Abstract
In 1959, Russell and Burch published The Principles of Humane Experimental Technique, which included concrete advice on factors that they considered would govern progress in the implementation of these principles (enunciated as the 3Rs [Replacement, Reduction, and Refinement in animal-based studies]). One challenge to the implementation of the 3Rs was identified as information retrieval. Here, we further explore this challenge—the need for ‘research on research’—and the role that systematic reviews and reporting guidelines can play in implementation of the 3Rs. First, we examine the 2-fold nature of the challenge of information retrieval: 1) the identification of relevant publications spread throughout a large population of nonrelevant publications and 2) the incomplete reporting of relevant details within those publications. Second, we evaluate how systematic reviews and reporting guidelines can be used generally to address this challenge. Third, we assess the explicit reporting of the 3Rs in a cohort of preclinical animal systematic reviews. Our results show that Reduction methods are the most commonly reported by authors of systematic reviews but that, in general, reporting on how findings relate to the 3Rs is limited at best. Although systematic reviews are excellent tools for resolving the challenge of information retrieval, their utility for making progress in implementation of the 3Rs may be limited unless authors improve their reporting of these principles.
“We now have far too much information as a species to digest as individuals.”116
Since Russell and Burch published The Principles of Humane Experimental Technique, the 3Rs (replacement, reduction, refinement) have become the guiding principles for the ethical use of animals in science.6 In 1959, Russell and Burch deplored the long delay in the application of existing knowledge to improve experimental techniques. Currently, some 55 y later, a gap still remains between the guiding principles of the 3Rs and their application in practice.5,74,78,79,127,131 To help close this gap, we revisit Russell and Burch's advice regarding the “factors which govern its (the 3Rs) progress.”116
Russell and Burch acknowledged that an important factor for progress in the implementation of the 3Rs is the necessity for ‘research on research,’116 a general concept that continues to be advocated by contemporary authors, irrespective of any interest in the 3Rs.19 This factor was deemed to be so important that Russell and Burch suggested that “[a]s science continues to expand, this [research on research] will be seen more and more clearly as the only way to save it from grinding to a standstill.”117 Although Russell and Burch viewed interspecialist communication as a key factor governing progress in implementation of the 3Rs; the necessity for research on research stems from a wider problem: information retrieval. Here, we explore the challenges of information retrieval, the need for research on research, and the role that systematic reviews and reporting guidelines can play in closing the gap between the 3Rs principles and their implementation in practice.
Information Retrieval
The problem of information retrieval can be divided into 2 parts: 1) the identification of relevant publications spread throughout a large population of nonrelevant publications and 2) the incomplete reporting of relevant details within those publications.
Identification of relevant publications.
When searching for information on any subject only a finite proportion of information may be found because individual publications are spread throughout a wide range of periodicals. Russell and Burch recognized the challenges of information retrieval saying, “…there will be many such [publications] in a few periodicals and few [publications] in a great many [periodicals]”117. This challenge is also known as Bradford's Law of Scattering.14,94 Finding information by searching a few periodicals with many relevant publications is easy, but when the relevant information becomes spread through a widening range of periodicals, the task becomes more difficult.
Information retrieval is also made difficult by the sheer quantity of information; as Russell and Burch noted in 1959,117 when the total number of citations indexed in PubMed (from 1907 to 1959), was 1,475,600, of which 65,785 (4%) were related to animals (Figure 1). From 1960 to 2012, the total number of citations indexed in PubMed was 20,786,790, of which 5,451,234 (26%) were related to animals (Figure 1). Therefore the retrieval of information scattered among an ever-increasing number of publications is a daunting task for any investigator attempting to summarize all the relevant information on a given topic.
Figure 1.
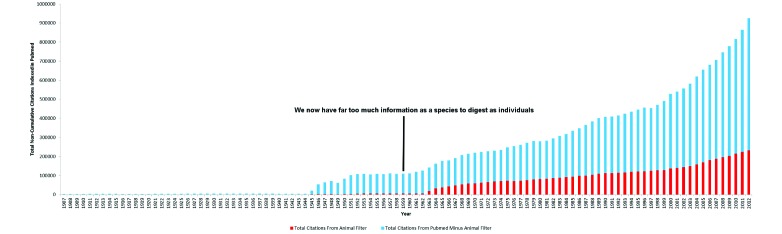
Total citations in PubMed and total animal citations in PubMed by Year 1907 – 2012. The total number of references indexed PubMed by year for the years 1907 to 2012 (noncumulative). Blue bars represent the total number of references in PubMed in a given year. The red shading overlying the blue bars represents the total number of animal-related references indexed in PubMed in a given year. A search of all indexed material in PubMed was conducted on 12 December 2013 by using the search string (“1900/01/01”[PDAT]: “2012/12/31”[PDAT]). The ‘results by year file’ (.csv) was downloaded from Pubmed, and the values for the total number of citations by year were entered into an Excel (Microsoft, Redmond, WA) file. For each year from 1907 to 2012, a search was conducted (for example, (“1959/01/01”[PDAT]: “1959/12/31”[PDAT])), and a validated search filter for animal-related research28 was applied and the number entered into an Excel file. The quoted material is from reference 118.
Incomplete reporting of relevant details.
The information retrieval challenge becomes more formidable given the incomplete descriptions of methodology often found in publications.117 Russell and Burch described the challenge by using a quote from Maurice Visscher, “…methodology is usually relegated to a place of smaller type and sharply abbreviated importance in journal publication of research. Numerous essential details are customarily omitted, either because they are considered to be common knowledge or simply for lack of space.”137 In recent years, increasing evidence has highlighted the prevalence of incomplete methodology reporting in preclinical animal research. For example, one cross-sectional study of the biomedical animal literature found that methodological details such as randomization and blinding often were not included in research publications.61 Another review of highly cited (at least 500 times) biomedical animal studies published in high-impact–factor journals found a similar pattern of missing methodologic details.44 Unfortunately, missing details are not limited to methodology but sometimes include such basic information as the hypothesis or objective.61
Synthesis of information.
To resolve the problem of information retrieval, Russell and Burch recommended the use of a form of synthesis: “One approach to this special problem [information retrieval] is to concentrate some attention on the particular branch of synthesis which takes the form of general methodological study.”117 For Russell and Burch, a “general methodological study” was a book review, such as The Principles of Humane Experimental Technique. Here, we depart from Russell and Burch's recommended approach and suggest 2 complementary approaches to the synthesis of 3Rs information (which have evolved in the clinical sciences field since the publication of the Principles of Humane Experimental Technique): the use of systematic reviews and reporting guidelines.
Systematic Reviews and Reporting Guidelines
Systematic reviews.
A systematic review has been defined as “…a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research, and to collect and analyze data from the studies that are included in the review. Statistical methods (meta-analysis) may or may not be used to analyze and summarize the results of the included studies.”91
In general, systematic reviews are conducted by a multidisciplinary team. These teams may include the funder, subject-matter experts (for example, preclinical stroke researchers), systematic-review experts, librarians with expertise in using unbiased search strategies, knowledge users (for example, clinical stroke researchers), and statisticians.93,105 Such multidisciplinary teams allow for what Russell and Burch called “interspecialist communication,”118 which they saw as fundamental for progress in the 3Rs.
Systematic reviews differ from traditional narrative reviews in several important ways. Narrative reviews are usually not framed around a specific question, whereas the question must be framed explicitly in a systematic review.46 In human health research, this framing often involves the use of a structured approach with 5 elements used to frame the question. These elements, known by the acronym PICOS,72 include: P, the patient population or disease being studied (for example, healthy men and women aged 16 to 65 y54); I, the intervention (for example, killed or live, attenuated vaccines administered via any route54); C, the comparator group (for example, placebo or no intervention54); O, the outcome or endpoint (for example, number and seriousness of cases of symptomatic influenza and of adverse effects54); and S, the study design chosen (for example, randomized control trials54). Use of the PICOS acronym facilitates the development of a clearly stated set of objectives, which allows review authors to develop a search strategy and set criteria for studies to be included and excluded in the review. In addition, the PICOS acronym is easily adaptable to different fields and can be used to frame questions that include other important elements, such as Context106 (PICOCS) in the field of social sciences or Exposure30 (PECO) in the field of etiology.
Narrative reviews and primary studies do not use systematic search strategies but instead rely on simple availability of periodicals and author preference to identify relevant publications. Such an approach cannot overcome the problem of information retrieval and may lead to a biased selection of publications because authors may fail to identify all those that are relevant. For instance, a systematic review of clinical trials of the drug aprotinin, which is used in cardiac surgery, found that the median percentage of previous trials cited per publication was only 20%.33 This low level resulted in the completion of redundant studies long after the effectiveness of aprotinin in reducing the need for perioperative transfusion had been established.33 The use of systematic methods—in particular, the search strategy and screening methods used to identify all relevant publications52,70,138—is the chief way in which systematic reviews overcome the problem of information retrieval. Furthermore, reporting the methods used for the search strategy and the criteria used to include or exclude studies ensures that the entire process is transparent. This transparency allows readers to critique the methods used to identify and select relevant studies instead of pitting one expert opinion against another.125
The ambition to “include a library of trial overviews, which will be updated when new data become available” was first articulated in 1986.18 Updating clinical systematic reviews is still a major component of the reviews themselves, although the methods for keeping reviews up to date are not as well-established as are the methods for conducting systematic reviews.27 Updating reviews, in contrast to authoring new ones, is a concept that can save time and wasted effort as it ensures that the synthesized knowledge maintains the greatest currency for potential users.
Meta-analysis of data.
Findings from studies included in a systematic review are often analyzed by using techniques such as meta-analysis. The importance of analyzing the results from the individual studies, rather than the authors’ opinions regarding their data, has been demonstrated in retrospective cumulative meta-analyses of clinical trials.58,68 For instance, retrospective analysis of all clinical trials that looked at the efficacy of streptokinase for acute myocardial infarction and the efficacy of tranexamic acid for blood transfusion showed that effectiveness of the treatments had been established years before trials were stopped.58,68 In these and other cases, small trials and incomplete literature reviews prior to starting new trials led to the false belief that efficacy had not been established definitively.40 Similarly, retrospective cumulative meta-analyses of animal studies have found that animal experiments are unnecessarily replicated; for example, in establishing the efficacy of tissue plasminogen in stroke models.122 The use of systematic reviews with prospective cumulative meta-analysis would prevent unnecessary replication by highlighting when a question has already been answered definitively (that is, when the addition of data from additional studies do not affect the cumulative findings), ensuring that all important findings are considered, as well as contributing to the reduction of redundant and unnecessary animal use.
Limitations of systematic reviews and meta-analyses.
One identified limitation of systematic reviews is publication bias:115 the tendency for positive findings to be published more often than are negative findings. Techniques are available that allow systematic reviews to assess the likelihood that publication bias has skewed the overall results of the review.46 For example, evaluation of a systematic review of animal models for acute ischemic stroke revealed that publication bias caused a statistically supported 30% over-exaggeration of the treatment effect.124 Publication bias represents an additional challenge to the primary problem of information retrieval (that of identifying relevant publications), but the use of systematic search strategies and statistical tools can at least estimate the extent of the publication bias and can even estimate the number of experiments and animals used in the nonpublished studies.124 Another complication, similar to publication bias, is selective outcome reporting, where authors select for publication only a subset of the analyses they performed (for example, the significant ones).20,134 Unlike publication bias, selective outcome reporting is a challenge to the secondary problem of information retrieval (that of incomplete reporting). Without preregistered protocols, as there are in clinical trials, the direct assessment of either publication bias or selective outcome reporting is difficult if not impossible.20,62,63
Another limitation of systematic reviews and meta-analyses is the accuracy of the reporting of the primary studies used in the synthesis. Systematic reviews formally assess the validity of the included studies through tools such as a risk of bias assessment.46 Such assessments help authors to objectively and transparently appraise whether findings are biased. For instance, in a systematic review of animal studies for the efficacy of NXY059 in ischemia, the authors found that primary studies that did not use randomization or concealment of treatment group allocation had significantly larger effect sizes than did those that used these methods.83 Because systematic reviews use formal approaches to the synthesis and analysis of both the methods and outcomes of included studies, they are able to detect biases such as those described earlier, whereas traditional narrative reviews cannot. However, many primary animal studies do not fully report the methods used,61 as the secondary challenge to information retrieval addresses.
Reporting guidelines.
In both preclinical and clinical research, poor reporting has been identified as a systemic source of waste.1,40,89 In response to concerns about the quality of reporting in randomized control trials involving humans, the CONSORT (CONsolidated Standards Of Reporting Trials) was developed.119 The CONSORT statement itself was not developed as a quality assessment instrument but was designed to improve the reporting of items related to the internal and external validity of trials.89 However, the statement has proven to be an effective tool to improve the quality of reporting in trials.56,90,135 Early in the development of reporting guidelines for randomized control trials, separate guidelines groups met and developed a common set of recommendations that became the CONSORT statement;132,111 this one common set of recommendations facilitated their adoption. The development and assessment of reporting guidelines in the clinical field is now well-established,92 whereas preclinical reporting guidelines are a recent phenomenon and one that needs urgent attention.
The accuracy of reporting preclinical research has come under scrutiny as questions about the reproducibility of research have been raised.10,108 The United States National Institute of Neurologic Disorders and Stroke held a workshop and recommended that key methodologic items, such as sample size calculations; method of randomization of animals; blinding to treatment; and data handling, should all be reported.67 Similarly, motivated by the irreproducibility of research, the Nature publishing group has instituted a checklist3 for all animal-based papers, including requirements for sample size calculations; inclusion and exclusion criteria; methods of animal randomization; blinding to treatment; information on species, strain, sex, and age of animals; and ethics compliance statement.96 Many of these reporting items relate to the design of animal experiments, which was identified as a problem more than 2 decades ago34 and is now receiving increased attention.21,35,37,45 In addition, reporting guidelines for animal studies have been developed, for example, the Animal Research: Reporting In Vivo Experiments60 (ARRIVE) guidelines, Gold Standard Publication Checklist48 (GSPC), and Description of Animal Research in Scientific Publications53 (DARSP) recommendations, as well as area-specific guidelines such as the REFLECT statement for randomized control trials in livestock and food safety.118
Although the ARRIVE guidelines include a specific reporting element on the 3Rs and were developed by the National Centre for the 3Rs in the United Kingdom, they were developed to ensure that “data from animal experiments could be fully evaluated and utilised.”95 Currently the ARRIVE guidelines are endorsed by more than 300 journals, funders, universities, and learned societies. However, reporting guidelines are not a panacea: improved training of authors, editors, and reviewers is required, as is the endorsement and implementation of the guidelines by journals and peer reviewers. The advent of digital publishing and the availability of space to report more methodologic detail should assist in removing the barriers to good reporting of research. The important next step is to evaluate the effect that these guidelines have on the reporting of animal studies to determine whether these statements help to achieve progress in the implementation of the 3Rs.4,120
Summary of tools to address information retrieval.
The combined use of systematic reviews and reporting guidelines likely will help to achieve progress in implementation of the 3Rs by providing a structured way to address the problem of information retrieval of animal studies. Specifically, the use of systematic search strategies, often conducted by or in consultation with an expert in these techniques, combined with validated search filters and a well-defined question (for example, PICO) make systematic reviews an excellent adaptable tool to identify all relevant studies in the growing deluge of information confronting investigators.
In addition, systematic reviews provide authors with the means to assess the validity of the studies and to synthesize their results through techniques such as meta-analysis. As with the problem of publication bias,24,62 for which the remedy is the publication of all results, it is better to preempt the formidable problem of retrieving missing information by including all relevant details in publications. Reporting guidelines, like systematic reviews, have proven effective in the clinical field to ensure adequate reporting of experimental details and are being adopted by investigators, journals, and funders.
We have argued in general terms that systematic reviews will help achieve progress in the 3Rs by ameliorating the problem of information retrieval as described by Russell and Burch. Because systematic reviews provide a formal method to evaluate multiple primary studies’ methods and outcomes (for example, through risk of bias assessment and meta-analysis), they also may identify opportunities for implementation of the 3Rs. To examine this possibility, we reviewed available systematic reviews of preclinical animal studies to identify the frequency of reporting on the 3Rs.
The 3Rs in Systematic Reviews and Reporting Guidelines
Introduction.
In 1990, at the 7th annual meeting of the Johns Hopkins University Center for Alternatives to Animal Testing, 30 persons from 13 countries active in animal issues or alternatives in research agreed that it was time to institute a World Congress on Alternatives and Animal Use in the Life Sciences.42 The first World Congress was held in Baltimore in 1994, and since then, scientists, veterinarians, policy makers, animal protectionists, and other interested parties have come together every 2 to 3 y to discuss progress in implementation of the 3Rs. In 1999, participants at the 3rd World Congress endorsed A Declaration of Bologna31, reaffirming the principles put forward by Russell and Burch in 1959: “Humane science is a prerequisite for good science and is best achieved in relation to laboratory animal procedures by the vigorous promotion and application of the Three Rs.” In 2011, participants at the 8th World Congress on Alternatives and Animal Use in the Life Sciences adopted the Montréal Declaration: on the Synthesis of Evidence to Advance the 3Rs Principles in Science. The declaration called for a “…change in the culture of planning, executing, reporting, reviewing, and translating animal research.”71 In particular, the Montréal Declaration called for the use of systematic reviews and reporting guidelines to be implemented in animal studies in a similar vein as had been done for clinical research decades earlier.
Currently, only the ARRIVE guidelines, implemented for primary animal studies, recommends that authors report “…any implications of your experimental methods or findings for the replacement, refinement or reduction (the 3Rs) of the use of animals in research.”60 We decided to evaluate whether the 3Rs are being implemented through reporting in systematic reviews of preclinical animal research. Specifically, our objectives were to determine how often the 3Rs are explicitly reported in published systematic reviews of preclinical animal studies.
Methods.
One previous systematic review of systematic reviews and meta-analyses of in vivo animal experiments, carried out to inform human health, searched for materials through 2005.104 In 2011, this earlier review105 was extended to include studies through 2010.65 The combined studies identified 185 systematic reviews without meta-analyses, 59 systematic reviews with meta-analyses, and 22 meta-analyses that did not include a systematic review. We selected the 59 systematic reviews that included a meta-analysis for our analysis (references 8, 12, 13, 23, 52, 25, 26, 32, 36, 41, 55, 57, 69, 73, 76, 80–82, 84, 97, 103, 107, 113, 116, 129, 130, and 141–143, which were included in the 2006 review,105 and references 2, 7, 9, 11, 22, 28, 38, 39, 47, 59, 64, 66, 75, 77, 83, 85–88, 98, 99, 101, 102, 110, 121, 133, 139, 140, 144, and 145, which were in the 2011 publication66). We used standard definitions of the 3Rs from the Canadian Council on Animal Care17 to develop a 4-item data extraction checklist (Figure 2) to evaluate reporting of the 3Rs. Our approach was to read the 3Rs as they were reported and not to read the 3Rs into the papers for the authors. Each item was operationalized such that we scored a result of Yes, No, or Partial for each (Figure 2).
Figure 2.
Data extraction checklist to evaluate reporting of 3Rs.
For both Yes and Partial scores, authors had to have explicitly described an effect regarding animals that fell within the definition of the 3Rs, but authors did not need to have explicitly used the term replacement, reduction, or refinement (for example, “alternative” or “fewer animals” was sufficient). Yes and Partial scores were differentiated by whether the discussion of the item was in reference to the results of the systematic review (Yes) or not (Partial). When a paper had information to support both a Yes and a Partial score for an item, we scored the item as Yes only. The year of publication and journal name were extracted also.
Two authors (MTA and NF) independently pilot-tested the data extraction checklist on 5 randomly selected included studies and refined the criteria. Disagreements were resolved by discussion between the extractors (MTA and NF); if no agreement could be reached, then the third author (GG) acted as the decider. The same 2 authors (MTA and NF) independently extracted data from the 59 articles and one author (MTA) also compiled the supporting text for decisions that scored Yes or Partial (Table 1). For analysis, we counted the total number of Yes, No, and Partial scores for each item and evaluated differences descriptively.
Table 1.
Supporting text from references that met the criteria for Yes or Partial
| Reference | Sub-item name | Text for Yes | Text for Partial |
| 26 | Reduction | Strains such as SHR are inbred and require only a few animals to achieve statistical significance. | |
| 70 | Reduction | Because the use of large numbers of animals necessary for a survival study may not be justified ethically, the effect of an intervention on mortality may require extrapolation from results of studies that may not be primarily designed to establish survival differences. Therefore, insufficient numbers of animals are often employed. The sample size limitations associated with extrapolating results of survival from small experimental studies have, until now, been considered an inevitable limitation of such designs. | |
| 81 | Reduction | As the number of drugs analyzed in this way increases, multiple regression modeling should allow identification of those factors which have greatest impact on estimates of effect size. In turn, this may allow identification of a subset of variables which are sufficient to describe the properties of a given drug, potentially reducing the number of experiments needed to characterize that drug. | |
| 82 | Reduction | 1. Based on our observations, to have 80% power to estimate the percentage improvement in outcome after treatment to the nearest 10% would require 115 animals per group. Such sample sizes may seem large, but the use of smaller cohorts represents a false economy and results are likely to be misleading; ultimately more animals would be required. | |
| 2. Using this approach, we now have data for 3092 animals from 208 individual comparisons. With the addition of further data, multiple regression modeling should allow identification of those factors which have greatest impact on estimates of effect size. In turn, this may allow identification of a subset of variables which are sufficient to describe the properties of an administered drug, potentially reducing the number of experiments needed to characterize that drug. | |||
| 103 | 3Rs | Medical Research Council found that most people support their use provided that there are benefits to human health care, no alternative exists, and no unnecessary suffering occurs. | |
| 114 | 3Rs | In a recent editorial, Smith promoted the 3 Rs of animal research first suggested by William Russell: replacement, reduction, and refinement. On methodological grounds, animal experimentation would better contribute to human health care if we promoted registration, randomisation, and systematic reviews. | |
| Reduction | In his book The Principles of Humane Experimental Technique, William Russell proposed the principle of reduction—that is, the use of methods to “reduce the number of animals needed to obtain information of a given amount and precision.” Meta-analyses of the results of previous animal experiments would increase the precision of estimates of treatment effects and therefore reduce the number of animals needed in future experiments. | However, animal researchers are encouraged to reduce the number of experimental animals to a minimum. Indeed, the need to use the minimum number of animals to obtain valid results is embodied in the Animals (Scientific Procedures) Act 1986 and European legislation. |
Results.
Overall, most of the systematic reviews (53 of 59, 90%) did not explicitly report on any aspect of the 4 items. Of the 6 (10%) systematic reviews that did report on at least one item, 2 reviews reported on the 3Rs in general, 5 reported on reduction, and none reported on replacement or refinement (Table 2). The number of systematic reviews of animal-based studies each year increased, but no discernable trend for the reporting of the 3Rs was visible. However, only 1 of the 6 systematic reviews that reported on the 3Rs was from the most recent (2006 to 2010) cohort of studies.
Table 2.
Systematic reviews that reported on 3Rs
| Reference | Year | 3Rs | Replacement | Reduction | Refinement |
| 26 | 1994 | No | No | Yes | No |
| 70 | 2003 | No | No | Partial | No |
| 81 | 2005 | No | No | Yes | No |
| 82 | 2005 | No | No | Yes | No |
| 103 | 2007 | Partial | No | No | No |
| 114 | 2002 | Partial | No | Yes | No |
Discussion.
We began this review by looking to Russell and Burch's advice on factors that would govern progress in the implementation of 3Rs principles. We argued that systematic reviews and reporting guidelines are ideal tools for tackling the problem of information retrieval, which Russell and Burch saw as central to progress in humane science. The advantages of systematic reviews are their use of structured methodology and, in particular, the use of systematic search strategies to identify all relevant information for the focus question. Reporting guidelines, when implemented and enforced, help to ensure that all the relevant information that is necessary to critically appraise a research report is available. These 2 tools—reporting guidelines and systematic reviews—complement one another by ensuring that all information is present in the primary publication and that all relevant publications are identified for the synthesis. We also argued that systematic reviews may be ideal tools for identifying opportunities to implement the 3Rs because they formally evaluate both the methods and outcomes of multiple studies. However, the majority of the systematic reviews that we examined did not take the extra step to explicitly report their findings in the context of the 3Rs. Because systematic reviews are general tools, there is no guarantee that authors will either interpret their findings in the context of the 3Rs or report on 3Rs explicitly. Our results show that reporting on the 3Rs by systematic review authors is limited at best and, although very few of the systematic reviews explicitly discussed the 3Rs, reduction was discussed by some authors. Despite this lack of explicit 3Rs reporting, we believe that systematic reviews are an excellent tool to address the challenge of information retrieval and thus aid in progress of the implementation of the 3Rs. If primary research reports are missing critical information, then the full use of the animals in those reports has not been achieved and may lead to unnecessary or misguided subsequent work. Similarly, if reviews of treatments do not assess all relevant papers in a systematic manner, then research may continue long after questions have been definitely answered, thereby resulting in the unnecessary use of animals. The use of cumulative meta-analysis in systematic reviews in particular has potential to avoid unnecessary testing by determining when a question has been answered definitely. One limitation of systematic reviews and reporting guidelines is that they cannot identify studies that are simply unpublished. Publication bias potentially harms both animals in the research, whose euthanasia never becomes part of the scientific discourse, and the scientific community at large, which is presented with a biased set of research findings.62
Reduction opportunities were reported in 5 of the 6 systematic reviews that reported on any type of the 3Rs, and 4 of these were in relation to their results. Reduction opportunities that were identified included: 1) combining studies data in meta-analysis or other techniques, a strategy that increases the precision of estimates for treatment effects and thus reduces the number of animals needed in future studies;69,113 2) the use of sample-size calculations to correctly detect effect sizes, a measure that may increase the number of animals per experiment in the short term but that reduces the overall number of animals used in the long term;81 3) the identification of factors that had the greatest impact on estimates of effect size;80,81 and 4) the identification of the animal strains that required fewer animals to detect statistical significance.26 The greater number of reduction opportunities reported relative to those for replacement and refinement suggests that systematic reviews and meta-analyses may be particularly useful for identifying areas for reduction.
According to our reporting criteria, there were no examples of replacement or refinement opportunities among the 59 reviews we evaluated. However, our review evaluated a limited subsample of systematic reviews with meta-analyses from a larger pool of systematic reviews without meta-analyses, a feature that may have biased our results. In addition, we relied on 2 systematic reviews65,104 that were focused on systematic reviews of in vivo animal studies to inform human health and thus will have missed systematic reviews from other fields (for example, veterinary medicine,43,100 conservation,128 and in vitro studies114). Our criteria also required authors to explicitly report about the 3Rs, but there is currently no reporting requirement for systematic review authors to do so.104,123 The 2 reviewers for this paper (MTA and NF) found that some of the systematic reviews did report on methods and outcomes that could be related to the 3Rs, but they were not discussed in that context. For example, when multiple species were compared and one species was found to be a better model for clinical translation,76 the potential replacement or reduction benefits were not mentioned (therefore the description did not meet our criteria). Another limitation of our review is that the systematic reviews are relatively old in a field that is rapidly developing and improving its methods.15,65
Another approach to identify 3Rs-related findings in systematic reviews is to purposely review systematic reviews to identify the methods and outcomes that could assist 3Rs objectives—even if they are not explicitly reported as such. This shifts the focus from examining how the 3Rs are reported, as we did, to how the 3Rs can be interpreted as the reports are written. This approach may be a useful first step to identify the common elements within existing systematic reviews that can be used to achieve 3Rs objectives.
In the systematic reviews that we evaluated, the 3Rs were not part of the questions being addressed. If the 3Rs were considered at the initial planning stage of a systematic review, when the research question is framed, it may be incorporated more easily into the review or even made the focus. However, we recognize that expecting authors of systematic reviews to explicitly interpret their findings in the context of the 3Rs may be arguable. The ARRIVE guidelines for primary animal studies already include a reporting item for interpreting results in the context of the 3Rs, and we believe that a similar criterion could be adopted for the reporting of preclinical systematic reviews. There would be some advantages to discussing the 3Rs in systematic reviews compared with primary studies, due to the nature of the reports. First, because systematic reviews evaluate many studies and assess heterogeneity in design and the animal models, they are a more advantageous venue for identifying 3Rs opportunities than are primary studies. Although the systematic reviews in our evaluation were not designed to assess the 3Rs, some authors identified clear opportunities to achieve reduction. Second, it is more likely that authors have both the space and prerogative to explore broader issues and implications in a review article than in a primary study, where the reporting requirement may be seen as burdensome. Third, a key challenge for the achieving the 3Rs is information retrieval of 3Rs-relevant literature itself. When systematic review authors do identify a 3Rs opportunity, using the terminology of the 3Rs and including appropriate keywords in the publication would greatly facilitate the identification of 3Rs opportunities to other researchers.
Conducting systematic reviews of animal studies is still a relatively new endeavor.49 In the preclinical field, the CAMARADES16 and SYRCLE109 groups are leading the way in conducting systematic reviews of animal studies and developing tools and methods.50,51,123,136,138 Recently, at the 21st Cochrane Colloquium in Québec City, a consensus was reached to establish a Preclinical Animal Study Methods Group jointly with the Cochrane Collaboration.112 In addition, the NC3Rs in the United Kingdom is actively promoting the use of systematic reviews in animal studies, providing funding opportunities, and acting as a leader in developing reporting guidelines.95 To address the need for the development, promotion, and support of research reporting guidelines (not solely for animal-based studies) an umbrella organization, the Equator Network,126 was formed, and its website is a valuable source of information for reporting guidance.29
Progress in implementation of the 3Rs principles in practice is dependent on many factors. In this review, we looked at the problem of information retrieval and identified currently available, complementary tools for solving it. Systematic reviews and reporting guidelines both hold great promise for progress in implementation of the 3Rs. We showed that, currently, the explicit reporting of the 3Rs is limited and that additional research is needed to assess the implicit reporting of the 3Rs in systematic reviews. In particular, systematic reviews may be a source for reduction opportunities, although the path to implementation will require focused effort. For persons using animals in science, these tools present an opportunity to improve the quality of their research, use limited resources more effectively, and contribute to progress in the implementation of the 3Rs, as Russell and Burch advised more than 50 y ago.
References
- 1.Altman DG, Moher D. 2013. Declaration of transparency for each research article. An antidote to inadequate reporting of research. BMJ 4796:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarasingh S, Macleod MR, Whittle IR. 2009. What is the translational efficacy of chemotherapeutic drug research in neurooncology? A systematic review and meta-analysis of the efficacy of BCNU and CCNU in animal models of glioma. J Neurooncol 91:117–125. [DOI] [PubMed] [Google Scholar]
- 3.Announcement: reducing our irreproducibility 2013. Nature 496:398–398. [Google Scholar]
- 4.Baker D, Lidster K, Sottomayor A, Amor S. 2014. Two years later: journals are not yet enforcing the ARRIVE guidelines on reporting standards for preclinical animal studies. PLoS Biol 12:e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balcombe J, Ferdowsian H, Briese L. 2013. Prolonged pain research in mice: trends in reference to the 3Rs. J Appl Anim Welf Sci 16:77–95. [DOI] [PubMed] [Google Scholar]
- 6.Balls M, Goldberg AM, Fentem JH, Caren L, Burch RL, Festing MFW, Frazier JM, Hendriksen CFM, Jennings M, Van Der MDO, Morton DB, Rowan AN, Russell C, Russell WM, Spielmann H, Stephens ML, Stokes WS, Straughan DW, Yager JD, Zurlo J, van Zutphen BF. 1995. The 3 Rs: the way forward. The report and recommendations of ECVAM Workshop 11. Altern Lab Anim 23:838–866. [PubMed] [Google Scholar]
- 7.Banwell V, Sena ES, Macleod MR. 2009. Systematic review and stratified meta-analysis of the efficacy of interleukin 1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis 18:269–276. [DOI] [PubMed] [Google Scholar]
- 8.Baron M, Haas R, Dörtbudak O, Watzek G. 2000. Experimentally induced peri-implantitis: a review of different treatment methods described in the literature. Int J Oral Maxillofac Implants 15:533–544. [PubMed] [Google Scholar]
- 9.Bath PM, Gray LJ, Bath AJ, Buchan A, Miyata T, Green AR, NXY059 Efficacy Meta-analysis in Individual Animals with Stroke Investigators 2009. Effects of NXY059 in experimental stroke: an individual animal meta-analysis. Br J Pharmacol 157:1157–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begley CG, Ellis LM. 2012. Drug development: raise standards for preclinical cancer research. Nature 483:531–533. [DOI] [PubMed] [Google Scholar]
- 11.Benatar M. 2007. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis 26:1–13. [DOI] [PubMed] [Google Scholar]
- 12.Bertani H, Gelmini R, Del Buono MG, De Maria N, Girardis M, Solfrini V, Villa E. 2002. Literature overview on artificial liver support in fulminant hepatic failure: a methodological approach. Int J Artif Organs 25:903–910. [DOI] [PubMed] [Google Scholar]
- 13.Biondi-Zoccai GGL, Abbate A, Parisi Q, Agostoni P, Burzotta F, Sandroni C, Zardini P, Biasucci LM. 2003. Is vasopressin superior to adrenaline or placebo in the management of cardiac arrest? A meta-analysis. Resuscitation 59:221–224. [DOI] [PubMed] [Google Scholar]
- 14.Bradford SC. 1953. Documentation. London (UK): Crosby Lockwood. [Google Scholar]
- 15.Briel M, Müller KF, Meerpohl JJ, Elm E von, Lang B, Motschall E, Gloy V, Lamontagne F, Schwarzer G, Bassler D, OPEN Consortium 2013. Publication bias in animal research: a systematic review protocol. Syst Rev 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CAMARADES [Internet] CAMARADES. [Cited 7 February 2014]. Available from: http://www.camarades.info/
- 17.Canadian Council on Animal Care. [Internet] About the 3 Rs. 2014 [Cited 12 February 2014]. Available from: http://3rs.ccac.ca/en/about/
- 18.Chalmers I. 1986. Electronic publications for updating controlled trial reviews. Lancet 328:287. [Google Scholar]
- 19.Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, Howells DW, Ioannidis JP, Oliver S. 2014. How to increase value and reduce waste when research priorities are set. Lancet 383:156–65. [DOI] [PubMed] [Google Scholar]
- 20.Chan AW, Krleza-Jerić K, Schmid I, Altman DG, Krlez K. 2004. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ. 171:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins FS, Tabak LA. 2014. Policy: NIH plans to enhance reproducibility. Nature 505:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corpet DE, Pierre F. 2005. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice, and men. Eur J Cancer 41:1911–1922. [DOI] [PubMed] [Google Scholar]
- 23.Craig JC, Wheeler DM, Irwig L, Howman-Giles RB. 2000. How accurate is dimercaptosuccinic acid scintigraphy for the diagnosis of acute pyelonephritis? A meta-analysis of experimental studies. J Nucl Med 41:986–93. [PubMed] [Google Scholar]
- 24.Dickersin K. 1990. The existence of publication bias and risk factors for its occurrence. JAMA 263:1385–1389. [PubMed] [Google Scholar]
- 25.Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA. 2003. Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer 106:766–770. [DOI] [PubMed] [Google Scholar]
- 26.Dumas P, Tremblay J, Hamet P. 1994. Stress modulation by electrolytes in salt-sensitive spontaneously hypertensive rats. Am J Med Sci 307 Suppl 1:S130–S137. [PubMed] [Google Scholar]
- 27.Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JPT, Mavergames C, Gruen RL. 2014. Living systematic reviews: an emerging opportunity to narrow the evidence–practice gap. PLoS Med 11:e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.England TJ, Gibson CL, Bath PMW. 2009. Granulocyte-colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: a systematic review. Brain Res Rev 62:71–82. [DOI] [PubMed] [Google Scholar]
- 29. EQUATOR. [Internet]. The EQUATOR network—enhancing the quality and transparency of health research. [Cited 7 February 2014]. Available from: http://www.equator-network.org/
- 30.European Food Safety Authority 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. p 1-90. EFSA J.8:1637 p 1–90. [Google Scholar]
- 31.Executive Committee of the Congress 2009. Background to the Three Rs Declaration of Bologna, as Adopted by the 3rd World Congress on Alternatives and Animal Use in the Life Sciences, Bologna, Italy, on 31 August 1999. ATLA 37: 286–289. [PubMed] [Google Scholar]
- 32.Fay MP, Freedman L, Clifford C, Midthune D. 1997. Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res 57:3979–3988. [PubMed] [Google Scholar]
- 33.Fergusson D, Glass KC, Hutton B, Shapiro S. 2005. Randomized controlled trials of aprotinin in cardiac surgery: could clinical equipoise have stopped the bleeding? Clin Trials 2:218–229. [DOI] [PubMed] [Google Scholar]
- 34.Festing MF. 1992. The scope for improving the design of laboratory animal experiments. Lab Anim 26:256–268. [DOI] [PubMed] [Google Scholar]
- 35.Festing MFW, Altman DG. 2002. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 43:244–258. [DOI] [PubMed] [Google Scholar]
- 36.Freedman LS. 1994. Meta-analysis of animal experiments on dietary fat intake and mammary tumours. Stat Med 13:709–718. [DOI] [PubMed] [Google Scholar]
- 37.Fry DJ. 2013. The need to improve experimental design. Altern Lab Anim 41:P61–P64. [DOI] [PubMed] [Google Scholar]
- 38.Gibson CL, Gray LJ, Bath PMW, Murphy SP. 2008. Progesterone for the treatment of experimental brain injury; a systematic review. Brain 131:318–328. [DOI] [PubMed] [Google Scholar]
- 39.Gibson CL, Gray LJ, Murphy SP, Bath PMW. 2006. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab 26:1103–1113. [DOI] [PubMed] [Google Scholar]
- 40.Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, Michie S, Moher D, Wager E. 2014. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 383:267–276. [DOI] [PubMed] [Google Scholar]
- 41.Glatt SJ, Bolaños CA, Trksak GH, Jackson D. 2000. Effects of prenatal cocaine exposure on dopamine system development: a meta-analysis. Neurotoxicol Teratol 22:617–629. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg AM, van Zutphen LFM. 1995. Preface to the World Congress on Alternatives and Animal Use in the Life Sciences: Education, Research, and Testing. In: Alternative methods in toxicology. New York (NY): Mary Ann Liebert. [Google Scholar]
- 43.Habacher G, Pittler MH, Ernst E. 2006. Effectiveness of acupuncture in veterinary medicine: systematic review. J Vet Intern Med 20:480–488. [DOI] [PubMed] [Google Scholar]
- 44.Hackam DG, Redelmeier D. 2006. Translation of research evidence from animals to humans. JAMA 296:1731–1732. [DOI] [PubMed] [Google Scholar]
- 45.Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG. 2013. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med 10:e1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins J, Green S. 2011. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane-handbook.org. [Google Scholar]
- 47.Ho KM, Duff O, Chambers D, Murray R. 2008. Meta-analysis of nebulized amphotericin B to prevent or treat pulmonary aspergillosis in immunosuppressed animals. Transpl Infect Dis 10:168–176. [DOI] [PubMed] [Google Scholar]
- 48.Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. 2010. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the 3 Rs, and to make systematic reviews more feasible. Altern Lab Anim 38:167–182. [DOI] [PubMed] [Google Scholar]
- 49.Hooijmans CR, Ritskes-Hoitinga M. 2013. Progress in using systematic reviews of animal studies to improve translational research. PLoS Med 10:e1001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. 2014. SYRCLE's risk-of-bias tool for animal studies. BMC Med Res Methodol 14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooijmans CR, Tillema A, Leenaars M, Ritskes-hoitinga M. 2010. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 44:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horn J, Williams L, Health K, Horn J, de Haan RJ, Vermeulen M, Luiten PGM, Limburg M. 2001. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke 32:2433–2438. [DOI] [PubMed] [Google Scholar]
- 53.Institute for Laboratory Animal Research 2011. Guidance for the description of animal research in scientific publications, p 1–31. Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- 54.Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. 2010. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 7:CD001269. [DOI] [PubMed] [Google Scholar]
- 55.Jiao LR, Seifalian AM, Mathie RT, Habib N, Davidson BR. 2000. Portal flow augmentation for liver cirrhosis. Br J Surg 87:984–991. [DOI] [PubMed] [Google Scholar]
- 56.Kane RL, Wang J, Garrard J. 2007. Reporting in randomized clinical trials improved after adoption of the CONSORT statement. J Clin Epidemiol 60:241–249. [DOI] [PubMed] [Google Scholar]
- 57.Kelley G. 1996. Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. J Appl Physiol 81:1584–1588. [DOI] [PubMed] [Google Scholar]
- 58.Ker K, Edwards P, Perel P, Shakur H, Roberts I. 2012. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 344:e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ker K, Perel P, Blackhall K. 2009. β2 receptor antagonists for traumatic brain injury: a systematic review of controlled trials in animal models. CNS Neurosci Ther 15:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. The ARRIVE guidelines animal research: reporting in vivo experiments. PLoS Biol 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, Hutton J, Altman DG. 2009. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE 4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimmelman J, Anderson JA. 2012. Should preclinical studies be registered? Nat Biotechnol 30:488–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimmelman J, Mogil JS, Dirnagl U. 2014. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLoS Biol 12:e1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kook S, Kim G, Choi K. 2009. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J Med Food 12:552–560. [DOI] [PubMed] [Google Scholar]
- 65.Korevaar DA, Hooft L, ter Riet G. 2011. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim 45:225–230. [DOI] [PubMed] [Google Scholar]
- 66.Lamontagne F, Meade M, Ondiveeran HK, Lesur O, Fox-Robichaud AE. 2008. Nitric oxide donors in sepsis: a systematic review of clinical and in vivo preclinical data. Shock 30:653–659. [DOI] [PubMed] [Google Scholar]
- 67.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. 2012. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. 1992. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 327:248–254. [DOI] [PubMed] [Google Scholar]
- 69.Lee DS. 2003. Meta-analysis of the effects of endothelin receptor blockade on survival in experimental heart failure. J Card Fail 9:368–374. [DOI] [PubMed] [Google Scholar]
- 70.Leenaars M, Hooijmans CR, van Veggel N, ter Riet G, Leeflang M, Hooft L, van der Wilt GJ, Tillema A, Ritskes-Hoitinga M. 2012. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim 46:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leenaars M, Ritske-hoitinga M, Griffin G, Ormandy E. 2012. Background to the Montréal Declaration on the Synthesis of Evidence to Advance the 3Rs Principles in Science. ALTEX 1:35–38. [Google Scholar]
- 72.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linde K, Jonas WB, Melchart D, Worku F, Wagner H, Eitel F. 1994. Critical review and meta-analysis of serial agitated dilutions in experimental toxicology. Hum Exp Toxicol 13:481–492. [DOI] [PubMed] [Google Scholar]
- 74.Lloyd MH, Foden BW, Wolfensohn SE. 2008. Refinement: promoting the 3 Rs in practice. Lab Anim 42:284–293. [DOI] [PubMed] [Google Scholar]
- 75.Lorente JA, Marshall JC. 2005. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock 24:107–119. [DOI] [PubMed] [Google Scholar]
- 76.Lucas C, Criens-Poublon LJ, Cockrell CT, de Haan RJ. 2002. Wound healing in cell studies and animal model experiments by low-level laser therapy: were clinical studies justified? A systematic review. Lasers Med Sci 17:110–134. [DOI] [PubMed] [Google Scholar]
- 77.Lui AJ, Byl NN. 2009. A systematic review of the effect of moderate intensity exercise on function and disease progression in amyotrophic lateral sclerosis. J Neurol Phys Ther 33:68–87. [DOI] [PubMed] [Google Scholar]
- 78.van Luijk J, Cuijpers Y, van der Vaart L, de Roo TC, Leenaars M, Ritskes-Hoitinga M. 2013. Assessing the application of the 3Rs: a survey among animal welfare officers in The Netherlands. Lab Anim 47:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Luijk J, Leenaars M, van Dongen AM, van der Vaart L, Ritskes-Hoitinga M. 2012. Outcomes of a Dutch workshop on improvements for the 3Rs in daily practice. ALTEX 29:440–443. [DOI] [PubMed] [Google Scholar]
- 80.Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. 2005. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res 38:35–41. [DOI] [PubMed] [Google Scholar]
- 81.Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. 2005. Systematic review and meta-analysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 25:713–721. [DOI] [PubMed] [Google Scholar]
- 82.Macleod MR, O'Collins T, Howells DW, Donnan GA. 2004. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35:1203–1208. [DOI] [PubMed] [Google Scholar]
- 83.Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. 2008. Evidence for the efficacy of NXY059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 39:2824–2829. [DOI] [PubMed] [Google Scholar]
- 84.Mapstone J, Roberts I, Evans P. 2003. Fluid resuscitation strategies: a systematic review of animal trials. J Trauma 55:571–589. [DOI] [PubMed] [Google Scholar]
- 85.Marshall JC. 2005. The effects of granulocyte colony-stimulating factor in preclinical models of infection and acute inflammation. Shock 24:120–129. [DOI] [PubMed] [Google Scholar]
- 86.Matthan NR, Jordan H, Chung M, Lichtenstein AH, Lathrop DA, Lau J. 2005. A systematic review and meta-analysis of the impact of ω3 fatty acids on selected arrhythmia outcomes in animal models. Metabolism 54:1557–1565. [DOI] [PubMed] [Google Scholar]
- 87.Minnerup J, Heidrich J, Rogalewski A, Schäbitz W-R, Wellmann J. 2009. The efficacy of erythropoietin and its analogues in animal stroke models: a meta-analysis. Stroke 40:3113–3120. [DOI] [PubMed] [Google Scholar]
- 88.Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schäbitz W-R. 2008. Meta-analysis of the efficacy of granulocyte colony-stimulating factor in animal models of focal cerebral ischemia. Stroke 39:1855–1861. [DOI] [PubMed] [Google Scholar]
- 89.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. 2010. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moher D, Jones A. 2001. Use of the CONSORT statement and quality of reports of randomized trials. JAMA 285:1992–1995. [DOI] [PubMed] [Google Scholar]
- 91.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moher D, Schulz KF, Simera I, Altman DG. 2010. Guidance for developers of health research reporting guidelines. PLoS Med 7:e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moher D, Tricco AC. 2008. Issues related to the conduct of systematic reviews: a focus on the nutrition field. Am J Clin Nutr 88:1191–1199. [DOI] [PubMed] [Google Scholar]
- 94.Nash-Stewart CE, Kruesi LM, Del Mar CB. 2012. Does Bradford ’ s Law of Scattering predict the size of the literature in Cochrane Reviews? J Med Libr Assoc 100:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.National Centre for the Replacement Refinement & Reduction of Animals in Research. [Internet] 2014. NC3Rs–ARRIVE guidelines. [Cited 30 May 2014]. Available from: http://www.nc3rs.org.uk/page.asp?id=1357
- 96.Nature. [Internet] 2013. Reporting checklist for life sciences articles. [Cited February 7 2014]. Available from: http://www.nature.com/authors/policies/checklist.pdf.
- 97.Nava-Ocampo AA, Reyes-Pérez H, Bello-Ramírez AM, Mansilla-Olivares A, Ponce-Monter H. 2000. For ischemic brain damage, is preclinical evidence of neuroprotection by presynaptic blockade of glutamate release enough? Med Hypotheses 54:77–79. [DOI] [PubMed] [Google Scholar]
- 98.Neitzke U, Harder T, Schellong K, Melchior K, Ziska T, Rodekamp E, Dudenhausen JW Plagemann A. 2008. Intrauterine growth restriction in a rodent model and developmental programming of the metabolic syndrome: a critical appraisal of the experimental evidence. Placenta 29:246–254. [DOI] [PubMed] [Google Scholar]
- 99.Norberg MM, Krystal JH, Tolin DF. 2008. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63:1118–1126. [DOI] [PubMed] [Google Scholar]
- 100.Olivry T, Mueller RS. 2003. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol 14:121–146. [DOI] [PubMed] [Google Scholar]
- 101.Pandit JJ, O'Gallagher K. 2008. Effects of volatile anesthetics on carotid body response to hypoxia in animals. Adv Exp Med Biol 605:46–50. [DOI] [PubMed] [Google Scholar]
- 102.Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. 2007. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perna A, Remuzzi G. 1996. Abnormal permeability to proteins and glomerular lesions: a meta-analysis of experimental and human studies. Am J Kidney Dis 27:34–41. [DOI] [PubMed] [Google Scholar]
- 104.Peters JL, Sutton A, Jones D, Rushton L, Abrams K. 2006. A systematic review of systematic reviews and meta-analyses of animal experiments with guidelines for reporting. J Environ Sci Health B 41:1245–1258. [DOI] [PubMed] [Google Scholar]
- 105.Petticrew M. 2001. Systematic reviews from astronomy to zoology: myths and misconceptions. BMJ 322:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petticrew M, Roberts H. 2006. Systematic reviews in the social sciences: a practical guide. Malden (MA): Blackwell Publishing. [Google Scholar]
- 107.Pries AR, Secomb TW, Sperandio M, Gaehtgens P. 1998. Blood flow resistance during hemodilution: effect of plasma composition. Cardiovasc Res 37:225–235. [DOI] [PubMed] [Google Scholar]
- 108.Prinz F, Schlange T, Asadullah K. 2011. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10:712. [DOI] [PubMed] [Google Scholar]
- 109.Radboud University Medical Center. [Internet] RadboudUMC–SYRCLE. [Cited 7 February 2014]. Available from: http://www.umcn.nl/research/departments/cdl/syrcle/Pages/default.aspx
- 110.Ramacciotti E, Myers DD, Wrobleski SK, Deatrick KB, Londy FJ, Rectenwald JE, Henke PK, Schaub RG, Wakefield TW. 2010. P-selectin/ PSGL1 inhibitors versus enoxaparin in the resolution of venous thrombosis: a meta-analysis. Thromb Res 125:e138–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rennie D. 1995. Reporting randomized controlled trials. JAMA 273:1054. [DOI] [PubMed] [Google Scholar]
- 112.Ritskes-Hoitinga M, Leenaars M, Avey M, Rovers M, Scholten R. 2014. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst Rev 3:ED000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberts I, Kwan I, Evans P, Haig S. 2002. Does animal experimentation inform human healthcare? Observations from a systematic review of international animal experiments on fluid resuscitation. BMJ 324:474–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenthal R. 1979. The file-drawer problem and tolerance for null results. Psychol Bull 86:638–641. [Google Scholar]
- 116.Rowlett JK, Woolverton WL. 1996. Assessment of benzodiazepine receptor heterogeneity in vivo: apparent pA2 and pKB analyses from behavioral studies. Psychopharmacology (Berl) 128:1–16. [DOI] [PubMed] [Google Scholar]
- 117.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Metheuen. [Google Scholar]
- 118.Sargeant JM, O'Connor AM, Gardner IA, Dickson JS, Torrence ME, Consensus Meeting Participants 2010. The REFLECT statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration. Zoonoses Public Health 57:105–136. [DOI] [PubMed] [Google Scholar]
- 119.Schulz KF, Altman DG, Moher D. 2010. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 7:e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwarz F, Iglhaut G, Becker J. 2012. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J Clin Periodontol 39 Suppl 12:63–72. [DOI] [PubMed] [Google Scholar]
- 121.Sena E, Wheble P, Sandercock P, Macleod M. 2007. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke 38:388–394. [DOI] [PubMed] [Google Scholar]
- 122.Sena ES, Briscoe CL, Howells DW, Donnan GA, Sandercock PA, Macleod MR. 2010. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab 30:1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. 2014. Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically. J Cereb Blood Flow Metab 34:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sena ES, van der Worp HB, Bath PMW, Howells DW, Macleod MR. 2010. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 8:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sense about science. [Internet] Systematic reviews. [Cited 7 February 2014]. Available at: http://www.senseaboutscience.org/resources.php/52/sense-about-systematic-reviews
- 126.Simera I, Moher D, Hirst A, Hoey J, Schulz KF, Altman DG. 2010. Transparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR Network. BMC Med 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith R. 2001. Animal research: the need for a middle ground. BMJ 322:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith R, Pullin A, Stewart G, Sutherland W. 2010. Is predator control an effective strategy for enhancing bird populations? CEE review 08-001 (SR38). Environmental Evidence: www.environmentalevidence.org/SR38.html. [DOI] [PubMed] [Google Scholar]
- 129.Sumner BE, Cruise LA, Slattery DA, Hill DR, Shahid M, Henry B. 2004. Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology (Berl) 171:306–321. [DOI] [PubMed] [Google Scholar]
- 130.Syeda B, Schukro C, Heinze G, Modaressi K, Glogar D, Maurer G, Mohl W. 2004. The salvage potential of coronary sinus interventions: meta-analysis and pathophysiologic consequences. J Thorac Cardiovasc Surg 127:1703–1712. [DOI] [PubMed] [Google Scholar]
- 131.Taylor K. 2010. Reporting the implementation of the 3 Rs in European primate and mouse research papers: are we making progress? Altern Lab Anim 38:495–517. [DOI] [PubMed] [Google Scholar]
- 132.The Standards of Reporting Trials Group. A proposal for structured reporting of randomized controlled trials 1994. JAMA 272:1926–1931. [PubMed] [Google Scholar]
- 133.Trksak GH, Glatt SJ, Mortazavi F, Jackson D. 2007. A meta-analysis of animal studies on disruption of spatial navigation by prenatal cocaine exposure. Neurotoxicol Teratol 29:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, Al-Shahi Salman R, Macleod MR, Ioannidis JP. 2013. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol 11:e1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. 2012. Does use of the CONSORT statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane Review. Syst Rev 1:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR. 2014. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221:92–102. [DOI] [PubMed] [Google Scholar]
- 137.Visscher MB. 1951. Methods in medical research, vol 4. Chicago (IL): The Year Book Publishers. [Google Scholar]
- 138.de Vries RBM, Hooijmans CR, Tillema A, Leenaars M, Ritskes-hoitinga M, De Vries RBM. 2011. A search filter for increasing the retrieval of animal studies in Embase. Lab Anim 45:268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L, Xun P, Zhao Y, Wang X, Qian L, Chen F. 2008. Effects of lead exposure on sperm concentrations and testes weight in male rats: a meta-regression analysis. J Toxicol Environ Health A 71:454–463. [DOI] [PubMed] [Google Scholar]
- 140.Wheble PCR, Sena ES, Macleod MR. 2008. A systematic review and meta-analysis of the efficacy of piracetam and piracetam-like compounds in experimental stroke. Cerebrovasc Dis 25:5–11. [DOI] [PubMed] [Google Scholar]
- 141.Willmot M, Gibson C, Gray L, Murphy S, Bath P. 2005. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: a systematic review. Free Radic Biol Med 39:412–425. [DOI] [PubMed] [Google Scholar]
- 142.Willmot M, Gray L, Gibson C, Murphy S, Bath PMW. 2005. A systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide 12:141–149. [DOI] [PubMed] [Google Scholar]
- 143.Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS. 2004. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22:241–247. [DOI] [PubMed] [Google Scholar]
- 144.Worp HB van der, Sena ES, Donnan GA, Howells DW, Macleod MR. 2007. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130:3063–3074. [DOI] [PubMed] [Google Scholar]
- 145.Zhang J, Xie X, Li C, Fu P. 2009. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. J Ethnopharmacol 126:189–196. [DOI] [PubMed] [Google Scholar]



