Abstract
Background
The differentiation of constrictive pericarditis (CP) from restrictive cardiomyopathy (RCM) may be clinically difficult and may require multiple investigations. Even though brain natriuretic peptide (BNP) is shown to be higher in patients with RCM as compared to CP, the clinical utility is not fully established especially in Indian patients known to have advanced CP and myocardial involvement.
Methods and results
We measured NT-pro-BNP levels in 49 patients suspected of having either CP or RCM, diagnosed on the basis of echocardiography, computed tomography, magnetic resonance imaging, endomyocardial biopsy and cardiac catheterization data as needed. Twenty nine patients (Mean age – 26 yrs, 24 males) had CP and 20 patients (Mean age – 39 yrs, 14 males) had RCM. The median plasma NT-pro-BNP levels were significantly higher in RCM as compared to CP [1775 (208–7500) pg/ml vs 124 (68–718) pg/ml, respectively; p = 0.001]. A cut off value of 459 pg/ml had sensitivity, specificity and overall accuracy of 90%, 86% and 88% respectively, for differentiating CP from RCM.
Conclusions
The NT-pro-BNP levels are significantly elevated in RCM as compared to CP.
Keywords: Constrictive pericarditis, Restrictive cardiomyopathy, NT pro-BNP
1. Introduction
Differentiation between constrictive pericarditis (CP) and restrictive cardiomyopathy (RCM) has always been a difficult task for the clinicians. The differentiation is crucial as CP is curable surgically whereas RCM needs conservative management with poor prognosis. Patients need to undergo various investigations like echocardiography including tissue Doppler, computed tomography and magnetic resonance imaging, one after the other and may require cardiac catheterization many a times to differentiate between the two conditions. An individual test is often unable to differentiate the two conditions, for instance, hemodynamic assessment by cardiac catheterization may miss up to one fourth of the cases of CP.1
Brain natriuretic peptide (BNP) is secreted from ventricular myocyte in response to ventricular overload.2,3 Its secretion is influenced by various hemodynamic factors.4 BNP levels are increased in various conditions like cardiac diseases (heart failure, acute myocardial infarction, hypertension with left ventricular hypertrophy), pulmonary diseases (chronic obstructive pulmonary diseases, pulmonary embolism), endocrine diseases, renal diseases and cirrhosis of liver. It is secreted as a pre-prohormone that splits into BNP and functionally inactive NT-pro-BNP. Half life of BNP is much lesser than NT-pro-BNP hence concentration of BNP are lesser.5 The action of this peptide, like those of atrial natriuretic peptide, includes natriuresis, vasodilatation, inhibition of the renin-angiotensin-aldosterone axis, and inhibition of sympathetic nerve activity.6
In CP, there is no stretch on the myocardium because of the constrictive effect of the diseased pericardium, but in RCM the restrictive effect is in ventricular myocardium which produces significant atrial stretch. Due to this inherent difference in the physiology of these two conditions, the levels of BNP/NT-pro-BNP are found to be lower in CP in contrast to RCM.7 Indian patients with CP often present late with advanced myocardial involvement and have tubercular etiology most of the time.8 Hence, we conducted this study to evaluate the utility of NT-pro-BNP levels as a differentiating marker between Indian patients with RCM and predominantly tubercular CP.
2. Methods
All patients with a diagnosis of CP or RCM on the basis of clinical examination, echocardiography, computed tomography (CT), magnetic resonance imaging (MRI) and hemodynamic assessment were included in the study after an informed consent. CP due to radiation pericarditis and post cardiac transplant was excluded from the study. RCM due to various causes like idiopathic, endomyocardial fibrosis (EMF), amyloidosis, hemochromatosis and sarcoidosis were included in the study. Patients with features suggestive of both RCM and CP, e.g. radiation associated myopericardial involvement were excluded from the study as the basic aim of the study was to focus on the discriminatory value of NT-pro BNP for differentiating between RCM and CP. RCM patients with end stage disease and on transplant list were also excluded because multiple comorbidities associated with very advanced disease may confound the overall result of the study. End stage patients were defined as NYHA class IV patients who required hospitalization, intravenous medications and a median life expectancy of about 6 months. The study was approved by Institute's Ethics committee.
All patients suspected to have CP or RCM underwent complete clinical and echocardiographic evaluation. A 6-min walk test was done as per standard protocols. Echocardiogram was performed by a cardiologist with vast experience in this field. A comprehensive echocardiogram which included respiratory variation in mitral/tricuspid inflow velocities, atrial enlargement, septal thickness, mitral regurgitation (MR), tricuspid regurgitation (TR), TR gradient, pericardial thickness, pericardial calcification, myocardial architecture and tissue doppler was performed. Patients with primary diagnosis of CP were subjected to cardiac CT, while those with primary diagnosis as RCM were subjected to MRI/cardiac catheterization and endomyocardial biopsy for further confirmation and diagnosing the etiology of RCM. In case of diagnostic dilemma, usually a CT was done followed by MRI/cardiac catheterization if diagnosis is still elusive. CP diagnosis was finally confirmed on surgery for patients who underwent pericardiectomy.
All the patients with confirmed diagnosis of either condition on the basis of clinical evaluation, echocardiography, CT or MRI and hemodynamic assessment were enrolled in the study. The blood sample for NT-pro-BNP was obtained in the morning after at least 30-min rest. NT-pro-BNP measurement was done for all the patients using quantitative assay for NT-pro-BNP by Roche diagnostics, Basel, Switzerland.
3. Statistical analysis
Statistical analysis was performed using STATA 11.0 (College Station, Texas, USA) statistical software. Results are presented as mean (SD) when parametric tests were used, and median (IQR) when non-parametric tests were used. Student's t-test was done to compare the means of continuous variables. Two-sample Mann Whitney test was used to compare non-parametric variables. A p value of <0.05 was taken as significant. Nonparametric estimation of receiver operating characteristics curve was used to derive cut off values for NT-pro-BNP levels.
4. Results
We studied 49 patients with confirmed diagnosis of either RCM or CP based on various tests. No patient was excluded due to suspected radiation CP. Two patients with RCM were excluded as they were of end stage disease. All these patients were on a stable dose of standard therapy including diuretics, ACE inhibitors and beta blockers. All patients had normal renal functions. Table 1 summarizes the characteristics of the two groups of patients. Patients with CP were younger, had relatively lower hemoglobin values and higher ESR as compared to RCM. Various other parameters like duration of disease, NYHA class, extent of hepatomegaly, 6-min walk test and left ventricular (LV) function were not statistically different between the two groups. None of the patients in the CP group had atrial or ventricular enlargement on echocardiography. Similarly, none of the patients in the RCM group had pericardial thickening, calcification or effusion. Majority of patients [24 (82%)] in the CP group had significant respiratory variation in mitral/tricuspid inflow velocities while none in the RCM group had this finding. A mitral annular early diastolic velocity of >8 cm/s was seen in all patients with CP and in 3 (15%) with RCM. All patients with CP were of tubercular etiology and had completed or were receiving antitubercular treatment (ATT). In the RCM group 2 had cardiac amyloidosis, one secondary hemochromatosis, 4 patients had endomyocardial fibrosis (EMF) and the rest were idiopathic RCM. Echocardiography was diagnostic in all patients except in 2 patients, out of whom one turned out to be CP after cardiac CT while in other CT was noncontributory and a cardiac MR clinched the diagnosis of RCM. In the CP group two patients recovered with ATT without signs of CP in the follow up period and one was managed conservatively due to improvement in symptoms after ATT. Rest of the 26 patients underwent pericardiectomy and the diagnosis was confirmed intraoperatively and in biopsy specimens. NT-pro-BNP levels were significantly higher in the patients with RCM as compared to CP with a median (IQR) value of 1775 (208–7500) pg/ml vs 124 (68–718) pg/ml, respectively (p = 0.001). A cut off value of 459 pg/ml had sensitivity, specificity and overall accuracy of 90%, 86% and 88% respectively, for differentiating CP from RCM. In patients whom echocardiography was inconclusive, NT-pro-BNP was able to differentiate between the two with cut off value of 459 pg/ml. The sensitivity and specificity of various cut off values of NT-pro-BNP in differentiating the two conditions are summarized in Table 2.
Table 1.
Baseline characteristics and comparison of two groups.
| CP | RCM | P value | |
|---|---|---|---|
| No of patients | 29 | 20 | |
| Age (yrs) | 25.7 ± 13.2 | 39.2 ± 20 | 0.007 |
| Gender (male) | 24 (83%) | 14 (70%) | NS |
| Duration of disease (months)* | 12 (2–120) | 24 (1–60) | NS |
| NYHA class | 2.65 ± 0.63 | 2.7 ± 0.73 | NS |
| Hepatomegaly (cms) | 3.3 ± 1.0 | 2.8 ± 0.97 | NS |
| 6 min walk test (meters) | 372 ± 60 | 363 ± 29 | NS |
| Hemoglobin (gm/dl) | 11.3 ± 1.1 | 12.4 ± 2.1 | 0.05 |
| ESR | 27.7 ± 9.9 | 15.4 ± 3.7 | 0.0007 |
| NT-pro-BNP (pg/ml)* | 124 (68–718) | 1775 (208–7500) | 0.0001 |
| LV function (%) | 56 ± 4.7 | 58 ± 6.2 | NS |
Values displayed as mean ± standard deviation except for * values which are expressed as median (IQR).
CP = constrictive pericarditis, RCM = restrictive cardiomyopathy, NYHA = New York Heart Association, ESR = Erythrocyte Sedimentation Rate, LV = left ventricular.
Table 2.
Diagnostic values of various cut off values for NT-pro-BNP.
| NT-pro-BNP levels (pg/ml) | Sensitivity (%) | Specificity (%) | Overall accuracy (%) |
|---|---|---|---|
| ≥208 | 100 | 62 | 78 |
| ≥459 | 90 | 86 | 88 |
| ≥740 | 85 | 100 | 94 |
5. Discussion
Differentiation of CP from RCM is difficult and at times the diagnosis is only made in operation theater or on autopsy. Given the limitations of existing diagnostic modalities for differentiating the two conditions we studied the utility of NT-pro-BNP in this patient population as an ancillary diagnostic modality. The plasma level of BNP is elevated in patients with congestive heart failure and increases in proportion to the degree of left ventricular dysfunction and the severity of symptoms of heart failure.3,9 BNP and its amino-terminal portion (NT-pro-BNP), appears to be a powerful neurohormonal predictor of LV function and prognosis in various conditions including myocardial infarction and acute coronary syndrome.5,10,11 As NT-pro-BNP is relatively stable form than BNP, its levels are 1–3 times more than the corresponding BNP levels in the same patient.12 The age differences in NT-pro-BNP is also important but in various studies, population <50 yrs has been clubbed in one group and our study population included mainly patients under 50 yrs of age. Although, some minor changes in BNP levels may occur it will not change the utility of NT-pro-BNP as seen in this study.13,14
It has been hypothesized by Leya et al that NT-pro-BNP level should not be elevated in CP.7 On the contrary, RCM will have elevated NT-pro-BNP levels. An earlier study showed that plasma levels of BNP and BNP receptors are elevated in patients with RCM.15 Although atrial natriuretic peptide is known to be modestly increased in patients with CP compared to congestive heart failure from other etiology, few studies had evaluated direct comparison between CP versus RCM.16 The difference in natriuretic peptide levels in CP and RCM, despite having similar degree of congestion and elevated ventricular diastolic pressures is due to the basic difference in the BNP kinetics in constrictive versus restrictive physiology. In CP, thickened and constricting pericardium effectively counter-balances the intracardiac distending pressure and thus negating the stimulus for BNP release. In RCM the stretch/distending stimulus is unchecked resulting in higher BNP release.7 Sengupta et al compared the utility of mean early diastolic mitral annular tissue Doppler imaging and BNP in differentiating CP and RCM. They found tissue doppler imaging to have more discriminative value in differentiating the two conditions with little overlap (area under the curve 0.97 vs 0.76, respectively; p = 0.01), while larger overlap between the two was seen with BNP values less than 400 pg/ml A mean annular velocity of 5 cm/s correctly distinguished CP from RCMP, even when there was a large overlap of BNP between the 2 groups. In this study the subgroup of patients with idiopathic CP had lower BNP value and thus still maintaining the discriminatory value for RCM.17 The utility of BNP get blunted in the presence of renal failure as shown by Reddy et al in a subgroup of patients with RCM and CP with renal failure. In patients with GFR <90 ml/min the discriminatory value of BNP became insignificant. Elevation in BNP due to renal failure was the reason for this effect. The patients in this study were older than that of our study and most of the CP were post thoracic surgery.18 In our study none of the patient had renal dysfunction, younger in age and thus discriminatory value of NT-pro-BNP was better in our study.
In study by Babuin et al 11 patients each of idiopathic CP, secondary CP and RCM were compared. Median BNP was 80 (44–193) pg/ml for idiopathic CP, 278 (118–526) pg/ml for secondary CP, and 499 (361–606) pg/ml for RCM. Secondary CP patients were either post operative or post radiation therapy and had higher BNP levels.19 In our study, all patients were of tubercular CP and is in sync with Babuin's study regarding the discriminatory value of NT-pro-BNP between idiopathic CP and RCM.
Kapoor et al measured BNP levels in 24 tubercular CP patients before and after pericardiectomy and compared it with various parameters of ventricular functions. They found a good correlation in BNP levels and diastolic dysfunction in these patients while correlation with systolic function was poor. Patients in this study had very high initial value of BNP (mean 514 pg/ml) as these patients were much sicker, had advanced NYHA class (NYHA III 75%, NYHA IV 25%) and almost half of them had renal dysfunction.8 All these studies measured BNP levels, while Mady et al studied NT-pro-BNP levels in various cardiomyopathies like hypertrophic cardiomyopathy, chagasic cardiomyopathy, EMF, CP and pericardial effusion (Table 3). Mean NT-pro-BNP levels were 633 ng/ml and 588 ng/ml in EMF and CP respectively and this difference was not significant. Reasons for this finding could be because of patients in CP group were NYHA class I/II while most of the patients (80%) in EMF group were class III/IV. All patients in restrictive group were of EMF a finding much different from other studies where idiopathic RCM is predominant diagnosis.20
Table 3.
Comparison of various studies of BNP/NT-pro-BNP in CP and RCM.
| Study (Year) |
Diagnosis | No of pts | Pts with idiopathic CP | Age (yrs) | BNP/NT-pro-BNPa levels (pg/ml) |
|---|---|---|---|---|---|
| Leya FS 2005 |
CP | 6 | – | 64.2 ± 10.2 | 128.0 ± 52.7 |
| RCM | 5 | 54.2 ± 17.7 | 825.8 ± 172.2 | ||
| Babuin L 2006 |
Idiopathic CP | 11 | 51.7 ± 11.1 | 80 (44–193)† | |
| Secondary CP | 11 | 62.0 ± 10.3 | 278 (118–526)† | ||
| RCM | 11 | 58.8 ± 10.2 | 499 (361–606)† | ||
| Reddy PR 2007 |
CP (GFR <90 ml/min) | 9 | 2 | 65.9 ± 17.1 | 434 ± 307 |
| CP (GFR>(90 ml/min) | 8 | 2 | 53.8 ± 11.6 | 124 ± 97.5 | |
| RCM | 5 | – | 681 ± 400 | ||
| Sengupta PP 2008 |
CP | 16 | 7 | 61.8 ± 13 | 231 ± 104 |
| RCM | 15 | 60.5 ± 9 | 595 ± 499 | ||
| Mady C 2008 |
CP | 16 | – | 32 ± 16 | 568a |
| EMF | 26 | 49 ± 7 | 633a | ||
| Control | 40 | 36 ± 10 | 28a | ||
| Kapoor PM 2010 |
CP | 24 | 24 | 32 (15–55)† | 513.71 ± 147.21 |
| Parakh N 2014 |
CP | 29 | 29 | 25.7 ± 13.2 | 124 (68–718)†,a |
| RCM | 20 | 39.2 ± 20 | 1775 (208–7500)†,a |
CP, constrictive pericarditis; RCM, restrictive cardiomyopathy; All values are in mean ± SD except † values are expressed as median (IQR).
Studies measuring NT-pro-BNP.
In our study echocardiography by an expert cardiologist was able to differentiate between RCM and CP with accuracy of 95%. In two patients where echocardiography was inconclusive, NT-pro-BNP cut off value of 459 pg/ml was able to differentiate between the two. In centers/operators with lesser expertise in echocardiography, NT-pro-BNP may have much more important role. All our patients were of tubercular CP and generalizability of these findings to other groups need further studies. We found in our study that both the groups had similar degree of heart failure as judged by NYHA class and hepatomegaly. Despite that level of NT-pro-BNP were only mildly elevated or normal in patients with CP. Our study confirms the earlier finding of possible use of BNP/NT-pro-BNP as an ancillary tool in differentiating CP from RCM. A possible algorithm is proposed in Fig. 1.
Fig. 1.
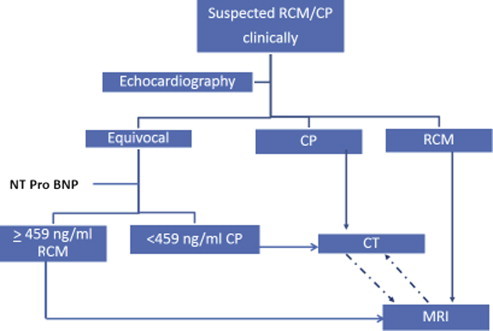
Algorithm for the differentiation of CP and RCM. CP – constrictive pericarditis; RCM – restrictive cardiomyopathy; CT – computerized tomography; MR – magnetic resonance imaging.
6. Limitations
Our study is also limited by small sample size, although the size is sufficiently large to conclude that the levels of NT-pro-BNP are higher in restrictive cardiomyopathy as compared to constrictive pericarditis.
7. Conclusions
We conclude from this study that NT-pro-BNP is a useful marker to differentiate restrictive cardiomyopathy from constrictive pericarditis and can be used in diagnostic protocol in cases where the differentiation between the two conditions is difficult by other tests.
Funding
The study was supported by intramural grant and resources from the All India Institute of Medical Sciences, New Delhi.
Conflicts of interest
All authors have none to declare.
Acknowledgment
We acknowledge Dr Kalaivani Mani for her statistical assistance in this study.
References
- 1.Vaitkus P.T., Kussmaul W.G. Constrictive pericarditis versus restrictive cardiomyopathy: a reappraisal and update of diagnostic criteria. Am Heart J. 1991;122:1431–1441. doi: 10.1016/0002-8703(91)90587-8. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H., Yoshimura M., Sumida H. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura M., Yasue H., Okumuro K. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 4.Kinnunen P., Vuolteenaho O., Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 5.Hunt P.J., Espiner E.A., Nicholls M.G., Richards A.M., Yandle T.G. The role of the circulation in processing pro-brain natriuretic peptide (pro-BNP) to amino-terminal BNP and BNP-32. Peptides. 1997;18:1475–1481. doi: 10.1016/s0196-9781(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 6.Francis G.S., Cohn J.N., Johnson G., Rector T.S., Goldman S., Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure: relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:40–48. [PubMed] [Google Scholar]
- 7.Leya F.S., Arab D., Joyal D. The efficacy of brain natriuretic peptide levels in differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Coll Cardiol. 2005;45:1900–1902. doi: 10.1016/j.jacc.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor P.M., Aggarwal V., Chowdhury U., Choudhury M., Singh S.P., Kiran U. Comparison of B-type natriuretic peptide and left ventricular dysfunction in patients with constrictive pericarditis undergoing pericardiectomy. Ann Card Anaesth. 2010;13:123–129. doi: 10.4103/0971-9784.62942. [DOI] [PubMed] [Google Scholar]
- 9.Cohn J.N., Levine T.B., Olivari M.T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 10.Richards A.M., Nicholls M.G., Yandle T.G. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 11.Richards A.M., Nicholls M.G., Yandle T.G. Neuroendocrine prediction of left ventricular function and heart failure after acute myocardial infarction. The Christchurch Cardioendocrine Research Group. Heart. 1999;81:114–120. doi: 10.1136/hrt.81.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mair J. Biochemistry of B-type natriuretic peptide – where are we now? Clin Chem Lab Med. 2008;46:1507–1514. doi: 10.1515/CCLM.2008.295. [DOI] [PubMed] [Google Scholar]
- 13.Wang T.J., Larson M.G., Levy D. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt P., Collinson P.O., Doughty R.N. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. Eur Heart J. 2010;31:1881–1889. doi: 10.1093/eurheartj/ehq163. [DOI] [PubMed] [Google Scholar]
- 15.Takemura G., Takatsu Y., Doyama K. Expression of atrial and brain natriuretic peptides and their genes in hearts of patients with cardiac amyloidosis. J Am Coll Cardiol. 1998;31:754–765. doi: 10.1016/s0735-1097(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 16.Anand I.S., Ferrari R., Kalra G.S., Wahi P.L., Poole-Wilson P.A., Hamis P.C. Pathogenesis of edema in constrictive pericarditis. Circulation. 1991;83:1880–1887. doi: 10.1161/01.cir.83.6.1880. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta P.P., Krishnamoorthy V.K., Abhayaratna W.P. Comparison of usefulness of tissue Doppler imaging versus brain natriuretic peptide for differentiation of constrictive pericardial disease from restrictive cardiomyopathy. Am J Cardiol. 2008;102:357–362. doi: 10.1016/j.amjcard.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 18.Reddy P.R., Dieter R.S., Das P., Steen L.H., Lewis B.E., Leya F.S. Utility of BNP in differentiating constrictive pericarditis from restrictive cardiomyopathy in patients with renal insufficiency. J Card Fail. 2007;13:668–671. doi: 10.1016/j.cardfail.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Babuin L., Alegria J.R., Oh J.K., Nishimura R.A., Jaffe A.S. Brain natriuretic peptide levels in constrictive pericarditis and restrictive cardiomyopathy. J Am Coll Cardiol. 2006;47:1489–1491. doi: 10.1016/j.jacc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Mady C., Fernandes F., Arteaga E. Serum NT pro-BNP: relation to systolic and diastolic function in cardiomyopathies and pericardiopathies. Arq Bras Cardiol. 2008;91:46–54. doi: 10.1590/s0066-782x2008001300008. [DOI] [PubMed] [Google Scholar]


