Abstract
Background
Chronic liver diseases (CLD) are quite prevalent throughout the globe. Its early and correct diagnosis is always a concern among physicians, especially the residual liver function. For this various substrates like caffeine are being investigated in body fluids like serum and saliva. Saliva as a study sample has its own advantages due to its non invasiveness; it can be very useful study sample.
Methods
30 Subjects with CLD and 15 healthy controls were administered 200 mg of caffeine. Subjects classified into severity groups (class-A-mild-n = 9, B-moderate-n = 11, and C-severe-n = 10) based on “Child-pugh classification” of severity of liver disease. After 17 h of dietary caffeine restriction and before drug administration, 0 h salivary sample was taken. After the dose of caffeine, 4 and 16 h saliva sample was taken. Blood sample was taken from controls only at same time points. These samples were analyzed on semi automated analyzer using Enzyme Multiplied Immunoassay Technique (EMIT) by spectrophotometric method. Caffeine clearance values were calculated and results were statistically analyzed.
Results
Significant correlation was found between serum caffeine clearance and salivary caffeine clearance (SCC). Controls showed higher mean of SCC value of 1.6 ± 0.2 ml/min/kg while SCC values of subjects were less, with mean of 0.5 ± 0.2 ml/min/kg. Significant correlation was found between degree of hepatic dysfunction and SCC values.
Conclusion
Saliva can be used for diagnosis of CLD and assessment of residual liver function in CLD as alternative to serum.
Keywords: Caffeine 1, 3, 7 tri-methyl xanthine; Child- pugh classification; EMIT; Liver cirrhosis
1. Introduction
The liver function assessment is important for estimation of the severity of liver disease, treatment efficacy and its prognosis. Conventional liver function tests, particularly various serum enzymes, provide important information on inflammation and cholestasis but give no indication of hepatic metabolic function.1 The sensitivity and specificity of these tests are also a concern. Some of commonly tested enzymes which rise in liver diseases may be seen raised in other conditions as they are present in these tissues also.2 Besides these, liver function tests may be normal in certain liver diseases also like congenital hepatic fibrosis, cirrhosis, non cirrhotic portal fibrosis etc. While serum albumin, which decreases in liver disease may decrease in nephritic syndrome and other chronic disease as well. Likewise it may be abnormal in persons with healthy liver; like aminotransferases which may be raised in cardiac diseases also. Thus researchers concluded that except for serum bile acids, the LFT are not specific for liver diseases and all the parameters may be elevated for pathological processes outside the liver.3,4 It is also worthy to mention that these tests provide only static assessment of the degree of liver injury, but give little information about the residual liver function.
It has been emphasized that the assessment of residual liver function is most important factor to determine the severity of acute or chronic liver diseases; irrespective of its aetiology, long-term prognosis, step-by step disease progression, surgical risk and efficacy of treatment procedure.5 It has been proposed that administration of a compound metabolized by the liver and study of its clearance from body affords a dynamic evaluation of residual metabolic capacity and thus the quantitative assessment of liver function.6 The first quantitative liver function tests to assess residual liver function were suggested in 1962.7 After that liver function test such as the galactose elimination, aminopyrine breath test and indocyanine green clearance have been developed and proved efficient by some studies.8,9 Studies have been reported that these tests are not popular either because of technical difficulties or adverse effects, like breath tests are not feasible in patients with severe restrictive lung disease, and in elderly patients with chronic heart failure.1,10
Thus a quantitative liver function test is required which uses test substance that is completely absorbed, harmless and has exclusive hepatic elimination and excretion. It is further desired that the test material and its metabolites can be easily assessed in patient's body fluids with economical, widely available and accurate equipments and techniques. Caffeine 1, 3, 7 tri-methyl xanthine, an exogenous substance appears to be almost an ideal substance for the purpose.11 This is due to its rapid and complete absorption12 after oral ingestion and almost exclusive metabolism in liver,13 caffeine clearance may show the residual metabolic capacity of liver in diseased patients giving quantitative assessment of liver function.
Blood serum is being used as standard diagnostic fluid for many body tests and clearance of caffeine can also be measured as the disappearance of the parent compound from the blood.1,5 However saliva is emerging as efficient and feasible diagnostic fluid for many diseases and also being popular among patients and clinicians due to easy collection and less chances of cross contamination and can be used as sample for the study of larger group like country surveys. Saliva has been proposed as suitable diagnostic fluid for caffeine clearance assessment which is substantiated by a number of studies that confirmed an excellent correlation between caffeine elimination in saliva and liver function14,15,16 In spite of these encouraging studies, the caffeine clearance test from saliva sample is not popular in clinical practice. This warrants further study to assess its feasibility as non invasive diagnostic modality for quantitative assessment of liver function and find out the possible shortcomings which is hindering its popularity.
Hence the study was aimed to validate the utility of saliva as study sample by evaluating the correlation of caffeine clearance in saliva and serum and then to correlate the salivary caffeine clearance with severity of chronic liver disease, using enzyme multiplied immunoassay technique (EMIT) with spectrophotometric method on a semi-automated analyzer.
2. Materials and method
2.1. Patient selection
Thirty hospitalized patients with chronic liver disease were selected. The diagnosis was confirmed on the basis of clinical findings and other relevant investigations. This group included twenty-five males and five females with age ranging from thirty-one to seventy-one years and mean age of forty-two years.
A detailed medical history of the patients was taken which included their presenting complaint and special attention was given on complications like malena, hematemesis, encephalopathy, esophageal varices and portal hypertension. Patients having such complications were not included in the study. Detailed drug history was also elicited. Personal history of indulgence in alcoholism, smoking, coffee and tea intakes were noted down and were asked to refrain from them during the study period. Along with chronic liver disease, a record of any other systemic illness like diabetes, hypertension, asthma, renal problems etc was also made. Permission was sought from the patient for the salivary caffeine clearance test and written informed consent was obtained from all the patients.
The chronic liver disease patients were classified into three severity groups based on child-pugh classification; as child-pugh class-A mild, B moderate, and C severe respectively.17 9 of the subjects belonged to child-pugh class-A, 11 belonged to child-pugh class B and 10 belonged to child-pugh class C. 15 healthy and young volunteers of either sex with age range of 20–35 years were selected as controls with no known history of chronic liver disease and were found normal on physical examination.
2.2. Sample collection
Study and control groups were individually informed in detail regarding the caffeine test protocol. They were instructed to follow exclusion of caffeine containing substance from their diet. (i.e. to avoid intake of tea, coffee, chocolate, commercial soft drinks) and refrain from smoking and alcohol and consumption for 17 h prior to the study period and throughout the study period.
2.3. Saliva sample collection
Following the 17 h caffeine exclusion period 1–3 ml saliva sample collected from the study and control group in a wide mouth sterile plastic container by drooling method. First sample was collected at evening 4 pm i.e usual coffee time and was leveled as 0 h sample. Following which a single dose of 200 mg capsule of anhydrous caffeine I.P. grade was administered. Second sample of saliva were obtained at evening 8 pm and leveled as 4 h sample and third sample of saliva were obtained next day at 8 am and leveled as 16 h sample.
2.4. Blood sample collection
1–2 ml sample of venous blood was also obtained from arms of the controls at time points similar to saliva collection.
The blood and saliva samples obtained were centrifuged immediately and clear serum and saliva was transferred to small storage plastic container with adequate labeling. The samples were then stored at −20 °C until analysis was performed.
2.5. Analytical method
Analysis of caffeine concentration in serum and saliva samples was done by enzyme multiplied immunoassay technique (EMIT) by a spectrophotometric method on a semi-automated analyzer. Caffeine serum and saliva concentrations were determined by the method described by Zysset et al18 using an automated enzyme immunoassay kit supplied by SYVA (Dade Behring,USA). The caffeine elimination constant (kel) was calculated assuming first order kinetics.19 Caffeine clearance was calculated as Cl = kel X Vd, using a constant volume of distribution of 0.61/kg body weight.
2.6. Statistical analysis
All the data was analyzed using the SPSS statistical package for windows. Patient and control data were expressed as mean ± standard deviation. Student's t-tests was performed to find significance of difference between patient and control for mean of caffeine concentration at 0, 4, 16 h and caffeine clearance. Comparison of mean caffeine clearance between different child pugh severity groups among patients was done using one way ANOVA. Correlation analysis was obtained between severity group and their caffeine clearance. Pearson's correlation analysis was done and scatter plot was drawn to assess the relationship between severity score and caffeine clearance.
3. Results
The mean of salivary caffeine clearance values among controls was 1.6 ± 0.2 ml/min/kg. The mean of serum caffeine clearance values among controls was 1.6 ± 0.3 ml/min/kg. The mean of salivary caffeine clearance values among study group (patients) was 0.5 ± 0.2 ml/min/kg [Table 1]. Caffeine clearance values in serum and saliva of controls showed a significant correlation with Pearson's correlation analysis (p < 0.001) [Fig. 1].
Table 1.
Mean Standard Deviation and Test of Significance of mean values between patients and controls.
| Variables | Patient saliva |
Controls saliva |
Controls serum |
p-value |
|---|---|---|---|---|
| Mean ± S.D | Mean ± S.D | Mean ± S.D | <0.001 | |
| 0 h Concentration μ g/ml |
2.4 ± 1.4 | 0.4 ± 0.2 | 0.5 ± 0.2 | <0.001 |
| 4 h Concentration μg/ml |
19.7 ± 1.7 | 5.9 ± 0.9 | 7.9 ± 1.2 | <0.001 |
| 16 h Concentration μg/ml |
11.7 ± 2.8 | 0.9 ± 0.2 | 1.2 ± 0.3 | <0.001 |
| Caffeine Clearance Ml/min/kg |
0.5 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.3 | <0.001 |
Fig. 1.
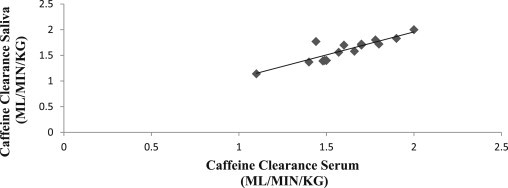
Scatter plot showing correlation between caffeine clearance in control serum and saliva: a positive slope with linear relation is evident; with scatter values distributed close to the graph.
The caffeine clearance of study group in Child pugh class A showed a mean of 0.6 ± 0.1 ml/min/kg while it was found 0.5 ± 0.1 ml/min/kg and 0.3 ± 0.1 ml/min/kg in Child pugh class B and Class C respectively [Table 2]. Caffeine clearance values in saliva of patients and severity of liver disease on child pugh severity scale showed a significant correlation with Pearson's correlation analysis (p < 0.001)[Fig. 2, Table 2].
Table 2.
Comparison of means of caffeine clearance among Child pugh severity groups and control.
| Child pugh class | Mean caffeine clearance ml/min/kg | p-value | Significant groups at 5% level |
|---|---|---|---|
| Class-A | 0.6 ± 0.1 | ||
| Class-B | 0.5 ± 0.1 | A vs B | |
| Class-C | 0.3 ± 0.1 | <0.001 | B vs C |
| Controls | 1.6 ± 0.2 |
Fig. 2.
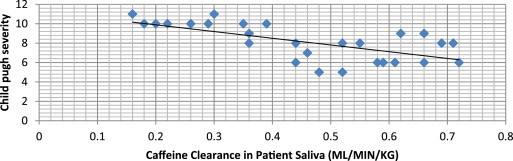
Scatter plot showing negative correlation between Child pugh severity and caffeine clearance in chronic liver disease patients.
4. Discussion
Various liver function assessment methods have been advocated. One of them is examination of clearance of some test material in body fluids which is metabolized in liver. Many techniques and substrates have been used to for this purpose but none of them are popular in routine clinical practice. This is either because of technical difficulties or adverse effects of metabolic substrates. This studied method utilizes the advantages of both caffeine as safe material and saliva as convenient sample fluid. Enzyme multiplied immunoassay technique (EMIT) which was employed in the current study to assay caffeine levels in plasma and saliva; makes it possible to measure caffeine concentration rapidly and accurately by a simple photometric procedure.
The method used in present study is an overnight caffeine clearance method. It has often been used for research and has been advocated for potential clinical use in subjects with liver dysfunction.5,11 It is convenient method for both patients and physicians. This method has some obvious advantage like resting at night and the absence of food or drugs intake.20 The present study employed intake of caffeine in capsule form to avoid the oral tissue contamination with caffeine and collecting saliva well past 4 h after the drug administration. This is due to the concern raised in a previous study regarding oral tissue contamination with the test substance administered directly by mouth, particularly when samples are collected soon after orally administered drug.21 As researchers reported doubtful results in presence of large availability of caffeine in the diet we followed the protocol of 17 h dietary restriction of any food containing caffeine for our study participants.22
During validating saliva as diagnostic fluid we noted a significant correlation (p < 0.001) between serum and salivary caffeine clearance with Pearson's coefficient values of r = 0.85 [Fig. 1/Table 3]. This is similar to the findings of Frederick W Lewis and William G rector Jr.23 in 1992 who reported significant (p < 0.001) but better correlation between the caffeine clearance in the two fluids, with Pearson's correlation coefficient value of r = 0.98 similar to findings of Wahllander et al21 (r = 0.963) and Scott et al15 (r = 0.965; p < 0.001).
Table 3.
Pearson's correlation analysis between serum and salivary caffeine clearance in controls.
| Time point | Pearson's correlation coefficient (r) | p value |
|---|---|---|
| 0 h | 0.99 | <0.001 |
| 4 h | 1.00 | <0.001 |
| 16 h | 0.85 | <0.001 |
| Clearance | 0.85 | <0.001 |
Our result showed that the salivary caffeine clearance value of all the thirty patients were less, with a mean of 0.5 ± 0.2 ml/min/kg. Controls showed a higher mean of salivary caffeine clearance value of 1.6 ± 0.2 ml/min/kg [Table 1]. This is comparable to the study done by Jost et al11 in 1987, with their mean of salivary caffeine clearance value of 0.58 ± 0.45 ml/min/kg in chronic liver disease patient and a mean of 1.53 ± 0.46 ml/min/kg in controls. Our findings can be also compared with findings of Wahlländer et al21 who showed that in the cirrhotic patients, clearance differed significantly from noncirrhotic liver disease and controls with 0.51 ± 0.45 ml/min per kg against 0.91 ± 0.44 ml/min per kg and 1.41 ± 0.56 ml/min per kg caffeine clearance respectively.
We also found significant correlation (p < 0.001) between child pugh score rating of severity of liver disease and salivary caffeine clearance value [Fig. 2/Table 2]. There was significant difference in salivary caffeine clearance between child-pugh category A (0.6 ± 0.1 ml/min/kg) and Child-pugh category C (0.03 ± 0.1 ml/min/kg) while there was less difference between child-pugh A (0.6 ± 0.1 ml/min/kg) and child-pugh category B (0.5 ± 0.1 ml/min/kg) [Table 2]. However, while studying Correlation of caffeine elimination and Child's classification in liver cirrhosis patients, Holstege et al24 reported that the caffeine clearance in cirrhosis decreased with increasing Child-Turcotte classification score. They further reported that Child's class A patients differed significantly from Child's class B or Child's class C patients, whereas the difference between Child's class B and C patients did not reach statistical significance (Wilcoxon's rank test) though strong correlation between the Child-pugh classification score and apparent caffeine clearance (p less than 0.001) was noted. The child-pugh classification adopted in the present study is also similar to that used by N.R. Scott et al15 in 1989, their study also showed a significant correlation (p < 0.001) between child-pugh score of severity of liver disease and salivary caffeine clearance values.
It is also evident from the results that salivary caffeine concentration in patients (study group) at 0 h, i.e. after cessation of caffeine intake for 17 h; were with a mean of 2.4 ± 1.4 μg/ml. While in the controls; the mean value of salivary caffeine concentrations at 0 h was much lower i.e. 0.4 ± 0.2 μg/ml [Table 1]. This shows that salivary caffeine elimination is delayed in chronic liver disease patients and caffeine can be detected in saliva even after cessation of caffeine intake for 17 h.
As we see in this study that caffeine is a good test material to assess the liver function but some issues needed to be addressed for standardization of test results. These issues are decreased caffeine metabolism in older people, smoking induced fast metabolism and effect of various drugs on caffeine metabolism for example impaired elimination by cimetidine. Besides that effect of caffeine test dose on the serious complications of liver disease like malena, hematemesis, encephalopathy and esophageal varices are not clearly understood thus require further study.
5. Conclusion
The results obtained in this study signify the fact that salivary and serum caffeine clearance are highly correlated and saliva as a sample is compatible with serum samples to detect caffeine clearance values. Further the caffeine clearance in saliva is found to decrease progressively with increased severity of liver disease and it is significantly correlated with Child pughs classification of severity of chronic liver disease. Thus with the salivary caffeine clearance values it is possible to discriminate chronic liver disease subjects from normal control group and it is also possible to find severity of chronic liver disease. The findings of the present study also indicate that this test is harmless, inexpensive, and easy to repeat and is feasible in hospitals for routine use.
Conflicts of interest
All authors have none to declare.
References
- 1.McDonagh J.E. Caffeine clearance by enzyme multiplied immunoassay technique: a simple, inexpensive, and useful indicator of liver function. Gut. 1991;32:681–684. doi: 10.1136/gut.32.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limdi J.K., Hyde G.M. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–312. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel S.P., Marshall M.K. 8th ed. JB Lippincott publications; USA: 1999. Evaluation of the Liver: Laboratory Tests. Schiff's Diseases of the Liver; pp. 205–239. [Google Scholar]
- 4.Sherlock S. 10th ed. Blackwell science ltd; London: 1997. Assessment of Liver Function Disease of Liver and Biliary System: Sheila Sherlock; pp. 17–32. [Google Scholar]
- 5.Tarantino Giovanni. Could quantitative liver function tests gain wide acceptance among hepatologists? World J Gastroenterol: WJG. 2009;15:3457–3461. doi: 10.3748/wjg.15.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman L.S., Martin P., Munoz S.J. Liver function tests and the objective evaluation of the patient with liver disease. In: Zakin D., Boyer T.D., editors. Hepatology: A Textbook of Liver Disease. 3rd ed. WB Saunders; Philadelphia: 1996. pp. 791–833. [Google Scholar]
- 7.Leevy C.M., Mendenhall C.L., Lesko W., Howard M.M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merkel C., Gatta A., Zoli M. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci. 1991;36:1197–1203. doi: 10.1007/BF01307508. [DOI] [PubMed] [Google Scholar]
- 9.Bircher J., Kupfer A., Gikalov I., Preisig R. Aminopyrine demethylation measured by breath analysis in cirrhosis. Clin Pharmacol Ther. 1976;20:484–492. doi: 10.1002/cpt1976204484. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman K., Zhang Y.Y., Gitt A. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 11.Jost G., Wahllander A., Mandach U., Preisig R. Overnight salivary caffeine clearance: a liver function test suitable for routine use. Hepatology. 1987;7:338–344. doi: 10.1002/hep.1840070221. [DOI] [PubMed] [Google Scholar]
- 12.Blancharad J., Sawers S.J.A. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol. 1983;24:93–98. doi: 10.1007/BF00613933. [DOI] [PubMed] [Google Scholar]
- 13.Arnaud M.J., Welsch C. IXth International Colloquium on the Science and Technology of Coffee. Association Scientifiques Intemationale du Cafd; London: 1981. Caffeine metabolism in human subjects; pp. 385–396. [Google Scholar]
- 14.Baker A., Girling A., Worthington D. The prognostic significance of caffeine half-life in saliva in children with chronic liver disease. J Pediatr Gastroenterol Nutr. 1995 Feb;20:196–201. doi: 10.1097/00005176-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Scott N.R., Stambuk D., Chakraborty J., Marks V., Morgan M.Y. The pharmacokinetics of caffeine and its dimethylxanthine metabolites in patients with chronic liver disease. Br J Clin Pharmac. 1989;27:205–213. doi: 10.1111/j.1365-2125.1989.tb05352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jover R., Carnicer F., Sánchez-Payá J., Climent E., Sirvent M., Marco J.E.L. Salivary caffeine clearance predicts survival in patients with liver cirrhosis. Am J Gastroenterology. 1997;92:1905–1908. [PubMed] [Google Scholar]
- 17.François, Valla Dominique. Assessment of the prognosis of cirrhosis: child–Pugh versus MELD. J Hepatology. 2005;42:S100–S107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Zysset T., Wahlländer A., Preisig R. Evaluation of caffeine plasma levels by an automated enzyme immunoassay (EMIT) in comparison with a high-performance liquid chromatographic method. Ther Drug Monit. 1984;6:348–354. doi: 10.1097/00007691-198409000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Bonati M., Latini R., Galletti F., Young J.F., Tognoni G., Garattini S. Caffeine disposition after oral doses. Clin Pharmacol Ther. 1982;32:98–106. doi: 10.1038/clpt.1982.132. [DOI] [PubMed] [Google Scholar]
- 20.Wahlländer A., Mohr S., Paumgartner G. Assessment of hepatic function. Comparison of caffeine clearance in serum and saliva during the day and at night. J Hepatol. 1990;10:129–137. doi: 10.1016/0168-8278(90)90041-o. [DOI] [PubMed] [Google Scholar]
- 21.Biederbick W., Joseph G., Rump A., Theisohn M., Klaus W. Caffeine in saliva after peroral intake: early sample collection as a possible source of error. Ther Drug Monit. 1997;19:521–524. doi: 10.1097/00007691-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Marchesini G., Checchia G.A., Grossi G. Caffeine intake, fasting plasma caffeine and caffeine clearance in patients with liver diseases. Liver Int. 09/1988;8:241–246. doi: 10.1111/j.1600-0676.1988.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 23.Lewis F.W., Rector W.G. Caffeine clearance in cirrhosis: the value of simplified determinations of liver metabolic capacity. J Hepatology. 1992;14:157–162. doi: 10.1016/0168-8278(92)90152-f. [DOI] [PubMed] [Google Scholar]
- 24.Holstege A., Staiger M., Haag K., Gerok W. Correlation of caffeine elimination and Child's classification in liver cirrhosis. Klin Wochenschr. 1989;67:6–15. doi: 10.1007/BF01736528. [DOI] [PubMed] [Google Scholar]


