Abstract
Practical implementation of the 3Rs at national and regional levels around the world requires long-term commitment, backing, and coordinated efforts by international associations for laboratory animal medicine and science, including the International Association of Colleges of Laboratory Animal Medicine (IACLAM) and the International Council for Laboratory Animal Science (ICLAS). Together these organizations support the efforts of regional organization and communities of laboratory animal science professionals as well as the development of local associations and professional colleges that promote the training and continuing education of research facility personnel and veterinary specialists. The recent formation of a World Organization for Animal Health (OIE) Collaborating Center for Laboratory Animal Science and Welfare emphasizes the need for research into initiatives promoting laboratory animal welfare, particularly in emerging economies and regions with nascent associations of laboratory animal science.
Abbreviations: CIOMS, Council of International Organizations of Medical Sciences; IACLAM, International Association of Colleges of Laboratory Animal Medicine; ICLA, International Committee on Laboratory Animals; ICLAS, International Council for Laboratory Animal Science; ILAR, Institute for Laboratory Animal Research; IUBS, International Union of Biologic Sciences; OIE, World Organization for Animal Health; WVA, World Veterinary Association; UNESCO, United Nations Educational, Scientific, and Cultural Organization
Promoting cross-cultural acceptance and implementation of 3Rs14 practices in biomedical science around the world requires consistent messaging, persistence, and long term support. This is a complex and time-consuming process that requires overall shifts in the belief systems and priorities of institutional officials (persons responsible for ensuring institutional compliance), scientists, veterinarians, regulatory oversight bodies, funding agencies, and animal care personnel, particularly in regions or countries without national animal welfare strategies or legislation. The ultimate goal of any such global harmonization process is adaptive change and widespread adoption of generally accepted best practices in the care and use of research animals. The incentive for countries or regions to participate in this process is both economic and ethical, in that consistent adoption of 3Rs practices may lead to broader acceptance of scientific output, increased opportunities for scientific collaborations and research funding, an enhanced ability to compete in the global scientific enterprise, more efficient achievement of scientific objectives, and enhanced internal and external public acceptance of the ethical nature of the scientific process as conducted in that region.
Establishing and sustaining multicultural acceptance of the need to enhance laboratory animal care and welfare requires the development of collaborative relationships with experienced and knowledgeable persons and organizations. Internationally based, not-for-profit, cross-cultural laboratory animal science organizations are strategically well placed to provide long-term peer support, encouragement, mentoring, and training to enhance 3Rs practices. As will be discussed, organizations such as the International Council for Laboratory Animal Science (ICLAS) and International Association of Colleges of Laboratory Animal Medicine (IACLAM) bring credibility, experience, and consistency to support global laboratory animal welfare messages, based on their permanent or ad hoc affiliations with other international associations, such as the World Organization for Animal Health (OIE) and World Veterinary Association (WVA). A particularly successful technique that these organizations have used to encourage uptake of 3Rs practices is the promotion of local, national, and regional ‘grassroots’ organizations of laboratory animal science composed of locally employed forward-thinking professionals and leaders in the field. This group works directly with, and influences the scientific community, which ultimately needs to lead in adoption of alternatives. Development of more organized research opportunities to support adoption of 3Rs practices, for example, through the initiation of an OIE Collaborating Center on laboratory animal science and welfare in conjunction with the Institute for Laboratory Animal Research (ILAR), will only enhance the process in the long term.
International Development and Organization of Laboratory Animal Science
History, mission, and organization of ICLAS.
In the wake of World War II, medical research and the associated use of animal models surged globally. With this rapid growth in animal-dependent research, concerns regarding disparities in animal quality and standards of care became apparent to a number of national and international science associations. From addressing these concerns came the creation of what is now ICLAS.
The original associations whose actions led to ICLAS's formation were the International Union of Biologic Sciences (IUBS), the Council of International Organizations of Medical Sciences (CIOMS), and the United Nations Educational, Scientific, and Cultural Organization (UNESCO). In 1955, IUBS appointed a committee to evaluate problems in science fields that relied on animal research subjects. Also that year, UNESCO requested international information on the production and use of laboratory animals; CIOMS was the lead on providing this information. At a meeting in Paris in 1956, members representing these and other international associations agreed to establish the International Committee on Laboratory Animals (ICLA) as an independent nongovernmental scientific committee, with the ideal of raising standards in laboratory animal use on a global basis. UNESCO provided financial backing for ICLA until 1962, at which time ICLA was able to establish its own support.
Initially, ICLA was composed of 7 members, representing CIOMS, IUBS, UNESCO, and constituent members of the associations comprising the IUBS. Over the ensuing years, the ICLA held annual meetings at which the membership and governance mechanisms were developed, and the constitution, bylaws, and policies were established.5 ICLA became a Scientific Associate member of the International Council of Scientific Unions (ICSU) in 1975, and in 1984 was recognized as an Inter-Union Commission by the ICSU. In 1975, ICLA changed its name to the International Council for Laboratory Animal Science (ICLAS). In 1980, ICLAS was granted Observer status in the World Veterinary Association (WVA).
The mission of ICLAS is to promote and coordinate the development of laboratory animal science throughout the world, particularly in developing countries. The council fosters international collaboration, quality definition and monitoring of laboratory animals, the collection and dissemination of information on laboratory animal science, and harmonization in the care and use of laboratory animals. ICLAS encourages humane use of animals in research by recognizing ethical principles and by promoting 3Rs tenets.14
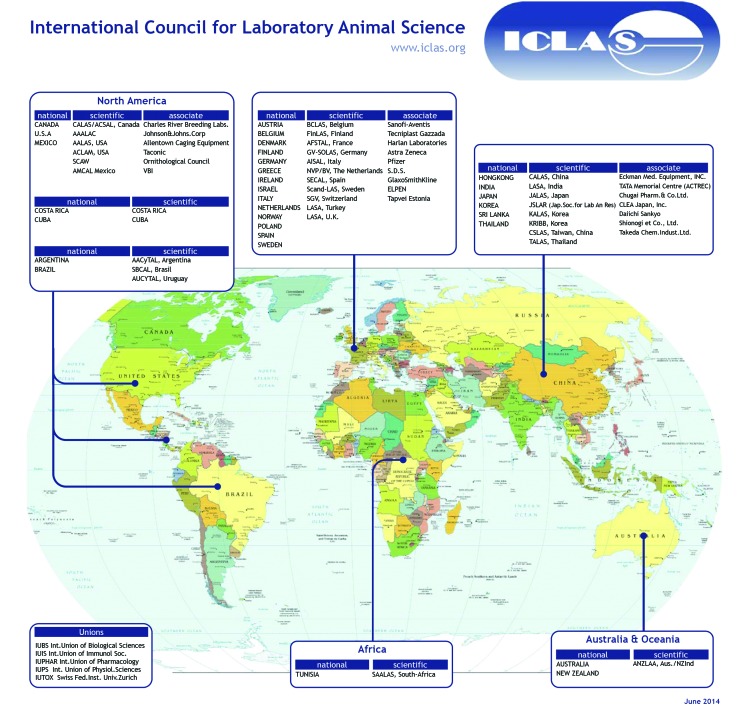
ICLAS membership categories have evolved over the years. Currently, there are 6 categories of membership. National Members are countries that are represented by a person appointed by an appropriate national body concerned with the direction and encouragement of scientific research within the scope of interest of ICLAS. Currently there are 27 National Members (Figure 1). Scientific or Union Members include laboratory animal science associations, scientific societies or unions, and other organizations that contribute to the work of ICLAS. There are 34 Scientific Members and 4 Union Members. Institutional Members (4 currently) are universities, research institutes, or other noncommercial organizations that support the aims of ICLAS. Association Members (18 currently) are organizations in sympathy with the aims of ICLAS. Organizations with which ICLAS has reciprocal relationships are Affiliate Members, and Honorary Members comprise persons who have made distinguished contributions to laboratory animal science, in particular to ICLAS.
Figure 1.
Global distribution of regional ICLAS members and associations. Membership categories (National, Scientific/Union, Institutional, Association, Affiliate, and Honorary) are defined in the text. Additional information about specific members and membership categories can be found at the ICLAS website (www.iclas.org).
The annual Governing Board and member meetings take place in a different global region each year and are held in conjunction with an international scientific meeting of a laboratory animal science association or federation of such associations. Elections of officers and Governing Board members are held every 4 y at an ICLAS General Assembly at one of these meetings.
ICLAS activities and initiatives.
The priorities of ICLAS have evolved over the 58 y since its inception. Initially, the main focus was on gathering information on laboratory animal use, conditions, and problems in various countries, with a goal of sharing information and facilitating communication of new ideas and progress in the field to help raise standards where needed. Communication to members occurred initially through a periodical, the ICLA Bulletin, which in 1991 was replaced by ICLAS News. Since 1999, the ICLAS website (www.iclas.org) has become the primary source of information for members, with intermittent news updates issued by email alerts. Governance documents, meeting minutes, policies, and committee reports are all available to members on the ICLAS webpage. ICLAS committees may publish guidelines, reports, or scientific papers in peer-reviewed journals.8
ICLAS’ priority has evolved from its original focus on collecting and sharing data on international development in laboratory animal science, to an active role of promoting ethical practices, harmonization of standards, improved animal quality, and access to educational opportunities. The primary work of ICLAS is carried out by its committees, consisting of designated members of the Governing Board plus interested and qualified persons recruited to assist the committee, often in ad hoc working groups with specific aims. For example, the ICLAS Harmonization Committee, created in 2004, reviews national guidelines relating to the care and use of animals for scientific purposes and recognizes those guidelines suitable for implementation internationally. The aim is to facilitate conduct of appropriate animal-based science on a global level and to protect the welfare of animals used in science. The committee's goal is not standardization; rather, ICLAS considers that each country should be able to maintain an animal welfare oversight system that reflects its cultures, traditions, religions, laws, and regulations. The Harmonization Committee and its working groups have created or adopted guidelines for euthanasia and humane research endpoints, ethical review of proposals for the use of animals, education and training of animal users in science, and genetically engineered animals.3,4,13 The committee is currently working on harmonization of experimental reporting standards for scientific journals. The aim is not to replace or supersede standards that have been created recently or are already in existence, such as the ARRIVE and ILAR Guidelines,7,10 but to provide a readily achievable first step that is practical enough to be universally adopted.
The ICLAS Ethics and Animal Welfare Committee bases its priorities on the principles that decisions regarding the welfare, care, and use of research animals should be guided by scientific knowledge and professional judgment, reflect ethical and social values, and consider the potential benefits and effects on the wellbeing of research animals. This committee has created Ethical Guidelines for Researchers and Guidelines for Reviewers and Editors, which are available on the ICLAS website (http://iclas.org/committees/ethics-and-animal-welfare-committee).
The ICLAS Education and Training Committee promotes and harmonizes education and training in laboratory animal science, particularly in regions where such opportunities are few or nonexistent. On the ICLAS webpage, the committee provides a resource that lists educational programs and resources by global region or country, according to information provided by regional contacts. A current initiative for this committee is a scholarship program (http://iclas.org/committees/education-and-training-committee/iclas-scholarship-program-for-veterinarians-in-laboratory-animal-science-and-medicine) for veterinarians who desire training in laboratory animal science and medicine but who live and work in regions where educational opportunities in this field are limited or unavailable. The scholarships are provided for designated programs in which the training is completed primarily online in a modular format, and onsite experiential learning requirements are of limited duration. Upon completion, these programs provide participants with a certificate or degree, which can be used to demonstrate specialized training or knowledge in laboratory animal medicine. The program is designed to assist veterinarians to complete meaningful training that requires minimal time away from their workplaces and homes. The program is endorsed by the OIE and the International Association of Colleges of Laboratory Animal Medicine (IACLAM) and has received financial support from various associations for laboratory animal science and medicine and from partners in industry.
ICLAS has supported the development of regional committees for Europe, Asia, the Americas, Africa, and Oceania (Figure 1). Regional committees are charged with supporting laboratory animal science educational opportunities and collaborations, providing assistance for national and regional associations, and aiding with the development of regulatory guidelines in regions where laboratory animal science is emerging. Diversity in language and cultural norms, economic disparities among nations, differences in the interest and power of governments to enact and enforce regulations, and large geographic distances in the regions present challenges for these committees. ICLAS has limited financial resources and, as such, requires regional committees to seek other sources of financial support for the majority of their regional activities.
The ICLAS Laboratory Animal Quality Network was established in 2006 as a joint initiative of ICLAS and various laboratories involved in monitoring the health or genetics of laboratory animals. This network has as its goal maintenance and improvement of the quality of animals used in research, as well as raising awareness of the importance of high quality laboratory animals within the international scientific community. The ICLAS Laboratory Animal Quality Network runs 2 programs: a Performance Evaluation Program for Diagnostic Laboratories, which enables diagnostic laboratories to monitor the sensitivity and specificity of health monitoring assays; and the Genetic Quality Monitoring Program, which is focused on colony monitoring for research facilities and suppliers breeding rodents. In addition to providing guidance and advice on genetic quality testing, the Genetic Quality Monitoring Program assists with developing self-assessment programs for genetic monitoring and promotes education and training to enhance awareness of the importance of genetic quality in research rodents.
Recently, ICLAS has collaborated with CIOMS in revising the International Guiding Principles for Biomedical Research Involving Animals.2 Like the original 1985 International Guiding Principles, the revised Principles are intended to be used by the scientific community to guide the responsible use of vertebrate animals in scientific or educational activities. In particular, the Principles are designed to assist countries in developing programs for the humane care and use of animals in research and education, especially those entities operating without national regulations. The revised Principles specifically state that “The tenets of the Three Rs—Replacement, Reduction, and Refinement—should be incorporated in the design and conduct of scientific and/or educational activities that involve animals.”2 The Principles reference the use of alternatives, using the minimum number of animals necessary to achieve the scientific or educational goals, and refinement of experimental techniques.
Future directions for ICLAS.
The ICLAS mission is central to advancing the 3Rs globally as animal research facilities are founded or expand in nations with emerging economies. The quality of science carried out with experimental animal models and its international acceptance benefit directly from the ideals, collaboration, and harmonization that ICLAS seeks to promote. That is, animals of defined genetic background and health status; the incorporation of ethical considerations in experimental design, review, and conduct; consistency in reporting of research results; and training and continuing education of laboratory animal scientists and researchers all promote the application of the 3Rs.
The Practice of Laboratory Animal Medicine Throughout the World
Laboratory animal veterinarians are professionals who by virtue of interest, experience, and training are specialized in working with and caring for laboratory animals. They are integral members of the research team and provide veterinary support for model development and implementation as they work to safeguard animal wellbeing.16 Although basic veterinary education imparts some of the specialized skills and information necessary to work with laboratory animals, the diversity and complexity of the laboratory environment and the species used within that environment generally require additional experience and training.1,21
Several associations and federations of laboratory animal veterinarians have been convened nationally and regionally (Figure 2), and these serve as forums for the exchange of information, recruitment of veterinarians into laboratory animal medicine, provision of continuing education, and development of guidelines and best practices, as well as other activities, including regional or national representation of laboratory animal veterinarians. There are minimal requirements for membership in most of these professional associations, and they do not conduct certifying activities to gauge the extent of knowledge and skill base of their members. These organizations are meant to be inclusive of all veterinarians with an interest in laboratory animal medicine and often serve as the umbrella organization within which the specialty is constituted. Members of Colleges of Laboratory Animal Medicine may also hold membership in such associations.
Figure 2.
National and regional associations for laboratory animal veterinarians. These associations require members to be veterinarians but represent professional interests only and do not certify competency in laboratory animal medicine.
Veterinarians undertaking the practice of laboratory animal medicine can demonstrate specific competency by becoming certified by an established group of specialists (also known as a College of Laboratory Animal Medicine [CLAM]) who have defined a set of skills and knowledge that each member, typically called a ‘Diplomate’, should possess. CLAM and their standards are often monitored by a regional veterinary specialty board, such as the American or European Board of Veterinary Specialties, to ensure consistent quality and consistency of processes for initial and continued certification of veterinary specialists. CLAM consist of specialists in veterinary medicine who have been certified to be competent in their specialty by virtue of training, experience, research, and publication, in addition to demonstration of the necessary knowledge and skill base required to pass an examination developed and administered by their peers. Independent CLAM exist on a country or limited regional basis. Each College has many members, who, in addition to their demonstrated proficiency in laboratory animal medicine, may possess subspecialization expertise, achieved through additional board certification or graduate training, in a variety of areas (for example, animal welfare, pathology, genetics, surgery, preventive medicine, animal behavior) that have direct bearing on the care, use, and wellbeing of laboratory animals.
History, mission, and organization of IACLAM.
The International Association of Colleges of Laboratory Animal Medicine (IACLAM; www.iaclam.org) was conceived in 2005 and formally constituted in 2006.9,19 It is a global organization that brings together national and regional CLAM with the goal of providing a common platform at the global level for communication among and representation of these colleges and their diplomates in various activities. IACLAM is an association of CLAM and, as such, does not conduct independent certification of veterinarians. Appointed representatives of these colleges serve on the IACLAM Board of Directors. IACLAM also serves as a global body of experts for those organizations and persons seeking advice and expertise from recognized veterinary specialists in the care, use, and welfare of laboratory animals. IACLAM assists individual laboratory animal medicine specialty organizations, including member colleges, to promote the welfare and responsible use of laboratory animals through the certification of veterinary specialists, development of educational programs, and dissemination of information relevant to the field and by serving as research partners.20
IACLAM currently consists of 4 member colleges, the American College of Laboratory Medicine (ACLAM, www.aclam.org), founded in 1957; European College of Laboratory Animal Medicine (ECLAM, www.eslav-eclam.org), founded in 1999; Japanese College of Laboratory Animal Medicine (JCLAM, http://plaza.umin.ac.jp/jclam/start/index.html), founded in 1998; and Korean College of Laboratory Animal Medicine (KCLAM, http://www.kclam.org/www/english/01.html), founded in 2006. Currently, the membership of the 4 colleges totals approximately 1155 active diplomates in 24 countries (Figure 3).
Figure 3.
Geographic locations of Diplomates from all CLAM.
Membership in a CLAM and its influence on the 3Rs.
All member Colleges of IACLAM impose additional postveterinary training or experience requirements that range from 2 to 6 y before eligibility is conferred to sit for the certifying examination. Internationally, formalized training programs have proven to be the most common route for veterinarians to become board-certified in laboratory animal medicine. Approximately 61% of all diplomates have gone through a formal training program, and the remaining 39% have used a practice option with mentoring to prepare for board certification. The practice option is a viable route for veterinarians to approach board specialization, and most colleges recognize this option. Therefore, board certification of veterinarians from countries without formal colleges of laboratory animal medicine is an attainable goal, particularly with mentoring support.
Diplomates of IACLAM member Colleges are employed in a variety of biomedical research activities in academia, industry, government, and not-for-profit research institutions. Roughly 66% of diplomates hold additional postDVM academic degrees, with 38% of diplomates holding doctoral degrees in a variety of subjects. This characteristic attests to their many years of training en route to specialization. Laboratory animal veterinary specialists therefore represent a knowledgeable group of persons who bring significant value to the research community.
The recruitment of veterinarians to this specialty and their retention is a common goal for all colleges of laboratory animal medicine and should be a goal of the scientific community internationally to ensure high standards of care and wellbeing of research animals. It is critical that provision of veterinary medical care and oversight is tailored to the unique needs and uses of the diversity of species housed in research facilities. IACLAM's promulgation of advanced veterinary medical training is central to ensuring that the veterinarian has the appropriate skill set and experience, and to ensuring implementation of the 3Rs in this highly specialized environment.
International efforts of IACLAM.
Because of its unique role in providing international expertise in laboratory animal medicine, IACLAM has developed both formal and informal relations with other pivotal organizations having an interest in the use of animals for research, testing, and education. These organizations include ICLAS, ILAR, OIE, and WVA. In addition, the American and Korean CLAM have established formal relationships with AAALAC through membership on AAALAC's Board of Trustees.
IACLAM has positioned itself to assist laboratory animal medicine specialists in countries where nascent colleges of laboratory animal medicine are beginning to organize (http://iaclam.org/documents/IACLAM%20Associate%20Membership%20-%20Guidance%20to%20Applicant%20Colleges.pdf). IACLAM can offer assistance in determining the structure of these new Colleges so that they are organized in a manner consistent with the current colleges in Asia, Europe, and the United States.20 In addition, IACLAM members are active in developing veterinary curricula in laboratory animal medicine as well as defining regional standards for veterinary care of laboratory species.1,15,17,20
IACLAM members have participated in the development of ICLAS guidelines on a range of topics including humane endpoints, animal user training, protocol review, and genetically modified animals. Most recently, IACLAM has collaborated with ICLAS to support an international scholarship that will provide training to laboratory animal veterinarians in countries with emerging economies.18
IACLAM Board members have served on the ILAR Council and, more specifically, on the International Committee of the Council. Such dual service ensures the coordination of activities and allows for collaboration on topics of mutual interest. For example, IACLAM and ILAR representatives coauthored an article that compared guidelines regarding adequate veterinary care for animals in research from around the world.22 In addition, IACLAM contributed to the ILAR-hosted 2008 conference, “Animal Research in a Global Environment: Meeting the Challenges,” by chairing sessions and providing speakers to address topics of global importance, such as difficulties of and opportunities for harmonization and operational and training issues associated with working across different standards in different countries.11
The OIE is recognized as a reference organization by the World Trade Organization and, as of May 2014, has a total of 180 Member Countries and Territories. IACLAM Board members have served on an OIE ad hoc working group to advise on the development of standards for laboratory animal care and welfare.12 The standards developed by this group for research animal care and welfare were incorporated into the Terrestrial Animal Health Code but now have become a global standard of care for research animals, exemplified by the implementation of the relevant section of the Code by AAALAC during its assessments of animal care and use programs. Codes and their associated manuals are reference documents used by the veterinary administrations or the competent authorities of the Member Countries and Territories, to assist them to establish regulations for their countries. Participation by IACLAM in this manner ensures that appropriate expertise is contributed to the discussion of international standards and that harmonization of efforts occurs.
Furthermore, in 2012, the first OIE collaborating center for laboratory animal science and welfare was established in partnership with ILAR with the support of IACLAM representatives. The OIE collaborating centers are internationally recognized centers of expertise that promote partnerships between developed and emerging economies in specific areas. The long-term aim of this laboratory animal science and welfare center is to promote research and educational initiatives to enhance the acceptance and adoption of 3Rs tenets around the world.
The WVA serves the veterinary profession—veterinary medicine and science—and promotes veterinary rights, standards, and competency at an international level. The organization's mission is to promote animal and human health and wellbeing through sustainable and humane use and management of animals. The WVA's constituency comprises more than 70 national veterinary associations and 10 international associations of veterinary specialists. IACLAM is the only international specialty college in the WVA. IACLAM members have served on the WVA Council and have worked with other Council members to develop a harmonized curriculum for veterinarians and to generate guidelines for veterinary leadership in promoting animal welfare.6
Overall, IACLAM, as an international organization, provides a readily accessible source of expertise from credentialed specialists in laboratory animal medicine. The wide spectrum of subspecialties coupled with the breadth of experience and familiarity with the range of laboratory animals used in biomedical research provides a platform for assisting the global biomedical research community and other international bodies in providing for the appropriate care, use, and wellbeing of animals in research environments. As noted previously, IACLAM partners national and international organizations to advance laboratory animal science and medicine. Finally, IACLAM is in a unique and enviable position to foster education, dialogue, and scientifically based analyses of components of laboratory animal care and use that will advance the implementation of the 3Rs.
Summary and Conclusions
Changing global attitudes about long-standing research practices and the welfare of laboratory animals is a long-term and incremental process. The end goal is harmonization of good management practices, with the aim of ultimately improving laboratory animal welfare, reducing laboratory animal use, and ensuring that research animals are incorporated into studies only when absolutely necessary. Changes in practices can be regulated and legislated, and although this process provides an impetus for amending practices to meet a minimal standard, true transformative change is often voluntary, brought about by increased education, training, and awareness and frequently occurs within a community. ICLAS and IACLAM seek to support such voluntary change in a global manner by promoting interconnectedness and a sense of responsibility for laboratory animal wellbeing among research animal users and caregivers.
Acknowledgments
We thank Guy DeVroey for assistance with the figure and past and current IACLAM representatives for survey comments.
References
- 1.Bayne K, Bayvel D, MacArthur Clark J, Demers G, Joubert C, Kurosawa TM, Rivera E, Souilem O, Turner PV. 2011. Harmonizing veterinary training and qualifications in laboratory animal medicine: a global perspective. ILAR J 52:393–403. [DOI] [PubMed] [Google Scholar]
- 2.Council for International Organizations of Medical Sciences and International Council for Laboratory Animal Science. [Internet] 2012. International guiding principles for biomedical research involving animals. [Cited 5 August 2014]. Available at: http://www.cioms.ch/images/stories/CIOMS/IGP2012.pdf.
- 3.Demers G, Brown M, Gauthier C, Rozmiarek H, Griffin G, Bedard M. 2013. International harmonization of guidance on the ethical review of proposals for the use of animals, and on the education and training of animal users in science. STAL 38: 73–79. [Google Scholar]
- 4.Demers G, Griffin G, De Vroey G, Haywood JR, Zurlo J, Bedard M. 2006. Harmonization of animal care and use guidance. Science 312:700–701. [DOI] [PubMed] [Google Scholar]
- 5.Erichsen S, Hopla CE. [Internet]. 2004. History of the International Council for Laboratory Animal Science. [Cited 5 August 2014]. Available at: http://iclas.eventconsulting.be/wp-content/uploads/2012/07/History-of-ICLAS.pdf [DOI] [PubMed]
- 6.Jorna T, Turner PV, Ostensson K. [Internet]. 2011. WVA position on global day-one competences. WVA/doc/010/007. [Cited 5 August 2014]. Available at: http://www.worldvet.org/library.php?item=64&cat=1&view=item.
- 7.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostomitsopoulos N, Carbone C, Demers G. 2008. Role of the International Council for Laboratory Animal Science (ICLAS) in the care and use of laboratory animals in Southeast Europe. Arch Biol Sci 60:175–179. [Google Scholar]
- 9.MacArthur Clark J. 2007. A global vision for laboratory animal medicine. ALTEX 14 Suppl:735–737. [Google Scholar]
- 10.National Research Council 2011. Guidance for the description of animal research in scientific publications. Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- 11.National Research Council 2011. Animal research in a global environment: meeting the challenges. Proceedings of the November 2008 International Workshop. Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- 12.OIE. [Internet] 2010. Chapter 7.8 Use of animals in research and education. Terrestrial animal health code. [Cited 19 January 2014]. Available at: http://web.oie.int/eng/normes/mcode/en_chapitre_1.7.8.htm.
- 13.Rose M, Everitt J, Hedrich H, Schofield J, Dennis M, Scott E, Griffin G. 2013. ICLAS Working Group on Harmonization: international guidance concerning the production care and use of genetically altered animals. Lab Anim 47:142–152. [DOI] [PubMed] [Google Scholar]
- 14.Russell WMS, Burch RL. [Internet]. 1959. The principles of humane experimental technique. London (UK): Methuen; [Cited 5 August 2014]. Available at: Available at: http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc. [Google Scholar]
- 15.Turner PV. 2008a. The CALAM/ACMAL standards of veterinary care and laboratory animal welfare. Can Vet J 49:86–88. [PMC free article] [PubMed] [Google Scholar]
- 16.Turner PV, Baar M, Olfert ED. 2009a. Laboratory animal medicine—needs and opportunities for Canadian veterinarians. Can Vet J 50:257–260. [PMC free article] [PubMed] [Google Scholar]
- 17.Turner PV, Colby LA, Vandewoude S, Gaertner DJ, Vasbinder MA. 2009b. Perspectives on curriculum needs in laboratory animal medicine. J Vet Med Educ 36:89–99. [DOI] [PubMed] [Google Scholar]
- 18.Turner PV, Vergara P, Kornerup-Hansen A, MacArthur Clark J, Pekow C. Global training programs for veterinarians in laboratory animal medicine. Proceedings of the OIE Global Conference on Veterinary Education. 4-6 December 2013, Foz do Iguacu, Brazil. [Google Scholar]
- 19.Turner PV, White W, Baneux P, Bayne K, MacArthur Clark J, Kurosawa TK, Ohwada K, Lee KJ, Park J-H, Ikeda T. 2008. Harmonization of a global vision for laboratory animal medicine—The International Association of Colleges of Laboratory Animal Medicine. J Am Assoc Lab Anim Sci 47:138. [PMC free article] [PubMed] [Google Scholar]
- 20.Turner PV, White W, MacArthur Clark J, Bayne K. 2013. Promoting international development of advanced training in laboratory animal medicine: IACLAM associate membership. Proceedings of the FELASA Symposium, Barcelona, Spain. J Am Assoc Lab Anim Sci 52:261. [Google Scholar]
- 21.Voipio HM, Baneux P, Gomez de Segura IA, Hau J, Wolfensohn S. 2008. Guidelines for the veterinary care of laboratory animals: report of the FELASA/ECLAM/ESLAV Joint Working Group on Veterinary Care. Lab Anim 42:1–11. [DOI] [PubMed] [Google Scholar]
- 22.Zurlo J, Bayne K, MacArthur Clark J. 2009. Adequate veterinary care for animals in research: a comparison of guidelines from around the world. ILAR J 50:85–88. [DOI] [PubMed] [Google Scholar]