tonsillar SCC in cervical CUP has not been established, however, in one study it accounted for 10 % of cases [28]. In practical terms, bilateral tonsillectomy adds little morbidity and also eliminates uptake from normal tonsillar tissue on post-treatment surveillance imaging, which may be mistaken for recurrent disease. The specimens received from these surgeries (tonsillectomy or base of tongue resection) should be entirely submitted in order to histologically evaluate all tissue for occult carcinoma. p16 IHC can be helpful to highlight small foci of SCC; but the expression must be strong and diffuse, as normal tonsillar crypt epithelium shows patchy p16 positivity. There is some evidence that identification of the primary site in a patient who initially presents with an unknown primary is associated with improved overall survival [29]. However, the improved survival may simply be because a primary site is more likely to be detected for HPV-related metastases that present as unknown primaries, and HPV-related SCC is known to have better outcomes than HPV-unrelated SCC. Nevertheless, finding the primary site may reduce the possibility of over treatment and provides reassurance for patients. A discussion of treatment options and outcomes for those patients with “true” CUP, for which the primary site remains undetected after a thorough search, is beyond the scope of this review.
Metastatic Thyroid Carcinoma with an Occult Primary
Papillary thyroid carcinoma (PTC) is the most common type of thyroid carcinoma and frequently metastasizes to neck lymph nodes. However, presentation as CUP is uncommon as the primary tumor is usually easily identified with ultrasound of the thyroid gland and FNA biopsy of suspicious lesions. In a review of 342 thyroid carcinomas of follicular origin, including 167 cases of papillary thyroid microcarcinoma (microPTC), only 4.4 % of patients (15 cases) had cervical lymph node metastases and an occult primary tumor on imaging [30]. Thirteen of the 15 occult tumors presented with lymphadenopathy [30]. These occult tumors are almost always microPTCs (<1 cm) and occasionally a primary tumor may not be detected even after total thyroidectomy. The latter group is presumably the result of very small tumors that were not identified histologically, that regressed, or that arose in ectopic thyroid tissue that was not removed from the patient. Other types of thyroid carcinomas rarely present with an occult primary tumor, but these will not be addressed here.
Papillary thyroid carcinomas (PTC) with occult primaries can be divided into two types—those that present as clinically detected neck masses and those that are discovered incidentally in lymph nodes removed for another indication. The former appears to be associated with a greater risk of recurrent disease [30]. In the study by Garrel et al. [30], clinically occult microPTCs that presented with lymphadenopathy were associated with a higher rate of extrathyroidal extension than other microPTCs (9/13 or 69 % vs. 15/154 or 10 %) and 2 of 13 developed subsequent recurrences after radioactive iodine treatment. Therefore, these tumors should be treated no differently than non-occult PTC. On the other hand (although data is relatively limited), incidental PTC in neck lymph nodes appears to be extremely indolent. It is well known that there is a significant incidence (up to 35 %, if the thyroid gland is thoroughly examined histologically) of occult PTC in the general population identified in autopsy studies [31]. Therefore, it is not surprising that metastatic PTC is occasionally encountered incidentally in neck dissections performed for other reasons, most commonly for head and neck SCC. Incidental metastatic PTC occurs in 1–2 % or less of neck dissections [32]. Many patients with incidental metastatic PTC, especially those with aggressive head and neck cancers, do not undergo (and probably do not need to undergo) total thyroidectomy and radioactive iodine therapy. The prognosis of incidental metastatic PTC with an occult primary appears to be excellent regardless of whether or not any treatment is undertaken [32].
Histologically, metastatic PTC may be quite bland and in some cases lack well-developed nuclear features in lymph node metastases. The differential diagnosis may include benign thyroid inclusions, which also occur in less than 2 % of neck dissections [32]. The criteria for diagnosing thyroid follicles in lymph nodes as metastatic PTC versus benign thyroid inclusions is controversial, although the latter should certainly lack nuclear features of PTC and psammoma bodies, and consist of only a few small thyroid follicles underneath the lymph node capsule. Even in such cases, metastatic PTC cannot be entirely excluded. It may just be that these are PTC follicles, but the disease is so focal and indolent that it never presents as clinically relevant disease. Cyst formation is also quite common in metastatic PTC, seen in up to 70 % of cases, and a subset may be entirely cystic [33] (Fig. 5). The differential diagnosis for cystic metastatic PTC includes benign cysts, especially thymic cysts, since they are also surrounded by lymphoid tissue and may be lined by squamoid, cuboidal or columnar cells. However, nodal architecture surrounding the cyst and nuclear irregularity, grooves and crowding of the epithelium (features of PTC) should be absent in a thymic cyst. Immunohistochemistry, such as for thyroid transcription factor-1 (TTF-1) or thyroglobulin, can be performed in difficult cases, but PAX-8 should be avoided as it is usually positive in both thyroid and thymic lesions. For metastatic PTC with well-developed nuclear features and more solid architecture, the differential diagnosis may include metastatic cribriform adenocarcinoma of the tongue and minor salivary glands (CATMSG) [34]. CATMSG is a rare salivary gland tumor that occurs, as its name implies, in the minor salivary glands of the oral cavity (especially the tongue). More than 50 % of patients have lymph node metastases at presentation [34]. It has similar nuclear features to PTC including nuclear clearing and contour irregularity, and may have papillary architecture as well as psammoma bodies in rare cases. They do not, however, contain colloid and are not immunoreactive for TTF-1 or thyroglobulin. Fortunately, CATMSG is quite rare, although a case of metastatic CATMSG mistaken for metastatic PTC with an occult primary has already been reported [34].
Fig. 5.
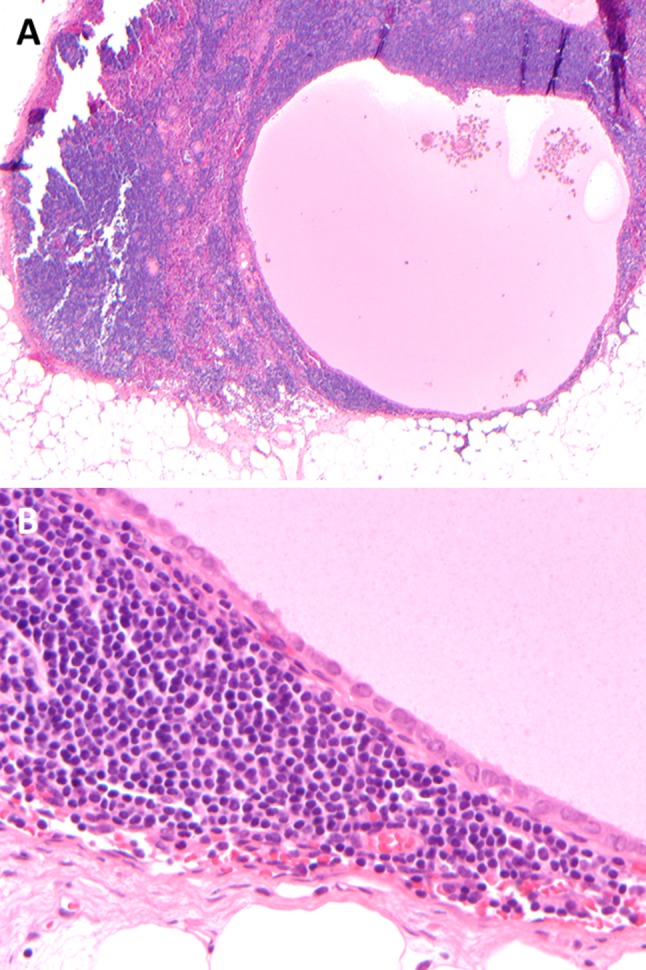
Metastatic PTC may be entirely cystic (a) and bland. Slight nuclear contour irregularity, overlap and grooves, as well as location within a lymph node suggest malignancy (b)
Salivary Gland Carcinoma: A Mimicker of CUP
It is rare for salivary gland carcinomas to present as a metastasis of unknown primary. However, the concept of ‘unknown primary’ is important in addressing salivary carcinomas for two reasons. The first is that primary salivary gland carcinomas may be mistaken for a metastasis of unknown primary, especially in the parotid gland. The second is that because the parotid gland in particular is rich in lymph nodes, it is a common site of non-salivary metastases, especially from cutaneous primaries. In some cases, these patients may present with a metastasis to the parotid gland and an unknown primary skin tumor. In this setting, the differential diagnosis may include both a primary parotid carcinoma and a metastasis from an unknown primary, often of cutaneous origin.
The reason that a primary salivary gland carcinoma may be mistaken as a CUP is twofold. First, intraparenchymal lymph nodes are intimately associated with the salivary gland tissue in the parotid gland and benign intranodal salivary tissue is a frequent finding in these lymph nodes (Fig. 6). In contrast, the sublingual and submandibular glands do not contain lymph nodes, although level IB neck lymph nodes may be in close proximity to the submandibular gland. Salivary inclusions may less commonly be seen in upper or even lower cervical lymph nodes as well. Salivary gland tumors may rarely arise from these intranodal salivary inclusions or, more commonly, secondarily involve a lymph node and thus may be mistaken for a metastasis of unknown primary. In a single institution review of salivary gland tumors arising in heterotopic tissue, Daniel et al. (2005) [35] identified three mucoepidermoid carcinomas, two acinic cell carcinomas and one salivary adenocarcinoma arising in periparotid or neck lymph nodes. Of course, primary nodal salivary gland carcinoma can only be diagnosed after a metastasis has been thoroughly excluded clinically. Another mimicker of metastatic salivary CUP is the presence of a lymphoid reaction surrounding the tumor that can be mistaken for a lymph node. A lymphoid reaction is common in acinic cell carcinomas but may also be seen in mucoepidermoid carcinomas (Fig. 7). Lack of nodal architecture (specifically lack of a capsule or sinus system) is an important distinguishing feature in such cases.
Fig. 6.
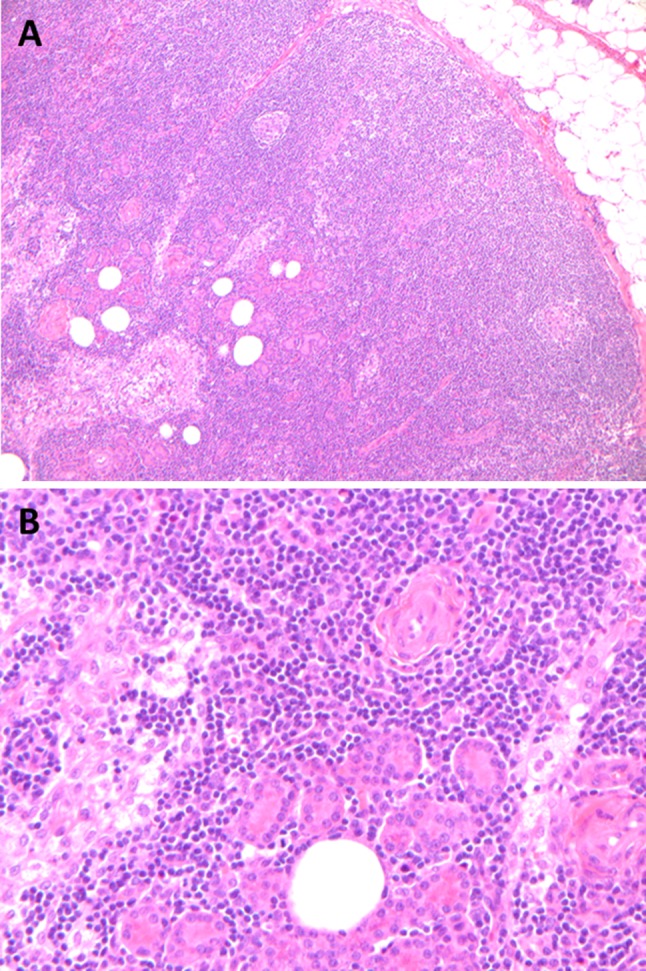
Intranodal salivary inclusions are a common finding in and around the parotid gland. On low power, numerous glandular structures are seen within the center (lower left) of a parotid lymph node (a). On higher magnification, the glands are very bland and uniform and represent atrophic salivary acini (b)
Fig. 7.
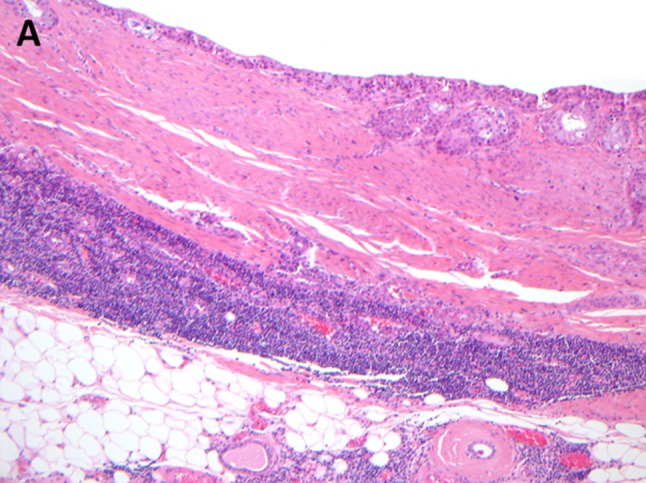
Primary mucoepidermoid carcinoma surrounded by lymphoid tissue, mimicking a lymph node metastasis
Metastases to the parotid lymph nodes, particularly from cutaneous head and neck tumors involving the lateral face and forehead, are not uncommon and may present as CUP. These also need to be distinguished from primary salivary carcinoma. In populations with high rates of skin cancer, such as in Australia, metastases to the parotid gland outnumber primary malignancies with metastases accounting for >70 % of parotid malignancies (57 % SCC, 39 % melanoma and 4 % Merkel cell carcinoma in one series) [36]. The incidence is likely much lower in other populations. In some patients with metastases to the parotid gland, the primary tumor may be occult. Occult or regressed primary malignant melanomas are well characterized and melanoma in the parotid gland is always considered to be a metastasis regardless of history of a cutaneous melanoma. Occult cutaneous metastases from SCC or Merkel cell carcinoma, however, may need to be differentiated from primary salivary gland tumors. Primary parotid SCCs as well as primary salivary high-grade neuroendocrine carcinomas with histologic and immunophenotypic similarities to cutaneous Merkel cell carcinoma, including cytokeratin 20 positivity, are rare but certainly do exist. The former likely arises from squamous metaplasia in salivary ducts. Yet, many tumors thought to be “primary parotid” SCCs likely represent cutaneous metastases for which the primary tumor was not recognized [37]. The diagnosis of a primary salivary SCC or neuroendocrine carcinoma should only be rendered after careful clinical exclusion of the possibility of a metastasis, including examination of the skin and review of past skin lesions that have been removed. Merkel cell polyomavirus, seen in up to 80 % of cutaneous Merkel cell carcinomas, appears to be less commonly found in primary salivary high-grade neuroendocrine carcinomas but cannot be relied upon as a distinguishing feature [38, 39].
Summary
The clinicopathologic features of the most common types of initial CUPs are important to recognize as the differential diagnosis may include both benign lesions and malignant neoplasms. The majority of cases are HPV-related SCCs. Recognition that HPV-related SCCs most often arise in the palatine tonsils and base of tongue has led to improved detection of occult primary tumors. Furthermore, HPV testing has become increasingly clinically important in SCC CUP. The best method for HPV testing of cytology specimens is still evolving but liquid-phase assays appear promising. p16 IHC, as a surrogate marker of transcriptionally-active HPV, can be useful on surgical specimens, if carefully applied. PTC may also present with an occult primary tumor. In such cases, it is important to distinguish between those that present with lymphadenopathy versus incidentally discovered tumors, the latter being a very indolent disease. Primary salivary carcinomas may mimic metastases of unknown primary and also need to be distinguished from cutaneous metastases to the parotid gland, a subset of which may not have a clinically apparent primary skin tumor. Finally, although not addressed here in detail, metastases from non-head and neck sites should always be considered in the setting of an unknown primary tumor in neck lymph nodes.
Conflict of interest
The authors have no conflicts of interest or funding to disclose.
References
- 1.Pavlidis N, Briasoulis E, Hainsworth J, Greco F. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990–2005. doi: 10.1016/S0959-8049(03)00547-1. [DOI] [PubMed] [Google Scholar]
- 2.Guntinas-Lichius O, Klussman J, Dinh S, Dinh M, Schmidt M, Semrau R, et al. Diagnostic work-up and outcome of cervical metastases from an unknown primary. Acta Otolaryngol. 2006;126(5):536–544. doi: 10.1080/00016480500417304. [DOI] [PubMed] [Google Scholar]
- 3.Cervin J, Silverman J, Loggie B, Geisinger K. Virchow’s node revisted. Analysis with clinicopathologic correlation of 152 fine-needle aspiration biopsies of supraclavicular lymph nodes. Arch Pathol Lab Med. 1995;119:727–730. [PubMed] [Google Scholar]
- 4.Cianchetti M, Mancuso A, Amdur R, Werning J, Kirwan J, Morris C, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119(12):2348–2354. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 5.Zengel P, Assmann G, Mollenhauer M, Jung A, Sotlar K, Kirchner T, et al. Cancer of unknown primary originating from oropharyngeal carcinomas are strongly correlated to HPV positivity. Vichows Arch. 2012;461(3):283–290. doi: 10.1007/s00428-012-1290-3. [DOI] [PubMed] [Google Scholar]
- 6.Tribius S, Hoffmann A, Bastrop S, Gorogh T, Haaq J, Rocken C, et al. HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol. 2012;48(11):1178–1184. doi: 10.1016/j.oraloncology.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Compton A, Moore-Medlin T, Herman-Ferdinandez L, Clark C, Caldito G, Wang X, et al. Human papillomavirus in metastatic lymph nodes from unknown primary head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2011;145(1):51–57. doi: 10.1177/0194599811400385. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi A, Engels E, Pfeiffer R, Hernandez B, Xiao W, Kim E et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. [DOI] [PMC free article] [PubMed]
- 9.Lewis J, Jr, Thorstad W, Chernock R, Haughey B, Yip J, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss D, Koopman M, Rudack C. Prevalence and impact on clinicopathological characteristics of human papillomavirus-16 DNA in cervical lymph node metastases of head and neck squamous cell carcinoma. Head Neck. 2011;33(6):856–862. doi: 10.1002/hed.21548. [DOI] [PubMed] [Google Scholar]
- 11.Ang K, Harris J, Wheeler R, Weber R, Rosenthal D, Nguyen-Tan P, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry M. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185(1):111–127. [PMC free article] [PubMed] [Google Scholar]
- 13.Chernock R, Lewis J, Jr, Zhang Q, El-Mofty S. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol. 2010;41(7):1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg D, Begum S, Westra W, Khan Z, Sciubba J, Pai S, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell J, Kumar B, Feng F, McHugh J, Cordell K, Eisbruch A, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakao K, Yuge T, Mochiki M, Nibu K, Sugasawa M. Detectionj of Epstein-Barr virus in metastatic lymph nodes of patients with nasopharyngeal carcinoma and a primary unknown carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(3):338–340. doi: 10.1001/archotol.129.3.338. [DOI] [PubMed] [Google Scholar]
- 17.Regauer S, Gogg-Kamerer M, Braun H, Beham A. Lateral neck cysts–the branchial theory revisited. A critical review and clinicopathological study of 97 cases with special emphasis on cytokeratin expression. APMIS. 1997;105(8):623–630. doi: 10.1111/j.1699-0463.1997.tb05063.x. [DOI] [PubMed] [Google Scholar]
- 18.Pai R, Erikson J, Pourmand N, Kong C. p16(INK4A) immunohistochemical staining may be helpful in distinguishing branchial cleft cysts from cystic squamous cell carcinomas originating in the oropharynx. Cancer Cytopathol. 2009;117(2):108–119. doi: 10.1002/cncy.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vent J, Haidle B, Wedemeyer I, Huebbers C, Siefer O, Semrau R, et al. p16 expression in carcinoma of unknown primary: diagnostic indicator and prognostic marker. Head Neck. 2013;35(11):1521–1526. doi: 10.1002/hed.23190. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J., Jr p16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–S82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernock R, Wang X, Gao G, Lewis J, Jr, Zhang Q, Thorstad W, et al. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26(2):223–231. doi: 10.1038/modpathol.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beadle B, William W, Jr, McLemore M, Sturgis E, Williams M. p16 expression in cutaneous squamous cell carcinomas with neck metastases: a potential pitfall in identifying unknown primaries of the head and neck. Head Neck. 2013;35(11):1527–1533. doi: 10.1002/hed.23188. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, El-Mofty S, Davila R. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer Cytopathol. 2008;114(2):118–123. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 24.Smith D, Maleki Z, Coughlan D, Gooi Z, Akpeng B, Ogawa T, et al. Human papillomavirus status of head and neck cancer as determined in cytologic specimens using the hybrid-capture 2 assay. Oral Oncol. 2014;50(6):600–604. doi: 10.1016/j.oraloncology.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller F, Psychogios G, Linke R, Lell M, Kuwert T, Iro H, et al. Carcinoma of unknown primary in the head and neck: comparison between positron emission tomography (PET) and PET/CT. Head Neck. 2011;33(11):1569–1575. doi: 10.1002/hed.21635. [DOI] [PubMed] [Google Scholar]
- 26.Karni R, Rich J, Sinha P, Haughey B. Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope. 2011;121(6):1194–1201. doi: 10.1002/lary.21743. [DOI] [PubMed] [Google Scholar]
- 27.Durmas K, Rangarajan S, Old M, Agrawal A, Teknos T, Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2014;36(6):848–852. doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch W, Bhatti N, Williams M, Eisele D. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124:331–333. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 29.Davis K, Byrd J, Mehta V, Chiosea S, Kim S, Ferris R, et al. Occult primary head and neck squamous cell carcinoma: utility of discovering primary lesions. Otolaryngol Head Neck Surg. 2014;151(2):272–278. doi: 10.1177/0194599814533494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrel R, Tripodi C, Cartier C, Makeieff M, Crampette L, Guerrier B. Cervical lymphadenopathies signaling thyroid microcarcinoma. Case study and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:115–119. doi: 10.1016/j.anorl.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Harach H, Franssila K, Wasenius V. Occult papillary carcinoma of the thyroid. A “normal” finding in Fibland. A systematic autopsy study. Cancer. 1985;56(3):531–538. doi: 10.1002/1097-0142(19850801)56:3<531::AID-CNCR2820560321>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Leon X, Sancho F, Garcia J, Sanudo J, Orus C, Quer M. Incidence and significance of clinically unsuspected thyroid tissue in lymph nodes found during neck dissection in head and neck carcinoma patients. Laryngoscope. 2005;115(3):470–474. doi: 10.1097/01.mlg.0000157841.63283.87. [DOI] [PubMed] [Google Scholar]
- 33.Kessler A, Rappaport Y, Blank A, Marmor S, Weiss J, Graif M. Cystic appearance of cervical lymph nodes is characteristic of metastatic papillary thyroid carcinoma. J Clin Ultrasound. 2003;31(1):21–25. doi: 10.1002/jcu.10130. [DOI] [PubMed] [Google Scholar]
- 34.Michal M, Kacerovska D, Kazakov D. Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. 2013;7(Suppl 1):S3–S11. doi: 10.1007/s12105-013-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel E, McGuirt W., Sr Neck masses secondary to heterotopic salivary gland tissue: a 25-year experience. Am J Otolaryngol. 2005;26(2):96–100. doi: 10.1016/j.amjoto.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Bron L, Traynor S, McNeil E, O’Brien C. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113(6):1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Roman S, Sosa J, Judson B. Prognostic factors for squamous cell cancer of the parotid gland: an analysis of 2104 patients. Head Nack. 2013;Epub ahead of print. [DOI] [PubMed]
- 38.Chernock R, Duncavage E, Gnepp D, El-Mofty S, Lewis J., Jr Absence of Merkel cell polyomavirus in primary parotid high-grade neuroendocrine carcinomas regardless of cytokeratin 20 immunophenotype. Am J Surg Pathol. 2011;35(12):1806–1811. doi: 10.1097/PAS.0b013e318236a9b0. [DOI] [PubMed] [Google Scholar]
- 39.Feng H, Shuda M, Chang Y, Moore P. Clonal integration of a polypomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]