Abstract
Neuroendocrine neoplasms represent a rare subset of tumors in the sinonasal tract. Combined tumors, with an endocrine and a non-neuroendocrine component, are exceedingly rare, and mainly consist of a combination of neuroendocrine carcinoma with adenocarcinomas. We present the clinico-pathologic and immunohistochemical features of a neuroendocrine carcinoma combined with squamous cell carcinoma, arising in the maxillary sinus. In addition, we evaluated the clonal origin of the two components through analysis of TP53 gene status. Both components were positive for cytokeratins AE1/AE3, while the squamous cell carcinoma was positive for cytokeratin 5/6 and p63, and the neuroendocrine carcinoma showed immunoreactivity for neuron specific enolase, chromogranin, synaptophysin and CD56. In situ hybridization for human papilloma virus and Epstein–Barr virus were negative in both components. A missense mutation in TP53 exon 7 (c.734G>C) and strong nuclear immunostaining for p53 were detected only in the neuroendocrine carcinoma. This suggests that the tumor either derived from one precursor cell with squamous differentiation, which underwent TP53 mutation and acquisition of a neuroendocrine phenotype, or it derived from two separate clones, one with mutated TP53 and neuroendocrine differentiation, and the other with wild type TP53 and squamous differentiation (collision tumor).
Keywords: Squamous cell carcinoma, Neuroendocrine carcinoma, Combined neuroendocrine carcinoma, Maxillary sinus, Immunohistochemistry, TP53 gene
Introduction
Tumors of the sinonasal tract are rare, representing approximately 3 % of the head and neck malignancies and less than 1 % of all malignant tumors [1]. The most frequent histologic subtype is squamous cell carcinoma, followed by adenocarcinoma, melanoma and olfactory neuroblastoma [1], while neuroendocrine tumors are exceedingly rare at these sites. A subset of neuroendocrine carcinomas may present with a combination of a small cell carcinoma and a squamous or adenocarcinomatous component. In the head and neck region, these tumors occur more frequently in the larynx, where they represent approximately 10 % of all neuroendocrine carcinomas [2, 3]. In the sinonasal tract only few examples have been reported, which consisted mostly of a combination of adenocarcinoma and neuroendocrine carcinoma [4], while cases of combined small cell and squamous cell carcinoma seem to be exceedingly rare [5, 6].
In this report we describe the clinico-pathological, immunohistochemical and molecular features of a combined neuroendocrine and squamous cell carcinoma arising in the maxillary sinus.
Case Report
A 75-year-old man was admitted to the emergency department of our hospital due to acute ischemic stroke. A non-contrast CT scan of the head was performed, which revealed a previously undisclosed 3.5 × 4 cm. lesion of the left maxillary sinus. CT scan with contrast showed that the lesion involved the left nasal cavity, the ethmoid sinus, extended anteriorly in the subcutaneous tissue and inferiorly in the hard palate and in the alveolar processes of the maxillary bone (Fig. 1). The patient was an heavy smoker (30 cigarettes per day for 60 years) and alcohol drinker.
Fig. 1.
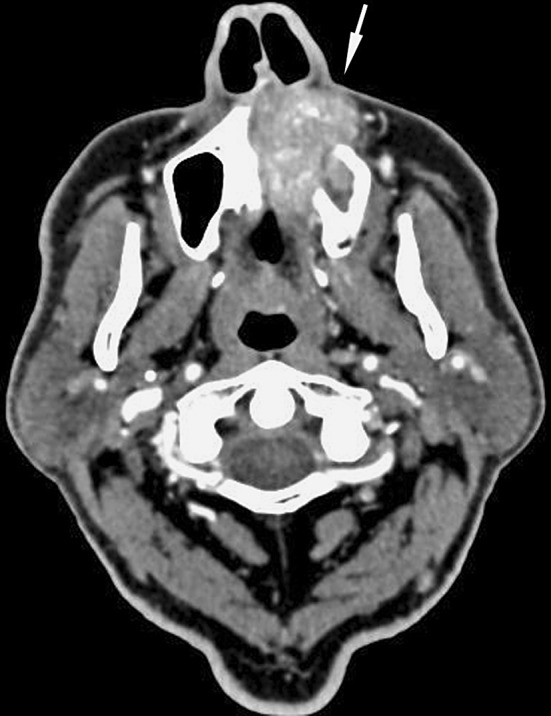
The pre-operative CT scan shows an irregular lesion involving the left maxillary sinus and the nasal cavity, and extending in the subcutaneous soft tissues (arrow)
An incisional biopsy was performed, and a diagnosis of squamous cell carcinoma was rendered.
The tumor was surgically removed using a transfacial approach with a left Weber-Fergusson incision. A type IIIC left maxillectomy and a type IIA right maxillectomy according to Brown were performed [7], together with removal of nasal septum and ethmoidal sinus. In consideration of the patient’s general conditions and age, the reconstruction consisted of pedicled temporalis muscle flap. Recovery was uneventful. Postoperative radiotherapy was planned in a 55 Gy dose, but was not completed due to complications. The patient showed no evidence of disease as of 20 months postoperatively.
Materials and Methods
Immunohistochemistry
For immunohistochemical staining, paraffin section (5 μm thickness) were dewaxed, hydrated and after inactivation of endogenous peroxidase were immunostained using the BenchMark® XT stainer and revealed with the iVIEW DAB detection kit, yielding a brown reaction product. Table 1 reports the antibody source, dilution and antigen retrieval protocols used. After the staining run was complete, the slides were removed from autostainer, counterstained with hematoxylin, dehydrated and mounted with permanent mounting medium. As negative controls, we substituted the primary antibody with a Ventana dispenser filled with non immune serum at the same concentration for each immunohistochemical reaction.
Table 1.
Summary of the features of the antibodies employed in this study
| Antibody | Clone and source | Titration | Antigen retrieval |
|---|---|---|---|
| Cytokeratin AE1/AE3 | PCK26, Ventana, Tucson, AZ, USA | Prediluted 32 min, 37 °C | Protease 4 min |
| Cytokeratin 5/6 | D5/16 B4, Chemicon Int., Temecula, CA, USA | 1:60 32 min, 37 °C | Microwave oven, citrate buffer pH 6.0 |
| p63 | 4A4, Ventana, Tucson, AZ | Prediluted 32 min, 37 °C | CC1 Mild (Ventana, Tucson, AZ, USA) |
| NSE | Clone E27, Cell Marque Co., Rocklin, CA | Prediluted 24 min, 37 °C | Microwave oven, citrate buffer pH 6.0 |
| Synaptophysin | Polyclonal rabbit, Cell Marque Co., Rocklin, CA | Prediluted 32 min, 37 °C | CC1 Mild (Ventana, Tucson, AZ, USA) |
| Chromogranin | LK2H10, Ventana, Tucson, AZ | Prediluted 32 min, 37 °C | CC1 Mild (Ventana, Tucson, AZ, USA) |
| CD56 | MRQ-42, Ventana, Tucson, AZ | Prediluted 32 min, 37 °C | Prediluted 32 min 37 °C |
| p16 | 16P04, Cell Marque Co., Rocklin, CA | Prediluted 32 min, 37 °C | CC1 Mild (Ventana, Tucson, AZ, USA) |
| p53 | DO7, Ventana, Tucson, AZ | 1:40 60 min RT | CC1 Mild (Ventana, Tucson, AZ, USA) |
The results of the immunohistochemical staining were reported semiquantitavely according to a four point scale: −, negative staining in tumor cells; +, positive staining in 1–25 % of tumor cells; ++, positive staining in 26–50 % of tumor cells; +++, positive staining in 51–100 % of tumor cells.
In Situ Hybridization
In situ hybridization (ISH) for HPV was performed using an automated ISH protocol with the Ventana HPV II family 6 which detects HPV genotypes 6 and 11, and the HPV III family 16 probes, which detects HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66 (Ventana, Tucson, AZ). The ISH iView detection system (Ventana, Tucson, AZ) was employed for detection. For Epstein–Barr virus-encoded RNA (EBER) ISH, we used an automated protocol with the EBER 1 DNP Probe (Ventana), along with the iView Plus detection system (Ventana). For both ISH tests, the Ventana XT automated staining platform was used.
TP53 Gene Sequencing
Ten serial sections (5 μm thickness) were obtained from one paraffin-embedded tissue block. Tissue fragments were microdissected using stereomicroscopic visualization from blank, deparaffinized slides, in order to separate the squamous carcinoma and the neuroendocrine areas of the tumor. The DNA was purified with QIAamp® DNA FFPE Tissue Kit (Qiagen, Hilden, DE) according to the manufacturer’s protocol and the DNA concentration was assessed spectrophotometrically using NanoDrop 2000 (Thermo Scientific).
PCR reaction for TP53 gene exons 4, 5, 6, 7, 8 and 9 was performed using the primers shown in Table 2. The reaction was carried out in a volume of 50 μl, with 1.5 mM MgCl2, 10 pmol of each primer and 2 U PerfectTaq Plus DNA Polymerase (5-PRIME) with 100 ng of DNA template. The cycle conditions were: 60 s at 95 °C, 60 s at specific annealing temperature (Table 2) and 60 s at 72 °C for 32 cycles. A 10 min final extension was added. The amplification was performed in a 2720 Thermal Cycler (Applied Biosystems, USA). The amplification products were purified with HiYield™ Gel/PCR DNA Fragments Extraction kit (RBC Bioscience) according to the manufacturer’s protocol.
Table 2.
Primers and annealing temperature employed in the analysis of the TP53 gene
| Primers | Annealing T (°C) | |
|---|---|---|
| Exon 4 forward | GCTCTTTTCACCCATCTACA | 53 |
| Exon 4 reverse | ACCGTGCAAGTCACAGACTT | |
| Exon 5 forward | CTCTTCCTACAGTACTCCCCTGCC | 62 |
| Exon 5 reverse | GCCCCAGCTGCTCACCATCGCTA | |
| Exon 6 forward | GATTGCTCTTAGGTCTGGCCCCT | |
| Exon 6 reverse | GGCCACTGACAACCACCCTTAAC | |
| Exon 7 forward | GTGGTTATCTCCTAGGTTGGCTCT | |
| Exon 7 reverse | CAAGTGGCTCCTGACCTGGAGTC | |
| Exon 8 forward | AGTAGTGGTAATCTACTGGGACGG | 54 |
| Exon 8 reverse | GCAGCTCGTGGTGAGGCT | |
| Exon 9 forward | CCCTTCAGGTACTAAGTCTTGG | 55 |
| Exon 9 reverse | CGAAATGCCCCAATTGCAGG |
Subsequently we performed cycle sequencing reaction of purified PCR products using the TP53 forward primers and BigDye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. The sequencing products were purified using ZR DNA Sequencing Clean-up Kit™ (Zymo Research, USA).
The samples were analyzed using the AbiPrism 310 Genetic Analyzer (Applied Biosystems, USA). The sequence results for each sample were analyzed using Seqscape® Software v2.5 (Applied Biosystems, USA) to verify the sequencing results and identify the possible mutations.
Results
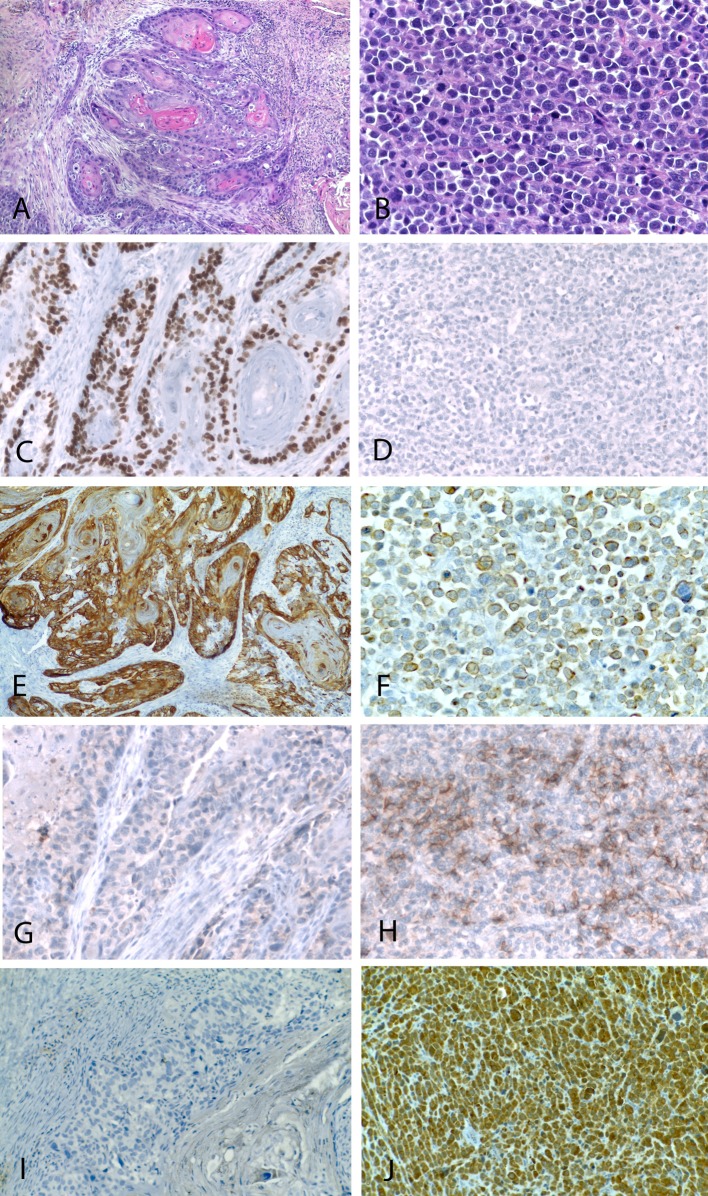
The biopsy specimen consisted of multiple mucosal fragments showing microscopic features of conventional squamous cell carcinoma. In the resection specimen, the tumor consisted of two morphologically distinctive components, which were in part clearly separated and in part strictly intermixed. The first component was a conventional well differentiated squamous cell carcinoma, with diffuse aspects of keratinization (Fig. 2), which represented approximately 70 % of the lesion. The second component consisted of a proliferation of large, closely packed, round to oval atypical cells, with large vesicular nuclei and prominent nucleoli, and scant pale eosinophilic cytoplasm, which were arranged mostly in solid sheets (Fig. 3) or focally in islands and chords (Fig. 3). This represented the remaining 30 % of the lesion.
Fig. 2.
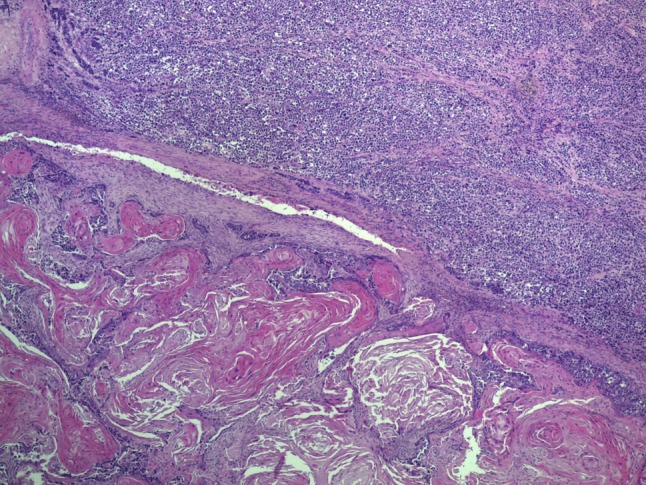
Low power view showing that the tumor consisted of two sharply separated components. The neuroendocrine carcinoma (top) consisted of solid sheets of poorly differentiated round to oval cells, while the squamous cell carcinoma component consisted of islands of atypical keratinizing epithelium (bottom)
Fig. 3.
The squamous cell carcinoma component was characterized by a proliferation of invasive irregular solid tumor islands with evidence of keratinization (a). The neuroendocrine carcinoma consisted of a diffuse proliferation of poorly differentiated intermediate to large cells (b). Immunohistochemically, the squamous component was positive for p63 (c) while the neuroendocrine component was negative (d). Cytokeratin 5/6 (e) was expressed only in the squamous component, while the neuroendocrine component was positive for AE1/AE3 (f). The neuroendocrine carcinoma showed positivity for CD56 and chromogranin (h, j), while these markers were negative in the squamous component (g, i)
Based on these findings, and on the results of the immunohistochemical studies (see below), the diagnosis of combined squamous and large cell neuroendocrine carcinoma was rendered.
Immunohistochemistry
The results of the immunohistochemical studies are summarized in Table 3 and illustrated in Fig. 3. The two components were markedly different in their immunoprofile. The squamous cell carcinoma component was moderately positive for cytokeratin AE1/AE3 and diffusely positive for cytokeratin 5/6 and p63, while neuroendocrine markers were negative. Conversely, the neuroendocrine carcinoma component was diffusely positive for cytokeratin AE1/AE3, negative for cytokeratin 5/6 and p63, while neuroendocrine markers were positive. Both tumor components were negative for p16, while p53 was strongly and diffusely positive in the neuroendocrine component (Fig. 4a), whereas the squamous cell component showed only focal and weak positivity (Fig. 4b).
Table 3.
Summary of the immunohistochemical studies according to the tumor component
| Marker | Squamous cell carcinoma | Neuroendocrine carcinoma |
|---|---|---|
| Cytokeratin AE1/AE3 | ++ | +++ |
| Cytokeratin 5/6 | +++ | − |
| p63 | +++ | − |
| Neuron specific enolase | − | +++ |
| Synaptophysin | − | ++ |
| Chromogranin | − | +++ |
| CD56 | − | ++ |
| p16 | − | − |
| p53 | − | +++ |
Fig. 4.
TP53 gene status. The neuroendocrine component showed intense nuclear positivity for p53 and a missense mutation in exon 7 (c.734G>C; arrow) was evidenced through DNA sequencing (a). The squamous cell carcinoma component showed negative immunostaining for p53 and no mutation of the gene was detected in the exons analyzed (b)
In Situ Hybridization
In situ hybridization for HPV and EBV was negative in both components.
TP53 Analysis
After microdissection, the two components were separately analyzed for the status of exons 4–9 of the TP53 gene. PCR amplification and subsequent DNA sequencing revealed a missense mutation in exon 7 (c.734G>C) with a heterozygous pattern, which was present only in the neuroendocrine carcinoma (Fig. 4), whereas no mutation was evidenced in the squamous cell carcinoma component. This mutation results in a glicin to alanin substitution and a non-functional protein (p.G245A).
Discussion
Combined neuroendocrine carcinomas are a rare but well known subset of tumors, that more frequently arise in the lung and in the digestive tract. In the head and neck region, these tumors are quite uncommon, and more frequently have been reported in the larynx. Here, they represented approximately 10 % of all neuroendocrine carcinomas and consisted of a combination of squamous cell and small cell carcinoma or atypical carcinoid [2, 3]. In the few cases reported in the sinonasal tract, the neuroendocrine carcinoma was more frequently combined with an adenocarcinoma not otherwise specified or with intestinal type adenocarcinoma, while inverted papilloma was associated in one instance [4], and squamous cell carcinoma in two instances [5, 6]. The present case consisted of a combination of well-differentiated squamous cell carcinoma and large cell neuroendocrine carcinoma. The two components were partly well separated and partly intermixed. This was well appreciated through the immunohistochemical studies performed, which showed that the squamous areas were positive for cytokeratin 5/6 and p63 and negative for neuroendocrine markers, whereas the neuroendocrine areas showed diffuse and intense immunoreactivity for specific markers such as chromogranin A and synaptophysin, and more limited expression of CD56, while being negative for cytokeratin 5/6 and p63.
The histopathological appearance and the immunohistochemical profile of the tumor allowed the distinction from other sinonasal malignancies showing round cell morphology and areas of squamous differentiation. In particular, NUT midline carcinoma, a recently described high grade malignancy which may involve in the sinonasal tract, is characterized by a proliferation of undifferentiated cells with focal areas of abrupt keratinization, showing diffuse positivity for p63 [8] and absent or focal positivity for neuroendocrine markers [9]. Sinonasal undifferentiated carcinoma (SNUC) is another entity which may be entered in the differential diagnosis, but in the present case it can be excluded based on the diffuse aspects of squamous differentiation, which are not part of the histopathological profile of this tumor.
Since a subset of neuroendocrine carcinomas arising in the oropharynx and a subset of sinonasal squamous cell carcinoma have recently been associated with high-risk HPV infection [10, 11], we searched for the presence of this virus by in situ hybridization. However, the results were negative in both components, as it was the immunostaining for p16, which is considered a surrogate marker for HPV infection. In addition, no evidence of EBV infection was also detected by in situ hybridization.
The frequency of TP53 mutation in sinonasal squamous cell carcinoma has ranged between 30 and 75 %, according to different studies [12, 13]. However, only sporadic cases of neuroendocrine carcinoma of the upper aerodigestive tract have been studied at the molecular level, while neuroendocrine carcinoma of the lung shows a high rate of TP53 mutations [14]. In the present case we observed a TP53 missense mutation in exon 7 in the tumor component with neuroendocrine differentiation, but not in the squamous cell carcinoma component. This mutation inactivates p53 oncoprotein, which accumulates in the nucleus. Accordingly, strong nuclear immunostaining for p53 was observed in the neuroendocrine component, but not in the squamous component. This mutation has been reported in several tumor types, including urinary bladder, lung, and esophageal carcinomas [15].
Few cases of combined neuroendocrine carcinoma have so far been subjected to molecular analysis of the TP53 gene. In a small cell neuroendocrine carcinoma of the lung combined with sarcomatous elements, TP53 codon V274F mutation in exon 8 was shared by either cell component, suggesting that the tumor probably derived for clonal evolution of a p53-mutated common ancestor lesion [16]. Similarly, a prostatic combined small cell carcinoma and adenocarcinoma showed an identical mutation in the DNA binding domain of TP53 in both components, although the acinar component apparently retained a wild-type allele while the small cell component showed a homozygous mutant form, which could be interpreted as a stepwise progression to the neuroendocrine phenotype [17]. In a nasal mixed exocrine-neuroendocrine carcinoma with histological features of an intestinal type adenocarcinoma and a poorly differentiated neuroendocrine carcinoma, the two components showed strong genetic relationships, although TP53 mutation was not detected in either neoplastic component [4]. These findings supported the hypothesis of a monoclonal origin of the tumor, with biphenotypic differentiation likely occurring during tumor progression [4]. Hiraki and coworkers studied the TP53 gene in two examples of combined small and non small cell carcinoma of the lung by PCR-SSCP, and in one case they found TP53 mutation in the small cell carcinoma tissue, but not in the non-small cell carcinoma tissue [18]. Similarly, in the present case the TP53 mutation, along with p53 immunohistochemical overexpression, were detected only in the neuroendocrine component. These findings can be interpreted according to two different hypotheses. First, the tumor could derive from one precursor cell acquiring squamous differentiation, and the occurrence of the TP53 mutation could represent a key event in originating a clone with neuroendocrine features during tumor progression. Alternatively, the tumor could derive from two separate clones, one with mutated TP53 and neuroendocrine differentiation, and the other with wild type TP53 and squamous differentiation, thus representing a “collision tumor”.
References
- 1.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population based data. Head Neck. 2012;34:877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal VR, Hoang MP. Primary combined squamous and small cell carcinoma of the larynx: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:1279–1282. doi: 10.5858/2004-128-1279-PCSASC. [DOI] [PubMed] [Google Scholar]
- 3.Davies-Husband CR, Montgomery P, Premachandra D, Hellquist H. Primary, combined, atypical carcinoid and squamous cell carcinoma of the larynx: a new variety of composite tumour. J Laryngol Otol. 2010;124:226–229. doi: 10.1017/S0022215109991228. [DOI] [PubMed] [Google Scholar]
- 4.La Rosa S, Furlan D, Franzi F, Battaglia P, Frattini M, Zanellato E, Marando A, Sahnane N, Turri-Zanoni M, Castelnuovo P, Capella C. Mixed exocrine-neuroendocrine carcinoma of the nasal cavity: clinico-pathologic and molecular study of a case and review of the literature. Head Neck Pathol. 2013;7:76–84. doi: 10.1007/s12105-012-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SF, Chuang WY, Cheng SD, Hsin LJ, Lee LY, Kao HK. A colliding maxillary sinus cancer of adenosquamous carcinoma and small cell neuroendocrine carcinoma. A case report with EGFR copy number analysis. World J Surg Oncol. 2010;8:92. doi: 10.1186/1477-7819-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barham HP, Said S, Ramakrishnan VR. Colliding tumor of the paranasal sinus. Allergy Rhinol (Providence) 2013;4:e13–e16. doi: 10.2500/ar.2013.4.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JS, Rogers SN, McNally DN, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22:17–26. doi: 10.1002/(SICI)1097-0347(200001)22:1<17::AID-HED4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, Aster JC, French CA. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 11.Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal carcinoma: systematic review and meta-analysis. Hum Pathol. 2013;44:983–991. doi: 10.1016/j.humpath.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Bandoh N, Hayashi T, Kishibe K, Takahara M, Imada M, Nonaka S, Harabuchi Y. Prognostic value of p53 mutations, bax, and spontaneous apoptosis in maxillary sinus squamous cell carcinoma. Cancer. 2002;94:1968–1980. doi: 10.1002/cncr.10388. [DOI] [PubMed] [Google Scholar]
- 13.Holmila R, Bornholdt J, Suitiala T, Cyr D, Dictor M, Steiniche T, Wolff H, Wallin H, Luce D, Husgafvel-Pursiainen K. Profile of TP53 gene mutations in sinonasal cancer. Mutat Res. 2010;686:9–14. doi: 10.1016/j.mrfmmm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Onuki N, Wistuba II, Travis WD, Virmani AK, Yashima K, Brambilla E, Hasleton P, Gazdar AF. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer. 1999;85:600–607. doi: 10.1002/(SICI)1097-0142(19990201)85:3<600::AID-CNCR10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.IARC TP53 database, R17, November 2013. http://p53.iarc.fr. Accessed 18 Nov 2013.
- 16.Pelosi G, Sonzogni A, Galetta D, Perrone F, Braidotti P, Manzotti M, Fabbri A, Spaggiari L, Veronesi G, Viale G. Combined small-cell carcinoma of the lung with quadripartite differentiation of epithelial, neuroendocrine, skeletal muscle, and myofibroblastic type. Virchows Arch. 2011;458:497–503. doi: 10.1007/s00428-010-1011-8. [DOI] [PubMed] [Google Scholar]
- 17.Hansel DE, Nakayama M, Luo J, Abukhdeir AM, Park BH, Bieberich CJ, Hicks JL, Eisenberger M, Nelson WG, Mostwin JL, De Marzo AM. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate. 2009;69:603–609. doi: 10.1002/pros.20910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraki A, Ueoka H, Yoshino T, Chikamori K, Onishi K, Kiura K, Bessho A, Mimoto J, Date H, Ando A, Shimizu N, Harada M. Synchronous primary lung cancer presenting with small cell carcinoma and non-small cell carcinoma: diagnosis and treatment. Oncol Rep. 1999;6:75–80. [PubMed] [Google Scholar]