Figure 1.
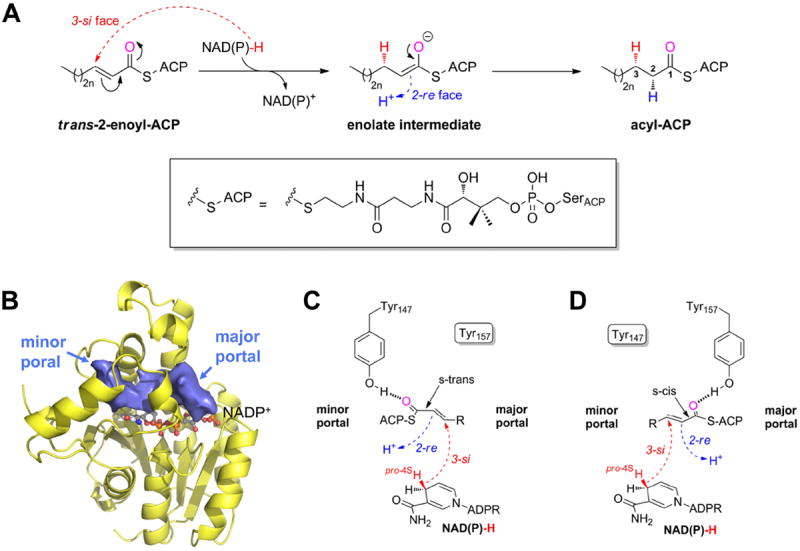
The FabI-catalyzed reaction 9-11. (A) Syn-addition of H2 by a two-step mechanism (n = 0-8). Dashed arrows indicate an attack from below the paper plane. The pro-4S hydride of NAD(P)H (red) is transferred from the 3-si face. In a second step, the enolate intermediate is protonated stereospecifically from the re face at C2 as observed for ecFabI and InhA (proton shown in blue) 9, 10. The thioester oxygen, which is hydrogen bonded to either Tyr147 (panel C) or Tyr157 (panel D), is indicated in magenta. During the reaction, the substrate is attached to ACP via a phosphopantetheine moiety (depicted in the box). (B) Binding-pocket topology including portals towards the solvent. Povme 47 was used to calculate the binding-pocket volume indicated as blue surface (PT400-bound saFabI structure). NADP+ is depicted as ball-and-stick model. (C) Substrate delivery via the minor portal. With the cofactor located below the substrate in the chosen view (see panel B), the trans-2-enoyl-ACP has to be in the s-trans conformation to enable the correct stereochemical outcome in a scenario where ACP binds close to the minor portal, and the thioester carbonyl is, thus, bound to Tyr147. Dashed arrows indicate attack vectors from below. (D) Substrate delivery via the major portal. If ACP is located at the major portal, the thioester carbonyl will be hydrogen bonded to Tyr157 and, thus, the substrate has to be in the s-cis conformation for consistency with the observed stereochemistry.
