Abstract
Adenosquamous carcinomas of the head and neck (ADSCs) are rare locally aggressive malignancies characterized by the presence of two distinctive components, a squamous cell carcinoma and an adenocarcinoma. The immunophenotype of the glandular component of ADSCs has only been rarely studied but has been reported as being positive for keratin 7 (CK7) and carcinoembryonic antigen (CEA) and negative for keratin 20 (CK20). Herein, we report a case of an ADSCs of the hypopharynx composed of a superficial squamous cell carcinoma and an adenocarcinoma with an intestinal phenotype. The patient was a 62 year-old male with a T2 N0 M0 squamous cell carcinoma (SCC) of uvula and palate and a T1 N0 M0 of right hypopharynx. The ADSCs of the hypopharynx was composed of a minimally invasive SCC and an adenocarcinoma with tubulo-glandular and cribriform architecture. The neoplastic glands were positive for CK7, CK20, CDX2, CEA and Villin. The patient underwent radiotherapy to both tumors and remains well with no evidence of recurrent disease 19 months after treatment. To the best of our knowledge, this is the first report of an ADSCs of the head and neck with an intestinal phenotype in its glandular component.
Keywords: Head and neck, Hypopharynx, Adenosquamous carcinoma, Intestinal-type adenocarcinoma
Introduction
Adenosquamous carcinomas of the head and neck (ADSCs) are uncommon neoplasms characterized by the presence of two distinctive components, a squamous cell carcinoma (SCC) and an adenocarcinoma [1–3]. Gerughty et al. [4] has been credited with establishing ADSCs as a distinctive variant of SCC of the head and neck. A recent literature review of 93 cases of ADSCs indicated a strong male prevalence (male/female ratio 6:1), with most cases involving the larynx (48.4 %) and rare tumors being associated with human papillomavirus (HPV) [2]. The immunophenotype of the glandular component of ADSCs has been investigated in only a small number of cases. In a study of 12 tumors, Alos et al. [5] reported that the neoplastic glands were positive for carcinoembryonic antigen (CEA) and keratin 7 (CK7) with keratin 20 (CK20) being negative. Similar results were described in two additional cases [3, 6]. ADSCs are aggressive malignancies with frequent cervical lymph node metastases and reported 3-year and median overall survivals of 52 % and 39 months [1, 3].
Adenocarcinomas of the head and neck with an intestinal phenotype (ITACs) is another group of uncommon neoplasms that affects the sinonasal tract. ITACs have distinctive epidemiologic, histopathologic, immunohistochemical and molecular pathology findings [7, 8]. ITACs may be seen as sporadic malignancies or as neoplasms associated to occupational exposure to wood or leather dust. ITACs resemble adenocarcinoma or adenomas of the intestines or rarely non-neoplastic small intestinal mucosa [7]. Immunohistochemically, ITACs stain diffusely for CK20, CDX2, CEA, Villin and are variably positive for CK7. Adenocarcinomas with an intestinal phenotype occurring outside of the sinonasal tract are extremely uncommon with only rare examples arising in the base of the tongue, major salivary glands, pharynx, and larynx [9–13]. To the best of our knowledge, an intestinal phenotype in ADSCs of the hypopharynx has not been previously reported. Herein we describe a primary adenosquamous carcinoma of the hypopharynx in which the glandular component exhibited an intestinal phenotype.
Case Report
Clinical Findings
The patient was a 62 year-old male that presented with painless bilateral neck lymphadenopathy. The patient’s previous medical history included a cerebrovascular event resulting in expressive aphasia, moderate alcohol and tobacco use. There was no history of occupational exposure to wood or leather dust.
Initial physical examination revealed bilateral enlarged cervical lymph nodes and an indurated uvular lesion, which measured 1.5 cm and extended to the right tonsillar pillar. CT scan of the head and neck confirmed a uvular swelling measuring 1.7 cm with right tonsil asymmetry and bilateral enlarged lymph nodes measuring up to 4.3 cm. A biopsy was performed and immunohistochemical and molecular analyses revealed a p16 and HPV negative, moderately-differentiated keratinizing squamous cell carcinoma. Fine needle aspiration of one of the enlarged cervical lymph nodes was positive for metastatic squamous cell carcinoma. The patient was referred to our centre for definitive therapy.
Physical examination and flexible laryngoscopy at our centre, revealed a second lesion involving the right aryepiglottic fold and piriform sinus. This second lesion was exophytic and measured 5 mm in maximum dimension. The patient underwent laser excision of the piriform sinus lesion and pathologic examination of the specimen demonstrated a moderately-differentiated adenosquamous carcinoma. The patient was staged as having a T2 N2c M0 SCC of the uvula and palate and a T1 N0 M0 adenosquamous carcinoma of the right hypopharynx. He underwent radiotherapy to both tumors with 70 Gy delivered in 35 fractionations. He remains well with no evidence of recurrent disease 19 months after treatment.
Pathologic Findings
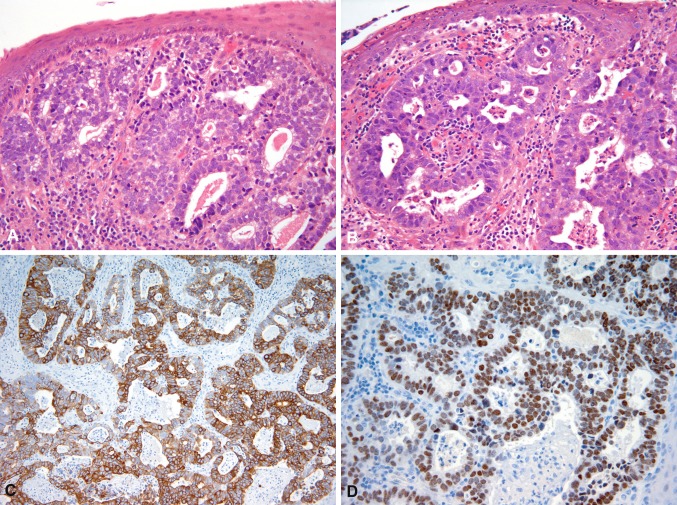
The tumor of the right piriform sinus measured 5 mm in maximum diameter with a thickness of 1 mm. Microscopically it demonstrated two distinctive components. The mucosa was involved by a moderately-differentiated, superficially invasive SCC lacking keratinization (Fig. 1a). The second element consisted of a submucosal infiltrate composed of neoplastic glands with tubulo-glandular and cribriform architecture lined by pseudostratified epithelium showing loss of cell polarity consistent with an adenocarcinoma (Figs. 2, 3a). Most of the squamous epithelium of the squamous epithelium covering the adenocarcinoma appeared reactive or partially ulcerated but in several foci the squamous carcinoma in situ was in direct contiguity with the underlaying adenocarcinoma (Fig. 2). Within the lumen of the tumor glands there was dense mucin associated with neutrophils. Cytologically, the glandular tumor cells showed increased nucleocytoplasmic ratio, nuclear hyperchromatism and moderate pleomorphism (Fig. 3b). Mitotic figures and apoptotic cells were readily identified. No tumor necrosis or atypical mitoses were noted. Mucicarmine and PAS stains revealed apical and occasionally intracytoplasmic mucin. No keratinization or any other indication of squamous differentiation was identified within the adenocarcinoma component.
Fig. 1.
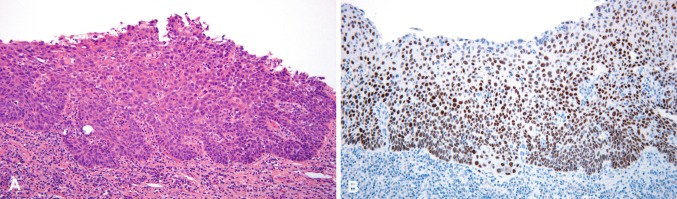
In situ and minimally invasive squamous cell carcinoma showing nuclear hyperchromasia and loss of cell polarity (a). Diffuse p63 staining in the squamous cell carcinoma (b)
Fig. 2.

Focus of squamous carcinoma in situ (left) associated with underlying adenocarcinoma (right)
Fig. 3.
Adenocarcinoma showing tubular and cribriform architecture. Some of the tumor cells demonstrate apical vacuoles. In this area the squamous epithelium appears unremarkable (a). Adenocarcinoma with cribriform architecture, nuclear hyperchromasia, and increased mitotic activity (b). Diffuse cytoplasmic CK20 and nuclear CDX2 expression by the adenocarcinoma (c, d)
The SCC component was diffusely positive for pankeratin (AE1/AE3), CAM5.2, and p63 (Fig. 1b), whereas CK7, CK20, CDX2, Villin, epithelial membrane antigen (EMA) and monoclonal carcinoembryonic antigen (mCEA) were negative. The adenocarcinoma was strongly positive for pankeratin, CAM5.2, CK7, CK20 (Fig. 3c), CDX2 (Fig. 3d), Villin, mCEA and EMA. The adenocarcinoma was negative for thyroid transcription factor-1 (TTF-1), MUC-2, MUC-5AC and MUC6. MLH1, MSH2, MSH6 and PMS2 expression was preserved in both components. P16 and p63 were negative in both components. P63 showed no basal or myoepithelial cells within the invasive glands.
Discussion
In this report we describe an ADSCs of the hypopharynx in which the glandular component exhibited an intestinal phenotype. The tumor had a minimally invasive SCC with an underlying adenocarcinoma positive for CK7, CK20, mCEA, and Villin [1]. To the best of our knowledge, this is the first documented example of an ADSCs of the head and neck in which the glandular component exhibited an intestinal phenotype. ADSCs of the head and neck are rare with less than 100 reported cases [3]. Perhaps due to its rarity, the expression of CK7, CK20 and CEA in the glandular component of ADSCs of the head and neck has been reported in less than 15 cases [3, 5, 6]. CK7 has been positive in 9 of 14 cases (79 %), CEA expression has been found in 13 of 14 cases (93 %), and CK20 was negative in all 12 cases investigated [3, 5, 6]. Testing of additional ADSCs of the head and neck is necessary to determine if the expression of CK20 and CDX2 in ADSCs is as uncommon as the current Literature suggests.
The main differential diagnoses of this ADSCs with intestinal phenotype includes non-sinonasal ITAC of the head and neck, metastatic adenocarcinoma from the gastrointestinal tract, and mucoepidermoid carcinoma. Metastatic adenocarcinomas from the gastrointestinal tract to the pharynx are uncommon [3, 14, 15] and are usually negative for CK7 [16]. The small size of the lesion in this patient, the absence of a malignancy in other sites at presentation and 19 months after completion of treatment argue against this tumor being a metastasis. The presence of surface SCC, lack of mucous and intermediate cells, and the CK7+/CK20+, Villin+ and CDX2+ phenotype exclude a diagnosis of mucoepidermoid carcinoma.
Primary non-sinonasal ITACs of the head and neck are exceedingly rare tumors with only seven cases described in the base of the tongue, major salivary glands, pharynx, and larynx [9–13]. Non-sinonasal ITACs have heterogeneous microscopic appearance and are composed of glands and tubules lined by columnar cells similar to those of colorectal adenocarcinomas [9, 12, 13], with some neoplasm containing a variable or prominent mucinous (colloid) component, signet-ring cells or poorly differentiated carcinoma [10, 11]. Unlike sinonasal and non-sinonasal ITACs, the case described herein has a superficial squamous cell carcinoma in addition to a deeper invasive glandular component in keeping with the typical appearance of an adenosquamous carcinoma [17]. It must be noted that the laryngeal ITACs recently described by Bell et al. [11] had a minor squamous component in addition to typical intestinal-type, mucinous and signet-ring cell components.
The origin of ADSCs remains unclear and controversial with suggested origins from squamous epithelium or excretory ducts of seromucous glands [4–6]. The intestinal phenotype observed in our case could have originated from metaplasia of surface epithelium or through a process where undifferentiated endodermal lining of the stomodeum might have been misplaced or entrapped, leading to the development of heterotopic intestinal epithelium [18]. The presence of surface squamous dysplasia or carcinoma in situ in many cases supports a surface origin [5] while the occurrence of ductal carcinoma in situ as described by Gerughty et al. [4] suggests a ductal origin. Interestingly, Fonseca [6] described the presence of both, surface squamous dysplasia and myoepithelial cells surrounding the glandular component of an ADSCs. Our case had a superficial SCC but there were no myoepithelial cells surrounding the glandular component, findings suggesting an origin from surface epithelium accompanied by transdifferentiation of its glandular component.
There are not enough cases published to determine the clinical significance of intestinal differentiation in ADSCs. ADSCs are aggressive malignancies with frequent cervical lymph node metastases and reported 3-year and median overall survivals of 52 % and 39 months [1, 3]. The tumor in this patient measured 5 mm and was classified as T1 N0 M0; however the long term prognosis is more likely to be determined by the concurrent T2 N2c M0 SCC of the oropharynx.
References
- 1.Keelawat S, Liu CZ, Roehm PC, et al. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23:160–168. doi: 10.1053/ajot.2002.123462. [DOI] [PubMed] [Google Scholar]
- 2.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5:108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schick U, Pusztaszeri M, Betz M, et al. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:313–320. doi: 10.1016/j.oooo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Geruthy RM, Hennigar RA, Brown FM. Adenosquamous carcinoma of the nasal, oral, and laryngeal cavities. A clinicopathological survey of ten cases. Cancer. 1968;22:1140–1155. doi: 10.1002/1097-0142(196811)22:6<1140::AID-CNCR2820220610>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Alos L, Castillo M, Nadal A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44:570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca FP, Ramos LM, Vargas PA, et al. Oral adenosquamous carcinoma: evidence that it arises from the surface mucosal epithelium. Histopathology. 2012;61:321–323. doi: 10.1111/j.1365-2559.2012.04257.x. [DOI] [PubMed] [Google Scholar]
- 7.Franchi A, Santucci M, Wenig BM, et al. Adenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 20–23. [Google Scholar]
- 8.Franchi A, Miligi L, Palomba A, et al. Sinonasal carcinomas: recent advances in molecular and phenotypic characterization and their clinical implications. Crit Rev Oncol Hematol. 2011;79:265–277. doi: 10.1016/j.critrevonc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Bell D, Kupferman ME, Williams MD, et al. Primary colonic-type adenocarcinoma of the base of the tongue: a previously unreported phenotype. Hum Pathol. 2009;40:1798–1802. doi: 10.1016/j.humpath.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slova D, Paniz MA, Moisini I, et al. Colonic-type adenocarcinoma of the base of the tongue: a case report of a rare neoplasm. Head Neck Pathol. 2012;6:250–254. doi: 10.1007/s12105-011-0301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell D, Holsinger CF, Ordonez N, et al. Intestinal-type adenocarcinoma of the larynx: a report of a rare aggressive phenotype and discussion of histogenesis. Head Neck. 2013. [DOI] [PubMed]
- 12.Gillenwater AM, Frank SJ, Fatani H, et al. Primary intestinal-like adenocarcinoma of major salivary glands: 2 instances of previously undocumented phenotype. Head Neck. 2013;35:E234–E236. doi: 10.1002/hed.23059. [DOI] [PubMed] [Google Scholar]
- 13.Lopez JI, Perez A. Pharyngeal adenocarcinoma with intestinal features. J Laryngol Otol. 1990;104:900–902. doi: 10.1017/S0022215100114306. [DOI] [PubMed] [Google Scholar]
- 14.Hong W, Wang X, Yu XM, et al. Palatine tonsillar metastasis of lung cancer during chemotherapy. Int J Clin Exp Pathol. 2012;5:468–471. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Wang SY, Li SM, et al. Metastatic tumors in nasal cavity and pharynx: a clinicopathological analysis of 11 cases. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:1030–1033. [PubMed] [Google Scholar]
- 16.Resto VA, Krane JF, Faquin WC, et al. Immunohistochemical distinction of intestinal-type sinonasal adenocarcinoma from metastatic adenocarcinoma of intestinal origin. Ann Otol Rhinol Laryngol. 2006;115:59–64. doi: 10.1177/000348940611500109. [DOI] [PubMed] [Google Scholar]
- 17.Cardesa A, Zidar N, Alos L. WHO classification of tumours. Head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 18.Morgan WE, Jones JK, Flaitz CM, et al. Congenital heterotopic gastrointestinal cyst of the oral cavity in a neonate: case report and review of literature. Int J Pediatr Otorhinolaryngol. 1996;36:69–77. doi: 10.1016/0165-5876(96)01331-6. [DOI] [PubMed] [Google Scholar]