Abstract
γ-aminobutyric acid type-A receptors (GABAARs) mediate the majority of fast synaptic inhibition and are the principle sites of action for anxiolytic, sedative, hypnotic and anti-convulsant agents that include benzodiazepines, barbiturates, neurosteroids and some general anesthetics. GABAARs are hetero-pentameric ligand-gated ion channels that are found concentrated at inhibitory postsynaptic sites where they mediate phasic inhibition. Specialized populations of extrasynaptic GABAARs that mediate tonic inhibition are also expressed by neurons. The efficacy of phasic inhibition and thus neuronal excitability is critically dependent on the accumulation of specific GABAAR subtypes at inhibitory synapses. Here we evaluate how neurons control the number of GABAARs on the neuronal plasma membrane together with their selective stabilization at synaptic sites. We then go on to examine the impact that these processes have on the strength of synaptic inhibition and their possible impact on behavior and the etiology of neuropsychiatric disorders.
I. Introduction
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the adult mammalian central nervous system (CNS). It is estimated that at least one third of all CNS neurons utilize GABA as their primary neurotransmitter. Most of these GABAergic neurons are interneurons and therefore uniquely able to alter the excitability of local circuits within a given brain region. GABA exerts its powerful inhibitory influence by acting on two distinct classes of receptors based on their electrophysiological and pharmacological properties. Ionotropic GABAARs are fast acting ligand-gated chloride channels (Sieghart 2006) while metabotropic GABAB receptors are coupled indirectly via G proteins to either calcium or potassium channels to produce slow and prolonged inhibitory responses (Bettler and Tiao 2006). GABAA receptors are clinically relevant drug targets for anti-convulsant, anxiolytic and sedative-hypnotic agents including benzodiazepines, barbiturates, neurosteroids, certain classes of general anesthetics and alcohol. These allosteric compounds bind to discrete sites distinct from the agonist binding site to either positively or negatively modulate receptor function (Sieghart et al., 2006).
The classic GABAAR-mediated hyperpolarization of the membrane potential is attributed to the direct activation of an integral ion channel and the resultant influx of chloride along its electrochemical gradient. The concentration-response curve exhibits positive co-operativity consistent with the presence of at least two GABA binding sites on the receptor complex (Baumann et al., 2003). Following brief exposure to high (millimolar) concentrations of GABA (released from presynaptic vesicles) activation of GABAARs located at synaptic sites transiently moves the membrane potential away from the spike threshold required for action potential generation underlying what is known as ‘phasic’ inhibition. In contrast, low (submicromolar) concentrations of ambient GABA in the extracellular space can persistently activate spatially and temporally less restricted extrasynaptic receptors to generate a persistent or ‘tonic’ inhibitory state (Farrant and Nusser 2005). Given the critical role of GABAARs in controlling neuronal excitability and animal behavior, it is of fundamental importance to understand the mechanisms used by neurons to regulate their function.
Emerging evidence indicates that activity-dependent changes in the number of postsynaptic GABAARs represent one of the most powerful mechanisms underlying the functional plasticity of GABAergic synapses. Moreover, deficits in the functional expression of GABAARs have been implicated in the pathogenesis of a wide range of neurological and psychiatric diseases including epilepsy, anxiety, depression, schizophrenia and substance abuse. A significant amount of research has thus focused on detailing the cellular and molecular mechanisms that regulate the stability of GABAARs on the cell surface and their accumulation at the inhibitory postsynapse.
II. GABAAR structure
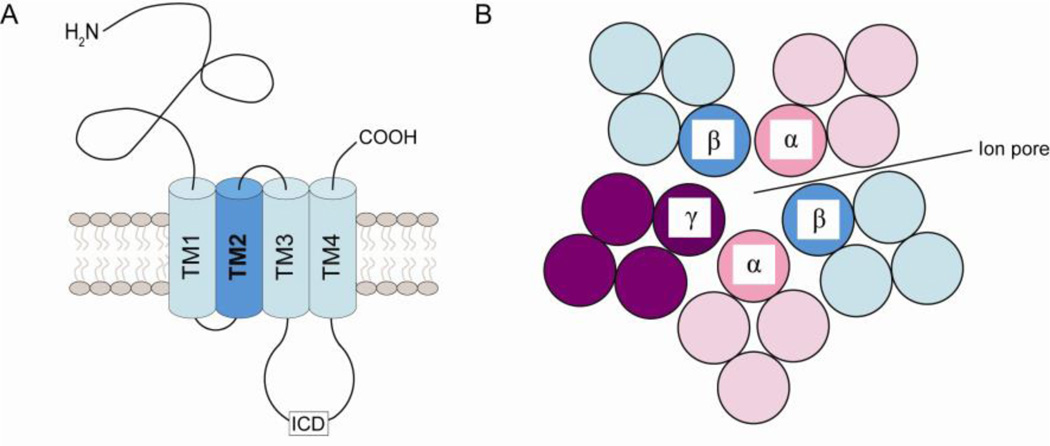
GABAARs belong to the superfamily of Cys-loop ligand-gated ion channels that comprises nicotinic acetylcholine (nACh) receptors, strychnine-sensitive glycine receptors and 5-hydroxytryptamine type-3 (5-HT3) receptors. Members of this family are heteropentameric glycoproteins composed of homologous subunits that specifically recognize one another and assemble around an intrinsic ion channel, which in the case of the GABAAR is permeable to chloride and, to a lesser extent, bicarbonate anions (Unwin, 1993). Each subunit is predicted to share a common membrane topology encompassing a large extracellular N-terminal ligand-binding domain and four transmembrane (TM)1–4 domains. A major cytoplasmic loop lies between TM3 and TM4 that is the most divergent part of the sequence among the GABAAR subfamily. This intracellular domain is the target for a number of post-translational modifications that can directly affect channel function and/or mediate the interaction with cytosolic proteins important for regulating receptor localization and/or membrane trafficking.
Comparative modeling of the GABAAR based on the 4-Å resolution model of the Torpedo nACh receptor for the first time provided an insight into the 3D organization of this ligand-gated ion channel (Ernst et al., 2005). The extracellular domain of each receptor subunit comprises a variable N-terminal domain and two β-folded sheets that form a twisted sandwich connected by a signature disulphide bridge. Each subunit has a principal (+) and a complementary (−) side. The GABA binding sites are located in solvent-accessible pockets at the two β+α− subunit interfaces and that of benzodiazepines at the α+γ− interface (Ernst et al., 2003). The TM domain of the receptor is made up of four loosely-packed helical bundles resulting in a considerable amount of solvent-accessible space within subunits and at the subunit interfaces. It has been proposed that the intersubunit pockets form a continuous groove with their extracellular counterparts, suggesting that they may not only play a role in the conformational mobility of the receptor but may also provide drug-binding sites.
The intrasubunit pockets contain a number of amino acid residues that have been shown to be of key importance in the binding and/or efficacy of a number of modulatory drugs. For example, a pocket within the α1 subunit, defined by the presence of S270 in TM2 and A291 in TM3, is thought to correspond to the site of action of volatile anesthetics (Nishikawa et al., 2002). Similarly, the presence of a homologous serine residue (S265) in the intrasubunit pocket of β1, which is replaced by an aspartic residue in the β2 and β3 subunit isoforms, is believed to account for the subtype-selectivity of etomidate and other related substances (Belelli et al., 1997). Future experiments leading to the improvement in the accuracy of the models will not only continue to provide a new perspective on existing data but also pave the way for structure-based drug design.
III. Subunit composition of synaptic and extrasynaptic GABAAreceptors
To date, 21 GABAAR subunits have been cloned and sequenced from the mammalian CNS. These have been divided into eight classes on the basis of sequence identity (Whiting et al., 1999): α(1–6), β(1–3), γ(1–3), δ, ε(1–3), π, θ and ρ(1–3). Subunit isoforms within a single class share approximately 70% sequence identity but between classes this falls to 30–40%. Alternatively spliced variants of several of these subunits have been reported, generating further subunit diversity and the potential for extensive molecular heterogeneity (Sieghart et al., 1999). For example, the γ2 subunit exists in short (γ2S) and long (γ2L) forms, which differ in an eight amino acid insert in the intracellular domain of the γ2L subunit (Whiting et al., 1990; Kofuji et al., 1991). There is a plethora of GABAAR subunit isoforms; however, due to the selective oligomerization mediated by receptor assembly, only a limited number of subunit combinations are in fact expressed on the neuronal plasma membrane (Sieghart and Sperk, 2002). The majority of GABAAR subtypes in the brain are composed of α1β2γ2, followed by α2β3γ2 and α3β3γ2 with a likely stoichiometry of 2α:2β:1γ, similar to that found for nACh receptors (Chang et al., 1996; Farrar et al., 1999; Knight et al., 2000; Tretter et al., 1997). To a lesser extent, δ, ε, π subunits replace the γ subunit to form benzodiazepine-insensitive receptor subtypes, whereas the θ subunit has been shown to replace the β subunit (Sieghart and Sperk, 2002). Conversely, ρ subunits (which are predominantly expressed in the retina) rarely co-assemble with other GABAAR subunits. Instead they homo- and hetero-oligomerize with other ρ subunits to form a family of pharmacologically distinct GABA-gated chloride channels that have been termed GABAC receptors (Bormann 2000).
In addition to molecular determination of GABAAR assembly, subunit composition is further restricted by the spatial and temporal pattern of subunit expression. In situ hybridization and immunohistochemical studies have demonstrated that each one of the subunits has a distinct regional and cellular distribution within the brain. Different subunits and/or subunit combinations further dictate the subcellular localization of these ligand-gated chloride channels and determine their biophysical and pharmacological properties. Detailed knowledge of the molecular composition and the exact anatomical expression of different GABAAR subtypes are therefore crucial in understanding the physiological actions of GABA within the brain and for developing potentially clinically useful subtype-selective drugs that are devoid of the side effects associated with the classical benzodiazepines.
In the cerebellum the γ2 subunit has been shown to be a component of all postsynaptic GABAARs, whereas the δ subunit is found almost exclusively at extrasynaptic sites (Nusser et al., 1998b). GABAARs incorporating a γ2 subunit together with α1–3 subunits (α1–3β2/3γ2) are thus the predominant receptor subtypes responsible for mediating phasic inhibition. It is important to note, however, that these receptor subtypes are also abundant at perisynaptic and extrasynaptic sites. This is consistent with recent evidence for the dynamic mobility and rapid exchange of γ2 subunit-containing receptors between extrasynaptic and synaptic receptor pools (Jacob et al., 2005; Thomas et al., 2005). The α5 subunit is unique in that, despite its coassembly with a γ2 subunit (α5β3γ2), it is enriched at extrasynaptic sites (Brunig et al., 2002). In accordance with this finding, deletion of the α5 subunit eliminates tonic conductance in cultured hippocampal neurons (Caraiscos et al., 2004). However, there is a pool of α5 subunit-containing GABAARs that concentrates at GABAergic synapses on CA1 pyramidal cell dendrites (Serwanski et al., 2006). In a recent study, diazepam-sensitive IPSCs elicited by dendrite preferring interneurons in the rat neocortex were blocked by the α5 subtype-selective inverse agonist (α5IA), suggesting that α5βγ2 receptor subtypes may also play also play a role in phasic inhibition (Ali and Thomson, 2008). α4 and α6 subunits form assembled channel complexes with δ subunits (α4βδ and α6βδ) that are exclusively extrasynaptic, accounting for tonic conductance in the thalamus and cerebellum respectively (Nusser et al., 1998b). On the other hand, the majority of phasic signaling in the cerebellum is due largely to synaptic α6 subunit-containing receptors of the α6β2γ2 combination (Nusser et al., 1998b).
IV. Functional significance of GABAAR structural heterogeneity
While the precise subcellular localization of different GABAAR subtypes undoubtedly contributes to their participation in phasic and tonic forms of signaling, this distinction alone is not sufficient to account for their differential activation. Subunit composition is a major determinant of the binding and gating properties of the ion channels, and hence the magnitude of the response following exposure to ligand. Studies using recombinant GABAARs have revealed that sensitivity to GABA is defined by the type of α subunit present. Extrasynaptic α6β3δ and α4β3δ subunit compositions display the highest affinities for GABA and synaptic α3β3γ2 subtypes the lowest (Bohme et al., 2004). For both αβγ and αβδ assemblies, the identity of the α subunit also affects the rates of activation, deactivation and desensitization (Bianchi et al., 2002; Caraiscos et al., 2004; Gingrich et al., 1995; Lavoie et al., 1997; McClellan and Twyman, 1999; Tia et al., 1996). Conversely, in αβ3γ2 subunit-containing receptors replacing the γ2 subunit with a δ subunit results in a dramatic reduction in single channel conductance independent of the type of α subunit present (Fisher and Macdonald, 1995). This supports the notion that GABA has a high affinity but low efficacy at δ subunit-containing extrasynaptic receptors. The presence of a γ2 or δ subunit also influences channel kinetics: αβδ receptors desensitize more slowly and less extensively than αβγ receptors (Bianchi and Macdonald, 2002; Haas and Macdonald, 1999; Saxena and Macdonald, 1996). Together the distinct biophysical properties of γ and δ subunit-containing receptors are wholly consistent with the involvement of these receptor subtypes in phasic and tonic signaling respectively.
Differences in subunit composition between synaptic and extrasynaptic receptors are reflected in a differential modulation of phasic and tonic signaling by a number of compounds of therapeutic importance. The most frequently cited example of this is the role of the α subunit in defining receptor affinity for benzodiazepines. GABAARs incorporating either an α4 or α6 subunit renders receptors insensitivity to fumctional modulation by benzodiazepines (Benson et al., 1998) as does elimination or substitution of the γ2 subunit. This difference can be attributed solely to the presence of a conserved arginine residue in α4 and α6 subunits, which in α1–3 and α5 subunits is a histidine (Wieland et al., 1992). Thus benzodiazepine site ligands selective for α1–3 subunits largely influence phasic signaling, whereas those selective for α5 subunits are capable of primarily modulating tonic conductance.
The diverse CNS depressant effects of benzodiazepines have been attributed to specific α subunit types of GABAARs (Rudolph and Möhler, 2004). A combined molecular genetic and pharmacologic approach has revealed that α1 subunit-containing receptors mediate the sedative, amnesic and, in part, anticonvulsant actions of diazepam, whereas α2 subunits contribute to its anxiolytic and muscle-relaxant effects. Strong pharmacological evidence for the involvement of the α3 subunit in anxiety comes from a study by Atack and colleagues (2005) who showed that the α3 subtype-selective inverse agonist (α3IA) induces an anxiogenic response in rats. The α5 subunit has been implicated in learning and memory following the observation that a single point mutation (H105R), which prevents the interaction of this receptor subtype with diazepam, abolishes its memory impairing effects (Crestani et al., 2002). The development of tolerance to the sedative actions of chronic diazepam is also associated with a downregulation of α5 subunit-containing GABAARs to which α5-H105R mice are resistant (Rijnsoever et al., 2004).
The diverse functions of GABA in the CNS are matched not just by the heterogeneity of GABAARs but also by the complex trafficking and clustering mechanisms that generate and maintain surface receptor populations accessible to the neurotransmitter at inhibitory postsynaptic specializations or extrasynaptic sites, depending on their subunit composition. The regulation of these mechanisms by GABAAreceptor-associated proteins is discussed below.
V. Membrane trafficking of GABAARs
GABAARs are not static entities on the neuronal cell surface but are believed to cycle continuously between the plasma membrane and intracellular compartments. The relative rates of receptor exo- and endocytosis are therefore key determinants in controlling the size of the postsynaptic pool accessible to GABA and GABAergic compounds and thus the strength of synaptic inhibition. Importantly, GABAARs have recently been reported to be inserted into and removed from the plasma membrane exclusively at extrasynaptic sites (Bogdanov et al., 2006; Thomas et al., 2005), highlighting the importance of lateral diffusion for their postsynaptic specialization.
VI. Exocytosis of GABAARs to the plasma membrane
GABAARs can be delivered to the cell surface either as newly assembled channel complexes via a de novo secretory pathway or reinserted following internalization. The oligomerization of GABAAR subunits into channel complexes is believed to occur in the endoplasmic reticulum (ER). Evidence suggests that this assembly process plays a critical role in determining the diversity of receptor subtypes expressed on the neuronal plasma membrane. Proteins cannot exit the ER until they have achieved their correctly folded conformation, and misfolded or unassembled proteins are retrotranslocated from this organelle for degradation in the proteasome, restricting the number of subunit combinations that can access the cell surface (Kittler et al., 2002). Following correct assembly, GABAARs are trafficked to the Golgi apparatus and segregated into vesicles for transport to and insertion into the plasma membrane facilitated by a number of receptor-associated proteins.
Yeast two-hybrid screens using the γ2 subunit ICD as bait isolated the first known GABAAR associated protein (GABARAP) (Wang et al., 1999), a 17 kDa cytosolic protein belonging to the family of membrane associated proteins (MAPs) involved in membrane trafficking. This includes the estrogen-induced protein first isolated in guinea-pig endometrial cells (GEC)-1 (or GABRAP like-1), Golgi-associated transport enhancer of 16 kDa (GATE-16, or GABARAP like-2), Apg8L and light chain (LC)-3 subunits of MAP1. Interestingly, GABARAP knockout mice do not show differences in punctate staining of γ2 subunit-containing GABAARs and lack an overt behavioral phenotype (O’Sullivan et al., 2005). GABARAP may thus be functionally redundant. Nevertheless, the notion that GABARAP is involved in the trafficking of GABAARs to the plasma membrane is supported by the finding that overexpression of GABARAP in heterologous expression systems and in cultured hippocampal neurons results in an increase in the cell surface expression of γ2 subunit-containing GABAARs (Leil et al, 2004). In Xenopus oocytes expressing α1β2γ2 subunit-containing GABAARs this was accompanied by an increase in GABAAR-mediated synaptic inhibition, an effect requiring the γ2 subunit- and microtubule-binding motifs as well as intact polymerized microtubules (Chen et al., 2005). More recently it has been demonstrated that GABARAP-mediated exocytosis of GABAARs is necessary for potentiation of inhibitory transmission by NMDA receptor activation, suggesting that GABARAP might have a role in the regulated delivery of GABAARs to the plasma membrane after activity rather than in the maintenance of basal surface receptor levels (Marsden et al., 2007).
Evidence in support of a function of GABARAP as a trafficking factor includes the identification of phospholipase C (PLC)-related catalytically inactive protein (PRIP)-1, a 130 kDa protein that is believed to competitively inhibit the binding of the γ2 subunit of GABAARs to GABARAP (Kanematsu et al., 2002). PRIP1 knockout mice show impairments in GABAAR modulation by benzodiazepines and zinc-sensitivity, indicating reduced activation of γ2 subunit-containing receptors (Kanematsu et al., 2002). These findings suggest that PRIP1 may play a role in the regulation of GABAAR trafficking by GABARAP, ensuring that only mature αβγ receptor complexes are delivered to the plasma membrane. In addition, PRIP1 has been shown to directly bind to the ICD of GABAAR β1–3 subunits, serving as an adaptor protein for the protein phosphatase (PP)-1α, and as such has been implicated in the phospho-dependent modulation of GABAAR functional expression (Terunuma et al., 2004). Recently, a second PRIP isoform (PRIP2) has been identified that, like PRIP1, binds both GABARAP and PP1α, suggesting a central role for all PRIP isoforms in modulating GABAAR functional expression (Uji et al., 2002).
GABAARs also interact with the protein that links the integrin-associated protein with the cytoskeleton (Plic)-1. Plic1 is a 67 kDa protein with a ubiquitin-like (UBL) N-terminal domain and a ubiquitin-associated (UBA) C-terminal domain (Kleijnen et al., 2000). It is able to bind ubiquitin ligases and components of the proteasome. As such it is thought to interfere with ubiquitin-dependent proteolysis of proteins (Kleijnen et al., 2000). Yeast two-hybrid screens and glutathione S-transferase (GST) affinity purification assays have shown that Plic1 interacts with the ICD of α1–3,6 and β1–3 (but not γ2 or δ) subunits of GABAARs (Bedford et al., 2001), indicating that Plic-1 function might be relevant for the majority of receptor subtypes expressed in the brain. This interaction has been demonstrated to be of significance in mediating the functional expression of GABAARs in human embryonic kidney (HEK-293) cells and in hippocampal slices as revealed using dominant negative peptides (Bedford et al., 2001). In a recent study by Saliba and colleagues (2008), the authors showed that Plic1 increases the accumulation of GABAAR β3 subunits on the cell surface in a manner independent of their rates of internalization. These findings suggest that Plic1 selectively modulates the secretory pathway. In accordance with this, Plic1 was found to significantly increase the half-life of polyubiquitinated GABAAR β3 subunits in the ER and facilitate their insertion into the plasma membrane (Saliba et al., 2008). By increasing the residence times of unassembled subunits in the ER, Plic1 may also increase subunit maturation and production of heteromeric receptors. Plic1 regulation of the ubiquitin-dependent proteasomal degradation of GABAARs may thus provide a dynamic mechanism for regulating the efficacy of inhibitory synaptic transmission.
The 190 kDa brefeldin A-inhibited guanine nucleotide exchange factor (BIG)-2 was also identified as a GABAAR β subunit interacting protein in a yeast two-hybrid screen (Charych et al., 2004). BIG2 is concentrated mainly in the trans-Golgi network but is also found in vesicle-like structures along dendrites and at the postsynaptic plasma membrane (Charych et al., 2004). Interestingly, coexpression of BIG2 with the GABAAR β3 subunit results in an increase in β3 exit from the ER, suggesting that BIG2 is involved in the post-Golgi vesicular trafficking of GABAARs (Charych et al., 2004). It is currently proposed that BIG2 may play a role in the transport of newly assembled GABAARs to the postsynaptic plasma membrane and also be involved in receptor recycling (Shen et al., 2006; Shin et al., 2004). Other GABAAR-associated proteins implicated in the forward trafficking of GABAARs include the GABAAR interacting factor (GRIF)-1 (Beck et al., 2002) and the multifunctional protein gC1qR (Schaerer et al., 2001). However, their functional significance remains unclear.
VII. Endocytosis of GABAARs from the plasma membrane
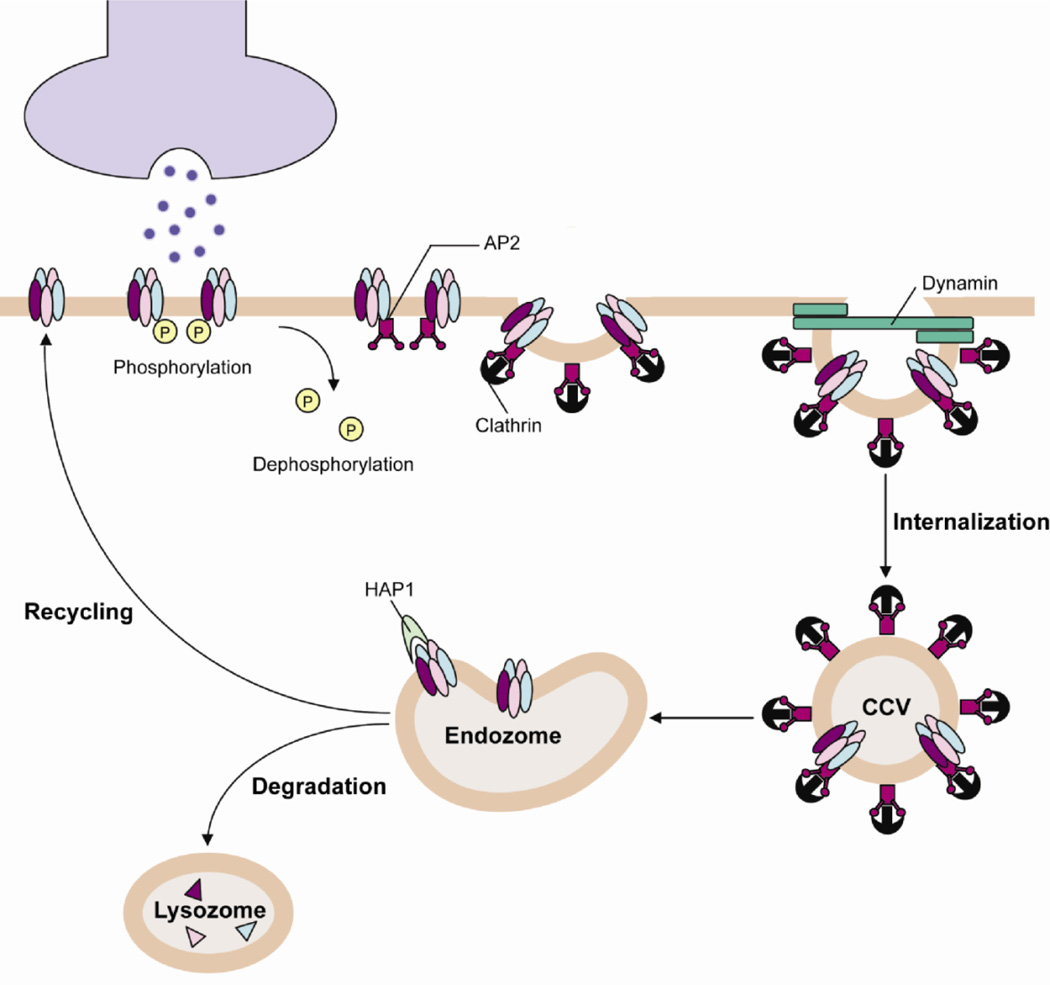
GABAARs have been shown to be localized in clathrin-coated pits, suggesting that they undergo clathrin-mediated endocytosis, a process that is further dependent on dynamin for endocytic vesicle formation. The clathrin adaptor protein (AP)-2 is a central component in the formation of these vesicles, forging a link between membrane proteins and clathrin, which forms the outer layer of the coat. AP2 is a heterotetrameric complex composed of two large (~100 kDa) α and β2 subunits, a medium (50 kDa) μ2 subunit, and a small (19 kDa) σ2 subunit, commonly referred to as adaptins. The α adaptin is responsible for targeting the protein to the plasma membrane, where the β2 adaptin interacts with clathrin to trigger clathrin assembly, forming coated pits. This in turn leads to the activation of μ2 adaptin phosphorylation, inducing a conformational change in the subunit that allows the complex to directly bind to endocytic motifs in cell surface receptors, clustering the protein cargo into the assembling coated pit. Cargo is bound by the β2 and μ2 adaptins that mostly recognize a dileucine motif or the canonical tyrosine-based YXXΦ motif (where X denotes any amino acid and Φ a bulky hydrophobic residue) (Clague, 1998; Le Borgne and Hoflack, 1998). The σ2 adaptin is responsible for stabilizing the complex at the core by mediating subunit interactions. The AP2 complex also interacts with several accessory proteins that form essential components of the endocytic machinery (Slepnev and De Camilli, 2000).
GABAARs are intimately associated with AP2 in the brain through a direct binding of the β1–3 and γ2 GABAAR subunits (Kittler et al., 2000). In the GABAAR β2 subunit a dileucine AP2-β2 adaptin-binding motif (L343L344) has been identified. This motif is important for clathrin-mediated endocytosis in HEK-293 cells and in cortical slices (Herring et al., 2003, 2005). It is also present in the ICDs of receptor β1 and β3 subunits, but evidence suggests that it is not involved in the interaction of these subunits with the AP2 complex (Kittler et al., 2005). In addition, an atypical AP2-binding motif conserved within the ICD of all GABAAR β subunit isoforms has been identified (KTHLRRRSSQLK in the β3 subunit) (Kittler et al., 2005). This motif, which is enriched in lysine and arginine residues, also incorporates the major sites of phosphorylation for PKA and PKC within this class of receptor subunits: S409 in β1, S410 in β2 and S408/9 in β3 (Moss et al., 1992). In vitro binding experiments have shown that phosphorylation and/or mutation of these residues confers a reduction in binding of the GABAAR β subunits to the μ2 adaptin of the AP2 complex (Jacob et al., 2009; Kittler et al, 2005). Moreover, neurons expressing GABAARs incorporating fluorescent β3S408/9A subunits exhibit reduced endocytosis and enhanced functional expression at both synaptic and extrasynaptic sites (Jacob et al., 2009). Intriguingly, while analyzing the cell surface distributions of wild-type and S408/9A mutant β3 subunit-containing GABAARs, the authors noted an apparent increase in long, filopodia-like spines on neurons expressing the latter. This deficit in spine maturity was reversed by pharmacological blockade of GABAARs. Regulating the efficacy of synaptic inhibition by modulating GABAAR membrane trafficking may thus play a critical role in regulating spinogenesis and synaptic plasticity (Jacob et al., 2009).
More recently a tyrosine based AP2-μ2 adaptin-binding motif in the GABAAR γ2 subunit (Y365GY367ECL) has been identified that is also conserved in the γ1 and γ3 subunits (Kittler et al., 2008). These tyrosine residues are the principal sites for phosphorylation by Fyn and Src kinase (Jurd et al; 2010; Brandon et al., 2001; Moss et al., 1995). Utilizing nonphosphorylated and phosphorylated peptides corresponding to Y365 and Y367 the authors showed that this high affinity interaction is phospho-dependent (Kittler et al., 2008). Introduction of the nonphosphorylated γ2 peptide into neurons induced a large increase in the mIPSC amplitude that was accompanied by an increase in the number of receptors on the cell surface (Kittler et al., 2008).
The physiological significance of GABAAR endocytosis has been further evaluated by creating a knock-in mouse in which Y365 and Y367 in the γ2 subunit have been mutated to phenylalanine mutations, significantly increasing the affinity for μ2-AP2. Homozygotes for these mutations die in utero. Heterozygotes are viable and do not exhibit any gross behavioral deficits. Consistent with the roles of Y365/7F in mediating high affinity binding to μ2-AP2 Y365/7F knock-in mice have elevated total and cell surface expression levels. In the hippocampus this results in an increase in the size of inhibitory synapses and efficacy of synaptic inhibition that is specific to CA3. This increase in inhibition correlates with a specific deficit in spatial memory in Y365/7F mice, a behavioral paradigm dependent upon CA3. Modifying GABAAR endocytosis clearly has behavioral consequences.
VIII. Post-endocytic GABAAR sorting
The fate of internalized receptors is another determinant of surface receptor levels. Following internalization GABAARs are either rapidly recycled back to the neuronal plasma membrane or, over longer time frames, are targeted for lysozomal degradation, an endocytic sorting decision that is regulated by the Huntingtin-associated protein (HAP)-1. Yeast two-hybrid screens have revealed that HAP1 interacts with the GABAAR β1 subunit but not the α1, γ2, or δ subunits (Kittler et al., 2004). Overexpression of HAP1 in cultured neurons has been shown to increase the number of receptors on the cell surface at steady-state, an increase that correlates with a dramatic increase in the mean amplitude of mIPSCs but that does not affect the frequency or kinetics of these events (Kittler et al., 2004). However, whether HAP1 promotes GABAAR recycling or prevents receptor lysozomal degradation is an unresolved issue. Nevertheless, the importance of HAP1-dependent regulation of GABAAR trafficking is clear, as evidenced by a study in which selective suppression of hypothalamic HAP1 by siRNA induced a decrease in feeding behavior in mice that was attributed to reduced surface expression and activity of GABAARs (Sheng et al., 2006). More recently it was established that HAP1 plays a role in regulating the targeting of GABAARs to inhibitory synapses dependent on the molecular motor-KIF5 (Twelvetrees et al., 2010).
IX. Postsynaptic targeting and clustering of GABAARs
The highly selective subcellular localization of GABAAR subtypes implies that subunit composition plays a major role in the postsynaptic targeting and clustering of these receptors. While the exact molecular mechanisms that govern the accumulation of GABAARs at inhibitory synapses are not yet fully understood, it involves a number of receptor-associated proteins and cytoskeletal elements that are concentrated at postsynaptic densities (PSDs).
The inhibitory synaptic marker gephyrin is a 93 kDa subsynaptic scaffolding protein that was originally implicated in regulating the postsynaptic clustering of glycine receptors in the spinal cord by directly binding to the receptor β subunit (Meyer et al., 1995). More recently, data derived from gephyrin knockout mice and knockdown experiments using antisense oligonucleotides or shRNAi have revealed that reducing gephyrin expression also leads to an extensive loss in the punctate staining of GABAARs incorporating a γ2 subunit together with α2 (but not α1) subunits (Essrich et al., 1998; Jacob et al., 2005; Kneussel et al., 1999; Levi et al., 2004). This suggests the existence of both gephyrin-dependent and -independent GABAAR clustering mechanisms. Gephyrin clusters are absent in γ2 subunit knockout mice. The remaining α and β subunit-containing receptors show diffuse staining (Essrich et al., 1998). Furthermore, transfection of γ2 subunit-deficient neurons with chimeric α2/γ2 constructs revealed that the TM4 of the γ2 subunit is sufficient for targeting these receptors to postsynaptic sites, but that both the TM4 and ICD of the γ2 subunit were necessary for recruiting gephyrin to the synapse and rescuing GABAergic inhibitory synaptic function (Alldred et al., 2005). A role for this receptor-associated protein in stabilizing previously clustered GABAARs at the cell surface, rather than their specialization at inhibitory synapses, has thus been proposed. Concurrent with this, postsynaptic GABAARs have been shown to be three times more mobile in cells where gephyrin expression has been impaired as measured by fluorescence recovery after photobleaching (Jacob et al., 2005). It is emerging that the rate of lateral mobility of GABAARs plays a central role in determining the number of these receptors at synaptic sites, a process revealed by the use of single particle tracking. This approach has illustrated that there is rapid exchange between extrasynaptic and synaptic populations of GABAARs and that this process is subject to modulation via the activity of protein phosphatase IIB. This Ca+-dependent mechanism activated via NMDA receptors leads to an increase in the lateral mobility of GABAARs and a reduction in the size of inhibitory synapses, a process that favors neuronal depolarization (Bannai et al., 2009).
Gephyrin is believed to anchor postsynaptic GABAARs to the plasma membrane by cross-linking the receptor molecule to the tubulin and actin cytoskeleton. Gephyrin binds with high affinity to tubulin and as such is viewed as a bona fide microtubule binding protein MAP (Ramming et al., 2000). Gephyrin also mediates an indirect interaction with the cytoskeleton by binding to LC1 and LC2 of the dynein motor proteins and the microfilament-associated proteins belonging to the Mena/VASP family (Fuhrmann et al., 2002). The membrane-associated protein collybistin II is a guanine-nucleotide exchange factor (GEF) that binds to and so permits the subsynaptic localization of the otherwise intracellular gephyrin, where it directly interacts with and immobilizes specific GABAAR subtypes on the plasma membrane (Harvey et al., 2004; Kins et al., 2000). In addition, the dystrophin glycoprotein complex (DGC) and neuroligin (NL)-2 bridge the synaptic cleft by interacting with presynaptic β-neurexin, promoting GABAergic synaptogenesis (Tretter and Moss, 2008).
Although gephyrin is found to be concentrated at GABAergic synapses in both hippocampal and cortical neurons (where glycine receptor expression is relatively low), in initial studies using standard biochemical methods it failed to co-purify with any of the GABAAR subunits. Hence an intermediate linker protein was postulated. Given its ability to directly bind the γ2 subunit ICD and gephyrin the prime candidate was GABARAP. However, subsequent analysis revealed that GABARAP is not clustered at gephyrin-rich postsynaptic sepecilizations but instead is mainly localized in intracellular compartments including the ER, Golgi apparatus and, to some extent, in subsynaptic secretory vesicles (Kittler et al., 2001; Wang et al., 1999). This is consistent with a role for this protein in intracellular trafficking rather than postsynaptic clustering.
In a recent study Tretter and colleagues (2008) for the first time provided evidence that gephyrin binds directly to the α2 subunit ICD in vitro. This interaction was blocked by low concentrations of detergent, providing a possible explanation as to why previous attempts to identify a direct association between gephyrin and GABAARs were unsuccessful. However, under the same conditions only very weak binding to β3 and γ2 subunits was observed (Tretter et al., 2008), suggesting that α subunits isoforms may play a predominant role in the synaptic accumulation of GABAARs via their ability to directly bind gephyrin
The 81 kDa actin-binding protein radixin, a member of the ezrin/radixin/moesin protein family, has recently been shown to directly link the α5 subunit to the cytoskeleton via a radixin-binding motif conserved within the ICD of α1–3 and α5 subunits, differing only by two amino acids in the α2 subunit (Loebrich et al., 2006). In neurons, both depletion of radixin and replacement of the radixin actin-binding motif dramatically decreased α5 subunit-containing GABAAR clusters without altering total surface expression levels. Radixin also showed limited colocalization with postsynaptic gephyrin, consistent with previous reports that GABAAR α5 subunits localize mainly at extrasynaptic sites (Brunig et al., 2002; Loebrich et al., 2006). However, the mechanisms responsible for the formation of radixin-dependent extrasynaptic GABAAR clusters remain to be elucidated.
X. Modulation of GABAAR function by post-translational modifications
The cell surface stability of GABAARs is further regulated by post-translational modifications such as palmitoylation, ubiquitination and phosphorylation. These modifications have been implicated in altering the biophysical and pharmacological properties of these ligand-gated ion channels (Arancibia-Carcamo and Moss, 2009).
A. Palmitoylation
Palmitoylation is the covalent attachment of the saturated fatty acid palmitate to cysteine residues of a given protein by the palmitoyl transferase Golgi-specific DHHC zinc finger domain protein (GODZ). Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. As such it has been shown to be involved in the postsynaptic clustering and subcellular trafficking of a number of proteins, including AMPA receptors (Hayashi et al., 2005) and the neuronal scaffold proteins PSD-95 and glutamate receptor-interacting protein (GRIP)-1 (DeSouza et al., 2002; Smotrys and Linder, 2004). It is unique in that it is the only reversible lipid modification and thus allows the cell to dynamically regulate the location of specific proteins. In a yeast two-hybrid screen, the 34 kDa protein GODZ was identified as a GABAAR γ2 subunit interacting protein that recognizes a 14 amino acid cysteine-rich domain conserved in the ICD of all γ1–3 subunits, N-terminal to the GABARAP binding site (Rathenberg et al., 2004). Analysis of Cys-Ala mutant γ2 constructs in transfected COS-7 cells revealed that the γ2 subunit is palmitoylated at all four cysteines within the GODZ binding domain (Rathenberg et al., 2004). Mutation of these cysteine residues resulted in a loss of GABAAR clusters at the cell surface, as did drug-induced global inhibition of palmitoylation by Br-palmitate (Rathenberg et al., 2004). Likewise, disrupting GODZ function or expression levels using dominant negative or RNAi approaches resulted in a significant reduction in the amplitude of mIPSCs attributed to a decrease in postsynaptic GABAAR number (Fang et al., 2006). From these studies it is evident that palmitoylation can dynamically regulate the efficacy of neuronal inhibition by controlling the accumulation of GABAARs at the postsynaptic membrane, although the exact mechanism by which this is achieved is unknown.
B. Ubiquitination
The regulation of GABAAR trafficking by the ubiquitin-related protein Plic1 suggests that GABAARs may also be direct targets for modification by the polypeptide ubiquitin. The covalent attachment of one or more copies of the 76-amino acid ubiquitin monomer to lysine residues of target proteins is referred to as ubiquitination. Monoubiquitination is reversible and serves as an active signal in diverse intracellular trafficking pathways, including as a trigger for endocytosis. In contrast, polyubiquitination is required for the translocation of proteins from the ER back into the cytosol, where they are degraded by the proteasome. Activity-dependent polyubiquitination of GABAAR β3 subunits has been shown to reduce the stability of newly-translated and assembled receptors in the ER via a mechanism dependent on the activity of the proteasome (Saliba et al., 2007).
Coincident with a loss of cell surface expression levels, chronic blockade of neuronal activity by tetrodotoxin (TTX) treatment reduced both the amplitude and frequency of mIPSCs (Saliba et al., 2007). TTX had no effect on the enhanced functional expression of GABAARs incorporating β3 subunits in which all twelve lysine residues within the ICD of this subunit had been mutated to arginines (β3K12R). These mutations did not alter GABAAR cell surface half-life or internalization rates but did significantly enhance receptor insertion into the plasma membrane (Saliba et al., 2007).
C. Phosphorylation
Protein phosphorylation is fundamental to the activity of cellular signaling networks. It is achieved through protein kinase catalyzed transfer of a phosphate group to serine, threonine and/or tyrosine residues of a given protein substrate that can be reversed in a dephosphorylation reaction catalyzed by protein phosphatases. GABAARs are well-established phosphoproteins. Diverse studies on GABAAR phosphorylation have implicated this process in altering channel gating, conductance and/or kinetics, sensitivity of the receptors to pharmacological agents, protein-protein interactions and membrane trafficking (Vithlani and Moss, 2009). Hence the coordinated activity of kinases and phosphatases plays a pivotal role in controlling neuronal excitability.
Studies in heterologous expression systems have demonstrated that GABAAR function, depending on the subtype analyzed, can be differentially modulated by phosphorylation of key residues within the ICDs of receptor β1–3 and γ2 subunits by a number of kinases, including cAMP-dependent protein kinase (PKA), calcium/phospholipid-dependent protein kinase (PKC), calcium/calmodulin-dependent kinase II (CaMKII), protein kinase B (PKB - also known as Akt), cGMP-dependent protein kinase (PKG) and tyrosine kinases of the Src family (summarized in Table 1.2). This is best illustrated by the differential modulation of GABAAR subtypes by PKA, dependent upon the identity of the β subunit. In vitro studies using purified bacterially expressed GST fusion proteins combined with site directed mutagenesis revealed that the consensus motif for PKA-induced phosphorylation conserved within the ICDs of GABAAR β1–3 subunits is RRRXSQLK, where S is a serine at position 409 in β1 and β3 subunits and at position 410 in the β2 subunit (McDonald and Moss, 1997) and X represents either an alanine residue in β1 and β2 subunits or a serine residue at position 408 in the β3 subunit. However, in contrast to in vitro findings, analysis of recombinant GABAARs in HEK-293 cells has demonstrated that PKA-induced phosphorylation of the β3 subunit occurs at both S408 and S409 (rather than at S409 alone), whereas the β2 subunit is not a substrate for this kinase in heterologous expression systems (McDonald et al., 1998). Interestingly, PKA was shown to depress GABA-activated currents in HEK-293 cells expressing β1 subunit-containing GABAARs, whereas in β3 subunit-expressing cells a potentiation was observed (McDonald et al., 1998). The latter was attributed to the presence of two juxtaposed serine residues (S408 and S409) in β3, as selective mutation of S408 to alanine (to structurally resemble the GABAAR β1 subunit at this site) converted this potentiation to a depression (McDonald et al., 1998). On the other hand, PKA had no effect on β2 subunit-expressing cells (McDonald et al., 1998), which can be accounted for by the selective recruitment of PKA to GABAAR β1 and β3, but not β2, subunits via the A-kinase anchoring protein (AKAP) of 79 (rat)/150 (human) kDa (Brandon et al., 2003).
Table 1.
GABAA receptor associated proteins
| Protein | Subcellular Localization |
Subunit specificity |
Proposed function |
|---|---|---|---|
| GABARAP | Golgi | γ | Facilitates exocytosis |
| PRIP1/2 | Synapses | β | Enhances cell surface stability by inhibiting PP1α mediated dephosphorylation |
| PLIC1 | Intracellular compartments | α/β | Facilitates exocytosis by inhibiting proteasome-dependent degradation |
| BIG2 | Intracellular compartments | β | Promotes ER exit and structural integrity of recycling endosomes |
| AP2 | Clathrin-coated pits | β/γ | Regulates clathrin-mediated endocytosis |
| HAP1 | Endosomes | β | Regulates post-endocytic sorting by inhibiting lysozomal degradation |
| GODZ | Golgi | γ | Palmitoylation |
| Gephyrin | Synapses | α1 | Facilitates clustering/scaffolding at synaptic sites |
| Radixin | Extrasynaptic sites | α5 | Facilitates clustering at extrasynaptic sites |
| GRIF1 | Intracellular compartments | β2 | Unknown |
| gC1qR | Intracellular compartments | β3 | Unknown |
Adapted from Arancibia-Carcamo and Moss, 2006
AKAPs have also been shown to interact directly with PP2B and PKC in addition to PKA (Klauck et al., 1996; Colledge and Scott, 1999); however, the targeting of PP2B and PKC to GABAARs by AKAP remains to be investigated. Interestingly, PP2B has been shown to bind directly to the ICD of the γ2 subunit, thereby dephosphorylating the receptor and inducing long-term depression (LTD) of synaptic inhibition in the CA1 region of the hippocampus, a mechanism that is believed to contribute to learning and memory (Wang et al., 2003). PKC is recruited to the GABAAR via the anchoring protein receptor for activated C kinase (RACK)-1. Like PKA it has different effects on receptor function depending on subunit composition. In agreement with the findings in vitro all of the β subunit isoforms were found to be a substrate for this kinase in HEK-293 cells (Krishek et al., 1994; McDonald et al., 1998; Brandon et al., 2000). Functional studies employing site-directed mutagenesis have demonstrated a downregulation of receptor activity in response to phorbol esters mediated by PKC-induced phosphorylation of β1 S409, β2 S410, γ2S S327 and γ2L S327/343. However, the use of a constitutively active catalytic domain of PKC (PKM) produced results contradicting those obtained with phorbol esters. In transfected mouse fibroblasts PKM enhanced the response to GABA in a manner that was dependent on the phosphorylation of the β1 subunit at S409 and the γ2L subunit at S327 and S343 (Lin et al., 1996). The reason for this discrepancy remains unresolved but it has been speculated that different PKC isoforms may produce different responses (Song and Messing, 2005). In contrast, brain-derived neurotrophic factor (BDNF)-induced PKC-mediated phosphorylation of the β3 subunit has been shown to transiently increase the amplitude of mIPSCs in cultured neurons, an effect paralleled by an increase in GABAAR cell surface stability (Jovanovic et al., 2004). Furthermore, the initial enhancement observed in receptor activity was followed by a prolonged depression that was attributed to PP2A-mediated dephosphorylation of the β3 subunit (Jovanovic et al., 2004).
No adaptor protein is known for CaMKII, although the kinase is capable of coimmunoprecipitating with GABAARs from detergent-soluble mouse brain extracts (McAinsh et al., unpublished data). In vitro studies have revealed that the serine/threonine kinase CaMKII directly phosphorylates specific residues within the ICDs of GABAAR β and γ2 subunits. These include S384/409 in β1, S410 in β2 and S383/409 in β3, as well as the γ2 subunit at S343 (γ2L only), S348 and T350. Interestingly, the intracellular application of purified, active CaMKII failed to modulate the function of GABAARs heterologously expressed in HEK-293 cells but significantly potentiated the amplitudes of whole-cell GABA-activated currents recorded from rat cultured cerebellar granule neurons and from recombinant GABAARs expressed in neuroblastoma-glioma hybrid (NG108-15) cells. These findings imply the contribution of some essential neuronal factor (Houston and Smart, 2006).
In addition to binding PKC and the GABAAR β subunits RACK1 also binds the tyrosine kinase Src to facilitate the phosphorylation of the γ2 subunit on Y365 and Y367 (Brandon et al., 2001; Kittler and Moss, 2003; Moss et al., 1995). Coexpression of Src with recombinant α1β1γ2 subunit-containing GABAARs has been shown to increase whole-cell GABA-activated currents in HEK-293cells, an effect abolished by site-specific mutagenesis of both of these tyrosine residues to phenylalanines (Moss et al., 1995). Interestingly, mutation of these sites led to increased phosphorylation of the β1 subunit at Y384 and Y386, a subunit that exhibits relatively low stoichiometry of phosphorylation in response to Src compared to wild-type control (Moss et al., 1995). In primary neuronal cultures intracellular application of sodium vanadate, a potent tyrosine phosphatase inhibitor, enhanced benzodiazepine-sensitive GABAAR function, suggesting high endogenous tyrosine kinase and phosphatase activity under basal conditions (Moss et al., 1995). Consistent with this studies using phospho-antibodies have revealed that Y365/7 are principally phosphorylated via the activity of Fyn and Src. Significantly, Fyn is able to bind specifically to phospho-Y367, providing a mechanism for the recruitment of this tyrosine kinases to GABAARs (Brandon et al., 2001; Jurd et al., 2010). Studies with phospho-antibodies have revealed significant variations in the stochiometry of Y367 phosphorylation in the brains of rodents, suggesting input-specific control of phosphorylation; however, the signaling pathways that regulate the phosphorylation of the γ2 subunit remain to be defined. In keeping with their roles in mediating high affinity binding to AP2 phosphorylation of Y365/7 increases the cell surface stability of GABAARs and enhances the amplitude and frequency of mIPSCs (Kittler et al., 2008; Tretter 2009).
XI. Concluding Remarks
GABAARs play a central role in mediating neuronal inhibition and mediating the effects of a broad range of anxiolytic, anticonvulsant, hypnotic and sedative agents. GABAARs mediate both phasic and tonic modes of inhibition, phenomena that are mediated via receptor subtypes with distinct molecular structures. Phasic inhibition is mediated largely by benzodiazepine-sensitive receptor subtypes that are highly enriched at synaptic sites and principally assembled from α1–3, β1–3 and γ2 subunits. A critical determinant of receptor number at inhibitory synapses is the steady-state cell levels of these receptors on the neuronal plasma membrane. This is likely determined by the relative rates of receptor exo- and endocytosis, processes that are intimately controlled by covalent modifications and interaction with specific binding partners. While the molecular details of these processes are being evaluated their significance for the efficacy of neuronal inhibition and ultimately for behavior remains to be established. Our comprehension of how GABAARs are selectively stabilized at inhibitory synapses is limited; however, a central role for gephyrin is emerging. The direct binding of gephyrin to the GABAAR subtypes containing α2 subunits has been recently (Tretter et al., 2008) The significance of this interaction for GABAAR subtypes containing other α-subunit isoforms warrants further investigation. Gephyrin-independent clustering mechanisms have also been postulated in particular for subtypes containing α5 subunits The importance of these emerging processes for other receptor subtypes remains to be demonstrated.
The cellular mechanisms that regulate the cell surface accumulation are under active investigation. Future studies to ascertain the significance of these molecular mechanisms in determining the efficacy of neuronal inhibition under both normal and pathological conditions are essential.
Figure 1. GABAAR structure.
(A) Transmembrane topology of the GABAAR. Each receptor subunit is composed of a large extracellular ligand-binding N-terminal region that is also the site of action of various drugs followed by four hydrophobic transmembrane (TM)1–4 α-helices and a short, barely extruding C-terminus. Each receptor subunit also contains a large intracellular domain (ICD) between TM3 and TM4 that mediates the majority of protein-protein interactions and is subject to a number of post-translational modifications. (B) Traverse view of the assembly of GABAAR subunits to form an ion channel. TM2 faces the lumen of the channel ion pore and TM4 is anchored in the lipid membrane. TM1 and TM3 interact with the neighboring subunits.
Figure 2. Clathrin-mediated endocytosis.
The receptors cluster in specialized sites at the plasma membrane known as clathrin-coated pits, which invaginate and pinch off to form clathrin-coated vesicles (CCVs), a process that is dependent on dynamin. The clathrin adaptor protein (AP)-2 is a central component in the formation of these vesicles, forging a link between membrane proteins and clathrin that forms the outer layer of the coat. The vesicles subsequently lose their coat and fuse together to form an early endosome. Internalized receptors are then either subject to rapid recycling or are targeted for lysosomal degradation, an endocytic sorting decision that is regulated by the Huntingtin-associated protein (HAP)-1.
Table 2.
GABAA receptor phosphorylation sites
| Subunit | Phosphorylation site |
Protein kinase | ||
|---|---|---|---|---|
| In vitro | Heterologous cell lines |
Primary neurons |
||
| β1 | S384 S409 |
CaMKII PKA, PKC, CaMKII, PKG |
PKA, PKC |
|
| β2 | S410 | PKA, PKC, Akt, CaMKII, PKG | PKC, Akt | Akt |
| β3 | S383 S408 S409 |
CaMKII PKC PKA, PKC, CaMKII, PKG |
PKA, PKC PKA, PKC |
PKA, PKC PKA, PKC |
| γ2 | S327 S343 |
PKC PKC, CaMKII |
||
| S348 T350 Y365 Y367 |
CaMKII CaMKII Src Src |
Src Src |
||
Adapted from Brandon et al., 2002
Acknowledgements
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01NS047478, R01NS048045, R01NS051195, R01NS056359 and R01NS054900 to SJM], and by the Wellcome Trust. MT is supported by a National American Heart Association Senior Scientist Award.
Non standard abbreviations
- AKAP
A-kinase anchoring protein
- GABAAR
γ-aminobutyric acid type-A receptor
- BIG-2
brefeldin A-inhibited guanine nucleotide exchange factor
- BDNF
Brain-derived neurotrophic factor
- PKA
cAMP-dependent protein kinase
- CaMKII
calcium/calmodulin-dependent kinase II
- PKC
calcium/phospholipiddependent protein kinase
- PKM
constitutively active catalytic domain of PKC
- DGC
dystrophin glycoprotein complex
- ER
endoplasmic reticulum
- GABARAP
GABAAR associated protein
- GEF
guanine-nucleotide exchange factor
- GODZ
Golgi-specific DHHC zinc finger domain protein
- GST
glutathione S-transferase
- ICD
intracellular domain
- HEK-293
Human embryonic kidney cell line A293
- 5-HT3
5-hydroxytryptamine type-3 receptor
- NL
Neuroligin
- (nACh)
nicotinic acetylcholine receptor
- Plic
phospholipase C-related catalytically inactive protein
- PP
protein phosphatase
- PSD
postsynaptic densities
- PKB/Akt
protein kinase B
- Plic-1
PP, protein that links the integrin-associated protein with the cytoskeleton
- RACK-1
Receptor for activated C kinase
- TTX
Tetrodotoxin
- TM
Transmembrane domain
- UBL
Ubiquitin-like
- UBA
ubiquitin-associated domain
References
- Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller AZ. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. 2009. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol. 2002;544:3–18. doi: 10.1113/jphysiol.2002.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohme I, Rabe H, Luddens H. Four amino acids in the alpha subunits determine the gamma-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem. 2004;279:35193–35200. doi: 10.1074/jbc.M405653200. [DOI] [PubMed] [Google Scholar]
- Bormann J. The 'ABC' of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ. Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABA(A) receptors with the activation of G-protein-coupled receptors. J Neurosci. 2002;22:6353–6361. doi: 10.1523/JNEUROSCI.22-15-06353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Hill J, Smart TG, Moss SJ. Constitutive tyrosine phosphorylation of the GABA(A) receptor gamma 2 subunit in rat brain. Neuropharmacology. 2001;41:745–752. doi: 10.1016/s0028-3908(01)00121-6. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Chang CS, Leil TA, Olcese R, Olsen RW. GABAA receptor-associated protein regulates GABAA receptor cell-surface number in Xenopus laevis oocytes. Mol Pharmacol. 2005;68:152–159. doi: 10.1124/mol.104.009878. [DOI] [PubMed] [Google Scholar]
- Clague MJ. Molecular aspects of the endocytic pathway. Biochem J. 1998;336(Pt 2):271–282. doi: 10.1042/bj3360271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- DeSouza S, Fu J, States BA, Ziff EB. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J Neurosci. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABA(A) receptors: limits, insights, future developments. Neuroscience. 2003;119:933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–1300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Fischer JK, Macdonald RL. Functional properties of recombinant GABA(A) receptors composed of multiple beta subunit types. Neuropharmacology. 1997;36:1601–1610. doi: 10.1016/s0028-3908(97)00133-0. [DOI] [PubMed] [Google Scholar]
- Fuhrmann JC, Kins S, Rostaing P, El Far O, Kirsch J, Sheng M, Triller A, Betz H, Kneussel M. Gephyrin interacts with Dynein light chains 1 and 2, components of motor protein complexes. J Neurosci. 2002;22:5393–5402. doi: 10.1523/JNEUROSCI.22-13-05393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514(Pt 1):27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, Smart TG, Luscher B, Rees MI, Harvey RJ. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharmacology. 2005;48:181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Houston CM, Smart TG. CaMK-II modulation of GABA(A) receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur J Neurosci. 2006;24:2504–2514. doi: 10.1111/j.1460-9568.2006.05145.x. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ. GABA(A) receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci U S A. 2009;106:12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. [2010/03/18];Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. EMBO J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, Gill G, Howley PM. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol Cell. 2000;6:409–419. doi: 10.1016/s1097-2765(00)00040-x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AR, Stephenson FA, Tallman JF, Ramabahdran TV. Monospecific antibodies as probes for the stoichiometry of recombinant GABA(A) receptors. Receptors Channels. 2000;7:213–226. [PubMed] [Google Scholar]
- Kofuji P, Wang JB, Moss SJ, Huganir RL, Burt DR. Generation of two forms of the gamma-aminobutyric acidA receptor gamma 2-subunit in mice by alternative splicing. J Neurochem. 1991;56:713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr Opin Cell Biol. 1998;10:499–503. doi: 10.1016/s0955-0674(98)80065-3. [DOI] [PubMed] [Google Scholar]
- Leil TA, Chen ZW, Chang CS, Olsen RW. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Angelotti TP, Dudek EM, Browning MD, Macdonald RL. Enhancement of recombinant alpha 1 beta 1 gamma 2L gamma-aminobutyric acidA receptor whole-cell currents by protein kinase C is mediated through phosphorylation of both beta 1 and gamma 2L subunits. Mol Pharmacol. 1996;50:185–195. [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 1999;515(Pt 3):711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature. 1995;377:344–348. doi: 10.1038/377344a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. Volatile anesthetic actions on the GABAA receptors: contrasting effects of alpha 1(S270) and beta 2(N265) point mutations. Neuropharmacology. 2002;42:337–345. doi: 10.1016/s0028-3908(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- O'Sullivan GA, Kneussel M, Elazar Z, Betz H. GABARAP is not essential for GABA receptor targeting to the synapse. Eur J Neurosci. 2005;22:2644–2648. doi: 10.1111/j.1460-9568.2005.04448.x. [DOI] [PubMed] [Google Scholar]
- Ramming M, Kins S, Werner N, Hermann A, Betz H, Kirsch J. Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proc Natl Acad Sci U S A. 2000;97:10266–10271. doi: 10.1073/pnas.97.18.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Pangalos M, Moss SJ. The ubiquitin-like protein Plic-1 enhances the membrane insertion of GABAA receptors by increasing their stability within the endoplasmic reticulum. J Biol Chem. 2008;283:18538–18544. doi: 10.1074/jbc.M802077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Schaerer MT, Kannenberg K, Hunziker P, Baumann SW, Sigel E. Interaction between GABA(A) receptor beta subunits and the multifunctional protein gC1q-R. J Biol Chem. 2001;276:26597–26604. doi: 10.1074/jbc.M102534200. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Xu KF, Fan Q, Pacheco-Rodriguez G, Moss J, Vaughan M. Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc Natl Acad Sci U S A. 2006;103:2635–2640. doi: 10.1073/pnas.0510599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Chang GQ, Lin JY, Yu ZX, Fang ZH, Rong J, Lipton SA, Li SH, Tong G, Leibowitz SF, Li XJ. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med. 2006;12:526–533. doi: 10.1038/nm1382. [DOI] [PubMed] [Google Scholar]
- Shin HW, Morinaga N, Noda M, Nakayama K. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell. 2004;15:5283–5294. doi: 10.1091/mbc.E04-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Front Mol Neurosci. 2008;1:7. doi: 10.3389/neuro.02.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Revilla-Sanchez R, Houston C, Terunuma M, Havekes R, Florian C, Jurd R, Vithlani M, Michels G, Couve A, Sieghart W, Brandon N, Abel T, Smart TG, Moss SJ. Deficits in spatial memory correlate with modified {gamma}-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci U S A. 2009;106:20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliff G, Schindler NL, Crites GJ, Goldenberg R, Yochum A, Malatynska E. The GABA(A) receptor complex as a target for fluoxetine action. Neurochem Res. 1999;24:1271–1276. doi: 10.1023/a:1020977123968. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Moss SJ. The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition. Biochem Soc Trans. 2010:1355–1358. doi: 10.1042/BST0371355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, Salter MM, Lu YM. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6:24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]