Abstract
Background
Diabetes mellitus is one of the most incessant metabolic afflictions with high prevalence rate in Indians. Diagnosis of diabetics in the initial stage helps to prevent its long term complications that are responsible for high morbidity and mortality. The aim of the present study was to assess whether glucometric analysis using Gingival Crevicular Blood (GCB) can be used for screening of diabetic patients in dental chair.
Materials and methods
Present study was a double blinded randomized controlled trial. A total of 50 patients, 25 diabetic and 25 non-diabetic with chronic periodontitis were enrolled in the study. Blood oozing from the gingival crevices after periodontal pocket probing of anterior teeth and Finger Prick Blood (FPB) was taken and analysed by glucometer.
Result
Mean ± S.D was 195.84 ± 27.19 and 138.78 ± 29.95 for GCB and FPB respectively in diabetic group. For non-diabetic group Mean ± S.D was 103.84 ± 12.56 and 84.36 ± 10.36 respectively for GCB and FPB. A Karl Pearson correlation coefficient value of r = +0.735 for diabetic and r = +0.802 for non-diabetic group comparing GCB and FPB.
Conclusion
GCB cannot be used for screening blood glucose during periodontal examination.
Keywords: Diabetic mellitus, GCB, Bleeding on probing
1. Introduction
Diabetes mellitus is one of the most incessant metabolic affliction with prevalence of 7% in industrialized countries.1 India has nearly 33 million diabetic subjects.2 Incidence of most common type of DM type II is expanding upto 6% per year.3
A large number of patients seek dental treatment being unaware of their undiagnosed diabetes mellitus. Thus, the dentist may contribute towards patient's health by participating in the search for undiagnosed asymptomatic diabetes mellitus patients. It is estimated that for every patient with known diabetes there is one with undiagnosed diabetes mellitus in dental patients.4 The issue of undiagnosed diabetes is especially important because early treatment and secondary prevention may prevent its long-term complications. Complications due to diabetes include diabetic retinopathy, neuropathy renal dysfunction, atherosclerotic cerebro-vascular, cardiovascular and peripheral vascular disease, Delayed healing etc.
Because of the frequent asymptomatic nature of diabetes in its early stages, many individuals with undiagnosed condition are likely to have the disease for several years before being diagnosed.5 Due to this, by the time of diagnosis, in many of these individuals, beta cell function may decline substantially6 and significant damage may already have occurred. So, there is a need to increase opportunities for diabetes screening, especially for those with a high risk. Periodontitis is considered to be the sixth complication of diabetes.7–9 Moreover, diabetes and periodontitis seem to interact in a bidirectional manner.10 The prevalence of diabetes mellitus is more than twice as high as in patients with periodontitis when compared to periodontally healthy subjects.
Periodontal inflammation is known to produce ample extravasated blood during diagnostic procedures with or without the complicating factor of diabetes mellitus.11 Routine probing during periodontal examination is more familiar to the practitioner and is less traumatic compared to finger puncture with a sharp lancet. It is possible that gingival crevicular blood (GCB) from probing may be an excellent source of blood for glucometric analysis using the technology of portable glucose monitors colloquially called Glucometer.12
Several methods have been developed to measure glucose level in biological fluids, but the search for more simple sensitive and specific method is still going on. Currently, portable glucose monitors are used in hospitals and domestically. These can be used for estimation of blood glucose in dental patient too.
Glucometers are reliable, rapid, affordable and commonly used for blood glucose determinations in diabetes screening and domestic monitoring. When utilized in a dental office, such a system may offer a more objective parameter in the diagnosis of diabetes mellitus. Dental office screening offers earlier treatment and possible minimization of serious complications associated with diabetes mellitus. Development of an intra-oral blood sampling technique could make such tests even more suitable for use by dental practitioners.13
Glucose monitoring system needs only 3 μl of blood and allows for non-invasive, convenient and painless method of determination in the blood oozing from the gingival crevices of patients with gingivitis or periodontitis during routine periodontal examination.
Therefore, the aim of the study was to evaluate the reliability of gingival crevicular blood as a diagnostic tool for diabetes by using glucose self monitoring device.
2. Materials and methods
A total of 50 subjects (19 female and 31 male; age range 29–68 years) with chronic periodontitis were randomly selected from patients visiting the Outpatient Department of Periodontics, Babu Banarasi Das College of Dental Sciences, Lucknow. Out of these, 25 individuals were known diabetic and 25 were reported to be systemically healthy.
First examiner did the periodontal examination and assessed the diabetic history. Second examiner collected (GCB) and Finger Prick Blood (FPB) to assess blood glucose level. Data collected by First examiner were not disclosed and each subject was allotted a unique code number. Subjects and examiner (second) were masked to the group allocation.
Prior to collection of the samples, following clinical parameters were assessed; Plaque Index (PI Silness and Loe, 1964), Gingival Bleeding Index (GBI Ainamo and Bay, 1975), and Probing Pocket Depth (PPD) were done. In addition, Orthopantomogram (OPG) was taken which aided in clinically diagnosing periodontitis.
Prior to probing, all the subjects were subjected to rinsing the oral cavity with 0.2% chlorhexidine in order to minimize microbial load. Subjects were excluded from the study if they required antibiotic premedication, any hematological disorder, systemic disease such as cardiovascular, renal, hepatic, immunologic, hematological disorders and any medication that may interfere with the coagulating system.
All patients were informed about the study and a written consent was obtained. Ethical clearance was taken from the Institutional Ethics Committee, according to the Helsinki Declaration of the 1975, as revised in 1983.
3. Sample collection
3.1. Gingival-Crevicular Blood (GCB)
Each subject was examined intra-orally for the visual signs of inflammation and bleeding sites. Site with more profuse bleeding was chosen for GCB. Sites with suppuration were excluded from the study. Maxillary anterior teeth were the site of interest, irrespective of their PPD, for the measurement of glucose as they offer better access for GCB. Care was taken to prevent contamination with saliva. To obtain a clean sample, probing was repeated, when necessary, until a sufficient quantity of blood was drawn into the capillary. Especially for participants with PPD ≥ 4 mm, collection of sufficient blood sample required no more probing than in routine to measure pockets and attachment loss in a standard periodontal examination. However, for participants with PPD ≤ 3 mm, it was at times necessary to probe three or more times at the site to obtain the GCB sample.
All possible attempts were made to obtain the blood sample with a fine capillary tube (2 mm) (Fig. 1a) without contact with gingival and periodontal tissues and then transferring the blood to the stick of glucose self-monitoring device. The test strip was held until the instrument beeped giving the blood glucose measurements in mg/dl. Glucometer was standardized by known sugar solution after every 10th reading.
Fig. 1.

(a) GCB sampling for glucose measurement. (b) FPB sampling for glucose measurement.
3.2. Finger-Prick Blood (FPB)
Samples for finger-capillary blood were drawn from preferably the index finger of the subject. The pad of the finger was wiped with alcohol, allowed to dry, and then punctured with sterile lancet. Sample was drawn onto the test strip preloaded in the glucometer and was held until the instrument gave a beep displaying the blood glucose measurements on the screen in mg/dl. The time of testing is approximately 10 s (Fig. 1)(b).
The data obtained were analysed using Statistical Package for Social Sciences, version 19.0 (SPSS). Mean and Standard Deviation (S.D.) were calculated for GCB and FPB glucose levels in study and control groups.
4. Result
50 subjects were divided in two groups of 25 each, Group 1 (diabetic group) and Group 2, (non-diabetic group). Range of 180–250 mg/dl for GCB and 150–210 mg/dl for FPB in diabetic group and a range of 90–140 mg/dl and 70–105 mg/dl respectively for GCB and FPB was found in non-diabetic group (Table 1). Mean ± S.D. (Range) was 210.56 ± 17.26 (180–250), 178.08 ± 17.66 (150–210) for GCB and FPB respectively in diabetic group. For non-diabetic group Mean ± S.D (Range) was 118.76 ± 13.83 (90–140) and 86.56 ± 10.17 (70–105) respectively for GCB and FPB (Table 1; Fig. 2). Within each group, on comparison the difference in Mean of GCB and FPB was found to be significant, indicating that the GCB is not similar to FPB. The result was same for both the Groups i.e. diabetic group and non-diabetic group (Table 1). A highly statistically significant difference was found between mean values of GCB and FPB between two groups of diabetic and non-diabetic on intergroup comparison (P < 0.001). A Karl Pearson correlation coefficient value of r = 0.735 for GCB and FPB for diabetic and r = 0.802 for non-diabetic group indicated a very low positive correlation (Table 2).
Table 1.
Mean, Standard Deviation and Range values of glucose levels measured at different sites in two groups.
| Groups | No. | Particulars | GCB (mg/dl) | FPB (mg/dl) | P value |
|---|---|---|---|---|---|
| Group I Diabetic |
25 | Mean | 210.56 | 178.08 | <0.001 |
| SD | 17.26 | 17.66 | |||
| Range | 180–250 | 150–210 | |||
| Group II Non-diabetic |
25 | Mean | 118.76 | 86.56 | <0.001 |
| SD | 13.83 | 10.17 | |||
| Range | 90–140 | 70–105 |
Fig. 2.
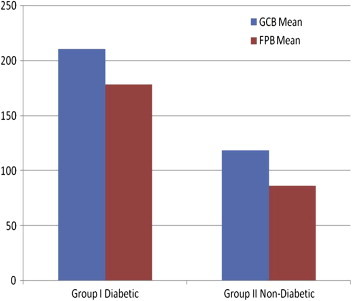
GCB & FPB glucose values mean in study groups.
Table 2.
Karl Pearson correlation coefficient for GCB Vs FPB in both Group I and Group II.
| GCB vs FPB | Pearson correlation coefficient (r) | P value |
|---|---|---|
| Group I Diabetic | 0.0735a | <0.001 |
| Group II Non-diabetic | 0.0802a | <0.001 |
Correlation is significant at the 0.01 level (2-tailed).
5. Discussion
According to the International Diabetes Federation every fifth diabetic in the world would be an Indian by the year 2025.14 The Asian Indian phenotype called Thrifty genotype predisposes Indian population to risk of developing diabetes. To prevent complications that result from diabetes, it should be diagnosed in the initial stage. For this, there should be screening of patients that are diabetic or non diabetic. There is a two way correlationship between diabetes and periodontitis. On the one hand, poorly controlled diabetes increases the risk for developing destructive periodontitis and impairs treatment outcome. On the other, chronic inflammatory periodontal disease considerably complicates diabetic control.9 Although certain microorganisms in dental plaque are considered to be the main cause of destructive periodontitis, ignoring established risk factors like smoking or diabetes would most probably lead to therapeutic failure.
Hence, dentists especially periodontists can play an important role in the screening. As the patient comes to a dental clinic for regular checkup, there is a periodontal probing that leads to oozing of blood that can be used for testing diabetes. Moreover, the technique described is more familiar and less traumatic to the patient than a finger puncture.
In the present study, there was a significant difference between GCB and FPB values indicating that both cannot be considered as alternative to each other. Any stimulation such as trauma, salivation, mastication and inflammation alters the reading of GCB. Gingivitis and periodontitis produce higher GCF. Thus, there being always a possibility of mixing of GCB with GCF, and the latter having its own glucose, the sugar content of blood cannot be assessed correctly.
In the present study, the FPB showed consistently lower measurements compared to GCB glucose. Recently, it has been shown that higher glucose level may be detected in GCF at the periodontally diseased site as compared to healthy which could explain the disparities observed.14
Our results were similar to those by Muller et al15 who concluded that there is no usefulness of GCB, as bleeding on probing was not sufficient in every third case. Kandwal et al also concluded that the GCB for testing blood glucose during routine periodontal examination is not useful.16
In contrast to our study, Parker et al examined diabetic patients with unknown periodontal status where a very strong correlation was observed between gingival crevicular, finger prick capillary and intravenous blood glucose measurements.12 In another study by Beiker et al, a pronounced association was observed between GCB and finger stick capillary measured blood glucose when diabetic and non diabetic patient with moderate to advanced periodontitis were examined.1 In the study by Gaikwad similar findings was reported.17 There was also a similar profile between GCB and FPB studied by Kaur et al.18
A potential reason for the possible disparity between our and the reported studies could be that our samples were collected only from blood oozing from the gingival crevices and finger capillary blood unlike the venous route. The latter is the established method routinely followed for quantifying glucose as done by Beikler et al and Kaur et al. Additionally, Beikler et al chose moderate or advanced periodontitis subjects for their study whereas, our study included in addition, subjects with mild periodontitis also. Further, the study by Parker et al consisted of subjects with diabetics with unknown periodontal status. Lastly, Gaikwad has conducted similar study in subjects with unknown diabetic and periodontal status.
On an overall basis, it can be concluded that our studies and those reported by others have some resemblances but have differences as far as the specific details are concerned which are critical for deriving a generalized conclusion. This study, therefore provides an approach to correlate periodontitis versus the metabolic status vis-à-vis diabetes which may have profound role(s) to play in the presentation and molecular regulation of the disease itself.
The present study failed to prove the authenticity of GCB in assessment of diabetic patients in dental chair using glucometer. The two values have significant difference showing that both cannot be considered same. However, in view of the possibilities that our study offers versus others does suggest that larger sample size will definitely shed more light in the area. The overall advantage of reducing the psychological trauma of venous blood harvest compares with that from the discharge site offers potential benefits and opens more vistas for investigation.
Conflicts of interest
All authors have none to declare.
References
- 1.Beikler T., Kuczek A., Petersilka G., Flemming T.F. In-dental-office screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol. 2002;29:216–218. doi: 10.1034/j.1600-051x.2002.290306.x. [DOI] [PubMed] [Google Scholar]
- 2.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 3.Rees T.D. Periodontal management of the patient with diabetes mellitus. Periodontol 2000. 2000;23:63–72. doi: 10.1034/j.1600-0757.2000.2230105.x. [DOI] [PubMed] [Google Scholar]
- 4.Stein G.M., Nebbia A.A. A chair side method of diabetic screening with gingival blood. Oral Surg Oral Med Oral Pathol. 1969;27:607–612. doi: 10.1016/0030-4220(69)90092-9. [DOI] [PubMed] [Google Scholar]
- 5.Harris M.I., Klein R., Welborn T.A., Knuiman M.W. Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care. 1992;15:815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 6.U. K. Prospective Diabetes Study Group U.K. Prospective Diabetes Study 16; overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 7.Ainamo J., Lahtinen A., Vitto V.J. Rapid periodontal destruction in adult humans with poorly-controlled diabetes. A report of 2 cases. J Periodontol. 1990;17:22–28. doi: 10.1111/j.1600-051x.1990.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura F., Takahashi K., Kurihara M., Takashiba S., Mura-yam Y. Periodontal disease as a complication of diabetes melli-tus. Ann Periodontol. 1998;3:20–29. doi: 10.1902/annals.1998.3.1.20. [DOI] [PubMed] [Google Scholar]
- 9.Grossi S.G., Genco R.J. Periodontal disease and diabetes melli-tus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Sicree R., Shaw J., Zimmet P. Diabetes and impaired glucose tolerence. In: Gan D., editor. Diabetes Atlas. International Diabetes Federation. 3rd ed. International Diabetes Federation; Belgium: 2006. pp. 15–103. [Google Scholar]
- 11.Tsutsui P., Rich S.K., Schonfeld S.E. Reliability of intraoral blood for diabetes screening. J Oral Med. 1985;40:62–66. [PubMed] [Google Scholar]
- 12.Parker R.C., Rapley J.W., Isley W., Spencer P., Killoy W.J. Gingival crevicular blood for assessment of blood glucose in diabetic patients. J Periodontol. 1993;64:666–672. doi: 10.1902/jop.1993.64.7.666. [DOI] [PubMed] [Google Scholar]
- 13.Ervasti T., Knuuttila M., Pohjamo L., Haukipuro K. Relation between control of diabetes and gingival bleeding. J Periodontol. 1985;56:154–157. doi: 10.1902/jop.1985.56.3.154. [DOI] [PubMed] [Google Scholar]
- 14.Position Paper Diabetes and periodontal diseases. J Periodontol. 1999;70:935–949. doi: 10.1902/jop.1999.70.8.935. [DOI] [PubMed] [Google Scholar]
- 15.Muller H.P., Behbehani E. Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract. 2004;13:361–365. doi: 10.1159/000080474. [DOI] [PubMed] [Google Scholar]
- 16.Kandwal A., Batra M. Gingival crevicular blood as a screening tool for diabetic patient: a randomized clinical trial. Ann Dent Speciality. 2014;2:6–8. [Google Scholar]
- 17.Gaikwad S., Jadhav V., Gaurav A., Shete A.R., Dearda H.M. Screening for diabetes mellitus using gingival crevicular blood with the help of a self monitoring device. J Periodontal Implant Sci. 2013;43:37–40. doi: 10.5051/jpis.2013.43.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur H., Jain S., Bansal S. Minimal invasive chair side procedure for the estimation of glucose level using gingival crevicular blood. Indian J Dent Sci. 2012;1:43–46. [Google Scholar]


