Abstract
The National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) is an independent scientific organization that is based in the United Kingdom, which was set up by the government to lead the discovery and application of new technologies and approaches that minimize the use of animals in research and improve animal welfare. The NC3Rs uses a range of strategies to improve and advance science through application of the 3Rs. These include funding basic research, open innovation (CRACK IT), and programs run by inhouse scientists. We present several case studies from the NC3Rs portfolio, featuring asthma research, the use of nonhuman primates in monoclonal antibody development, and CRACK IT. Finally, we anticipate the future, as we use our experience to move into new research fields and expand toward international collaboration. Here we highlight how equipping scientists with relevant and emerging 3Rs tools can help overcome the challenges and limitations of the use of animals in research to the benefit of the whole bioscience community.
Abbreviations: BBSRC, Biotechnology and Biological Sciences Research Council; mAb, monoclonal antibody; MRC, Medical Research Council; NC3Rs, National Centre for the Replacement, Refinement, and Reduction of Animals in Research; SME, small and medium-sized companies; UK, United Kingdom
The National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) is an independent scientific organization in the United Kingdom (UK) that was set up by the government to lead the discovery and application of new technologies and approaches that minimize the use of animals in research and improve animal welfare. The 3Rs provide a legal and ethical framework for in vivo research. The Centre aims to use the 3Rs as a catalyst to accelerate significant and innovative advances in science and business practice.
Historically, the 3Rs principles have been advocated for social and ethical reasons, with less focus on how they could be used to improve the quality of science. In 2001 the Medical Research Council (MRC), a UK-based publicly financed research-funding body, founded the Centre for Best Practice for Animals in Research, which aimed to improve the quality of animal work being undertaken in their own research units. This move was a significant step forward in recognizing the need to balance high animal welfare standards with world-leading scientific advances. In 2002, after a review into the use of animals in scientific procedures, a report was published by a House of Lords Select Committee (British parliamentary committees that investigate public policy, proposed laws, and government activity) recommending the establishment of a national center to focus on the 3Rs. Consequently, in September 2004, the NC3Rs was established, replacing and broadening the work of the Centre for Best Practice for Animals in Research.
Over the past 10 y, the Centre has expanded from a team of 2 to 25 staff, including 12 postdoctoral scientists. Funding is provided primarily by the UK government, but financial support is also received for specific programs and posts from the charitable and commercial sectors. Today, with an annual budget of around £7 million (approximately USD$11 million), the NC3Rs is the largest funder of 3Rs research in the United Kingdom. The Centre provides support and advice to scientists, regulators, and government bodies at both national and international levels on how to replace, reduce, and refine the use of animals in research and testing.
The NC3Rs’ work over the last 10 y has contributed to the current environment of increased openness among scientists to discuss and adapt their use of animals in research. Accompanying this openness is increasing scientific evidence that animal models may not always provide the most appropriate data to model human disease. Some animal models are poor predictors of human safety and disease, which may contribute to high levels of drug attrition, that is, the failure of candidate compounds to complete the pharmaceutical development process.17,23,26 Many pharmaceutical, chemical companies, and contract research organizations are pursuing ways to decrease their animal use. This shift in the environment supports the NC3Rs’ approach of actively encouraging collaboration across sectors, facilitating data sharing between companies through our role as an honest and unbiased broker, and bringing scientists together en masse to unite with a common voice and to stimulate change. Ensuring that the 3Rs remain topical and relevant by responding to and shaping the research landscape in turn helps to maintain a raised 3Rs profile. In this overview, we describe how the NC3Rs supports the science base, in the United Kingdom and beyond, through work that enables the successful application of the 3Rs.
The NC3Rs’ Tools and Strategies
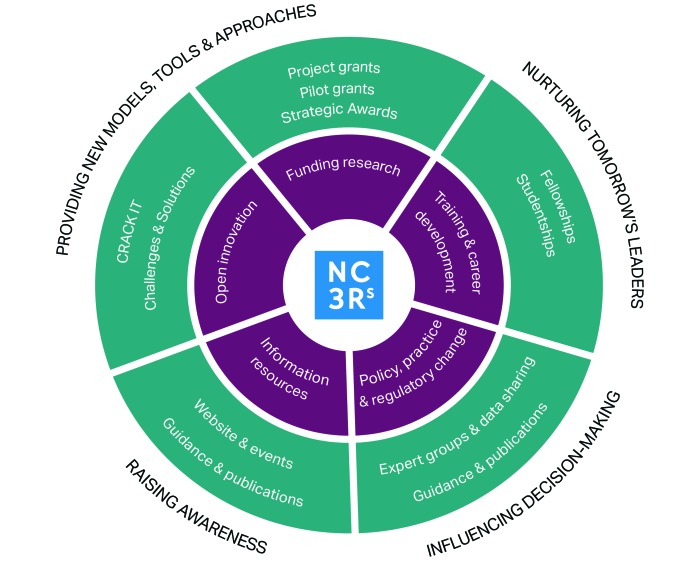
The NC3Rs operates a multifaceted approach, using different mechanisms to engage a diverse group of stakeholders (Figure 1). In addition to funding 3Rs-inspired basic research, the Centre employs a team of inhouse scientists who manage center-led research programs as well as the open-innovation platform CRACK IT.
Figure 1.
Schematic demonstrating the tools and overall strategy of the NC3Rs.
Funding research, training, and early career development.
The NC3Rs grant-funding strategy focuses on hypothesis-driven research to generate a pipeline of 3Rs ideas, approaches and technologies across the biosciences. Funding includes provision for 1) training and development, through PhD studentships and early career fellowships; 2) pilot studies; 3) projects grants; 4) investment into specific strategic areas; and 5) changes to infrastructure at an organizational level. The broad range of research schemes aims to ensure that the 3Rs are established in the minds and practices of the next generation of research leaders; to support research in areas where there are specific knowledge gaps; and to improve the infrastructure and resources that underpin UK research.
The research portfolio encompasses a wide range of technologies and approaches, across pharmaceuticals and chemicals, neuroscience and behavior, cells and systems, and infection, immunity and inflammation. Since 2004, more than £35 million (approximately USD$54 million) has been awarded across a total of 200 grants. Information about all of the projects funded to date can be found on the the NC3Rs website (www.nc3rs.org.uk).
Supporting open innovation.
One of the NC3Rs’ major goals is to ensure that technologies emerging from the basic science base are incorporated into practice. In 2011 the Centre launched the first open-innovation platform in the 3Rs, CRACK IT. This program supports research and development to replace, reduce, and refine the use of animals by delivering advanced tools and technologies that will provide commercial as well as scientific benefit.
CRACK IT is designed to spark collaborations between the pharmaceutical, chemical, and academic sectors, bringing together expertise in the bioscience, chemistry, mathematics, and engineering fields. This approach capitalizes on the shift within the pharmaceutical and chemical industries toward increased outsourcing and external collaboration. The platform has a particular focus on commercialization of products developed by small and medium-sized companies (SMEs) and academic spin-offs to create financial benefit at a national level. The scheme is split into 2 distinct parts: Challenges and Solutions.
Challenges.
CRACK IT Challenges is run in partnership with Innovate UK (formerly the Technology Strategy Board), as part of the Small Business Research Initiative. It is an annual competition in which the market-driven business needs related to animals in research are defined by Sponsors from the scientific community (the ‘end-users’). Sponsors provide ‘in-kind’ contributions (data, compounds, and equipment), expertise and advice, while the NC3Rs provides funding. The Sponsors define the exact problem and contribute extensively throughout the project to the design of the end-product. In this way, the 3Rs technique or technology has a ready-made market in which to pull from basic research toward successful commercialization and uptake. The NC3Rs brings together the relevant scientific communities and helps build teams or consortia to ‘solve’ the Challenge. Two distinct features of CRACK IT Challenges are that it is 1) separated into 2 phases to enable investment into multiple ideas before a ‘Dragon's Den–Shark's Tank’-style interview to select the final contractor and 2) contract-based milestone-driven research, where payment is linked to the delivery of key objectives.
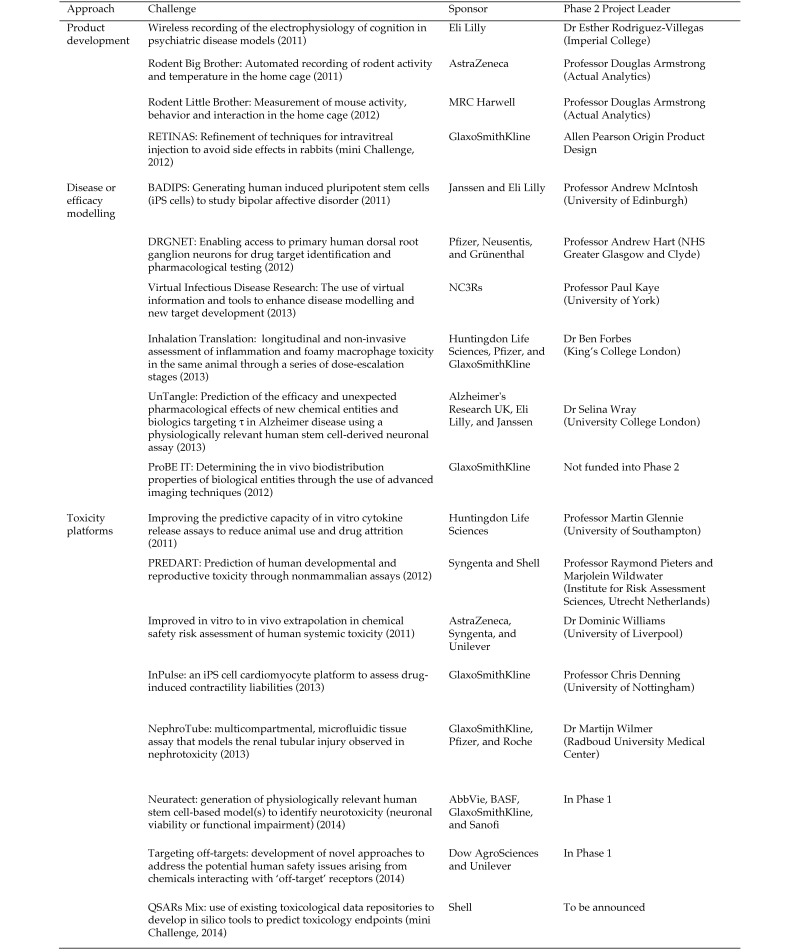
To date, 19 Challenges have been launched between 17 sponsoring organizations, with high levels of SMEs involvement. In 2013 alone, £7 million (approximately USD$11 million) was committed for 5 Challenges. The scheme now attracts funding from external sources, including Alzheimer's Research UK and the Department for Environment Food and Rural Affairs (Defra). An overview of the ongoing Challenges can be seen in Figure 2, and a case study of a completed Challenge is presented in the following section.
Figure 2.
Summary of CRACK IT Challenges to date.
Solutions.
CRACK IT Solutions is a technology-partnering hub, designed for academics or SMEs who are developing a novel technology to advance the 3Rs (a ‘Solution’) and who need new research partners or collaborators to support progress of the project. This forum can help developers to gain backing to validate the technology or to promote it to a wider audience. Research and development opportunities aligned to industry priorities are identified by the NC3Rs by working alongside university technology-transfer offices and life-science networks and with SMEs. Once Solutions have been identified, they are showcased on the dedicated CRACK IT website (www.crackit.org.uk) and distributed through partner networks such as One Nucleus and PraxisUnico or partnered directly with industry via the Centre's extensive contact network. The NC3Rs provides seed funding for a maximum of 1 y to help catalyze these new partnerships.
Influencing policy, practice, and regulatory change.
The NC3Rs inhouse programs of work are identified through horizon scanning and evaluation of emerging opportunities, for example, where there are planned regulatory guideline revisions or emergence of novel technologies (for example, ‘organ on a chip’). Programs are selected with input from expert scientists from industry and academia and prioritized on criteria such as short- and long-term impacts on the 3Rs, extent of innovation, benefit to UK business, cost and resources required, and alignment with global scientific advances.
There is a strong collaborative foundation to all projects, which are often based around data collection and analysis. Through expert working groups, data sharing is managed and facilitated across companies and sectors, as the NC3Rs provides a ‘safe harbor.’ Working groups also help to identify areas where awareness should be raised within the scientific community or where additional expertise is required to evaluate new strategic areas.
Figure 3 provides specific examples of selected The NC3Rs activities within each program area, with references to corresponding peer-reviewed publications.
Figure 3.
Examples of NC3Rs activities across different focus areas. *, Also applies to the category Influencing practices in the pharmaceutical industry.
Shaping the academic research landscape.
To help stimulate a culture shift within academia, the NC3Rs works closely with UK research councils and charitable bodies that fund bioscience research. These collaborations have funded basic research and have contributed to educational and peer-review activities. NC3Rs scientists sit on a wide range of expert review panels and have reviewed more than 400 research proposals involving nonhuman primates, cats, dogs, and horses that have been submitted to UK research funders such as the Biotechnology and Biological Sciences Research Council (BBSRC), MRC, and the Wellcome Trust.
Influencing practices within the pharmaceutical and chemicals industries.
The NC3Rs has a varied program of work to identify opportunities where the use of animals can be reduced in the pharmaceutical, chemicals, pesticides, consumer products, and cosmetic sectors. These projects rely on cross-company and cross-sector collaboration, in association with relevant regulatory bodies. Some of the evidence-bases collected during these projects have influenced regulatory changes (for example, in the use of nonhuman primates in monoclonal antibody development; see Case Studies).
The Centre's current work with the pharmaceutical industry spans 10 working groups involving 60 companies, in areas including microsampling, biosimilars, the use of recovery animals in toxicology, and human tissue for safety assessment. Ten years of collaboration with the pharmaceutical industry was showcased at an event at the UK Parliament in 2014 and in an accompanying publication (www.nc3rs.org.uk/news/ten-years-partnering-pharmaceutical-industry). Some of these projects have led to copublication with representatives from the major pharmaceutical companies and regulatory bodies, such as the Medicines and Healthcare Products Regulatory Agency, European Medicines Agency, and the US Food and Drug Administration (Figure 3).
The program of work with the chemicals industry is expanding, and there are now working groups in the areas of ecotoxicology, adverse outcome pathways, and the use of the Fixed Concentration Procedure for inhalation toxicology studies. Collaborators include scientists from companies such as Unilever, Syngenta, and Dow AgroSciences, as well as regulatory and government bodies (for example, the Organization for Economic Cooperation and Development, Public Health England, and the UK Environment Agency) and academia.
Improving the science of animal experiments.
Reduction of animal use can be achieved by improving the design of experiments and statistical analysis. To help equip scientists with the tools to best plan their experiments and analyze data to achieve maximal 3Rs benefit, the NC3Rs provide education and training, as well as web-based systems (for example, an Experimental Design Assistant which will be launched in 2015). In addition, the ARRIVE guidelines22 (Animal Research: Reporting In Vivo Experiments; www.nc3rs.org.uk/arrive)—a 20-point The NC3Rs-developed checklist of the essential information that should be included in publications reporting animal research—have now been endorsed by more than 400 journals including the Nature group, PLoS, and Cell, as well as funders, universities, and learned societies. In addition, these guidelines have been translated into Chinese, Italian, and Portuguese.
Improving animal welfare.
The Centre undertakes an extensive program of work to improve the welfare of laboratory animals. Activities focus on both long-standing issues (for example, the refinement of scientific procedures and the transport and husbandry of nonhuman primates) as well as emerging concerns (for example, as the use of genetically modified mice became increasingly widespread). Particular attention and funding (approximately £1.4 million [USD$2million]) has been provided to address welfare issues around the use of animals in neuroscience research. In 2011, the NC3Rs also participated in expert independent review panels assessing the quality, output, and effects of the nonhuman primate research funded by the United Kingdom's major funding bodies.
Providing information resources.
Many of the center-led activities around animal welfare have resulted in the publication of training materials to aid scientists and animal technicians in best practice techniques. These materials are routinely accessed via the NC3Rs website, which in 2014 received visitors from 196 different countries.
The NC3Rs undertakes a variety of engagement activities with the general public, politicians, and the media through a dedicated communications team. These activities are complemented with social media platforms, including the NC3Rs blog and dedicated YouTube channel (youtube.com/user/NC3Rs1), as well as Facebook (facebook.com/NC3Rs) and Twitter (twitter.com/NC3Rs) sites.
The Centre also promotes and recognizes scientific excellence in the 3Rs. One key example is the annual award of an international 3Rs Prize in collaboration with GlaxoSmithKline, through which outstanding original contributions to scientific and technologic advances are recognized.
Case Studies
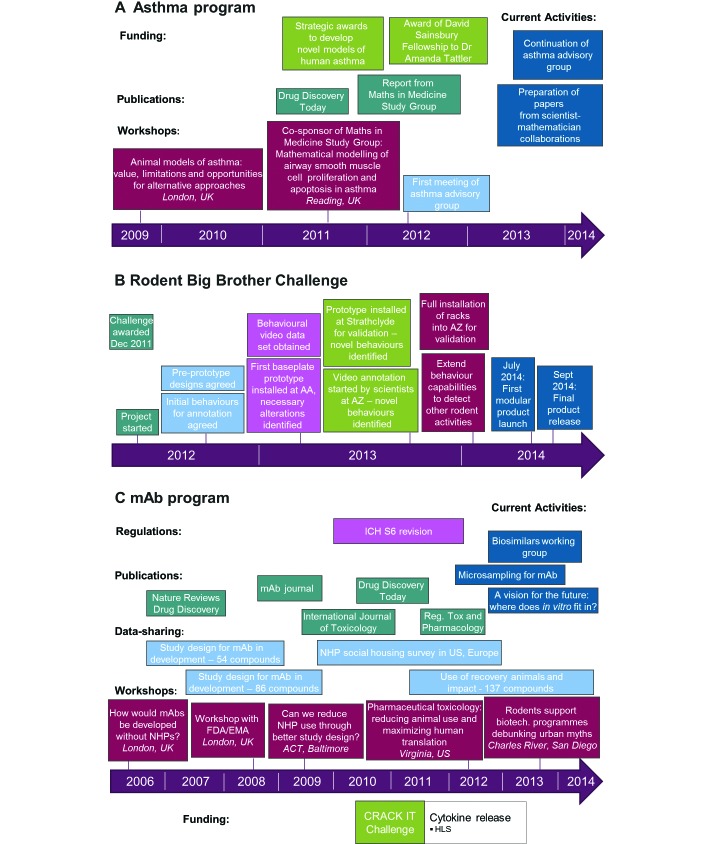
Here we present some detailed examples of how some of the NC3Rs-led programs have been carried out and how they have supported the scientific community. The accompanying timelines in Figure 4 demonstrate how different approaches were applied across each of the examples.
Figure 4.
Timelines demonstrating the progress of 3 NC3Rs programs. (A) Applying the 3Rs in asthma research. (B) Rodent Big Brother CRACK IT Challenge. (C) Minimizing the use of nonhuman primates (NHP) in the development of monoclonal antibodies (mAb). AA, Actual Analytics; AZ, Astra Zeneca; HLS, Huntingdon Life Sciences.
Applying the 3Rs in asthma research.
Why invest in asthma research?
Asthma is a complex disorder of considerable unmet medical need. It is considered to be one of the most common respiratory diseases, with 300 million people currently undergoing treatment globally, representing a significant economic burden.20 Despite its high prevalence and the considerable effort and investment in trying to understand the underlying pathologies of the various asthma phenotypes, very few novel drug treatments have been successfully launched in the past 50 y. However, many new drug classes have been tested and proved efficacious in a range of preclinical animal models displaying ‘asthma-like’ phenotypes, from mice to nonhuman primates. The fact that asthma is a uniquely human disease has underpinned the failure in translation of promising drug candidates into the clinic, raising many questions about the utility of in vivo studies in this disease area. This failure has led to demands for more predictive models and tools based on the latest technologies, many of which are likely to come from more sophisticated human tissue-based models (for example, ‘organs on chips’).
The challenge in this area is 2-fold: 1) to deliver new research models that will combine all aspects of the disease (for example, environmental exposure, immune system components, mechanical stimulation of breathing, and microfluidics to mimic the circulatory system) and 2) to develop, in collaboration with the asthma research community, the best way forward to integrate these in the quest to develop effective new treatments and reduce animal use.
Initiation of the NC3Rs program in asthma.
In 2009, working with the MRC, the NC3Rs brought together research leaders from academia and industry to identify opportunities for accelerating the development of new model systems and to overcome the scientific and technical hurdles necessary to establish models that better mimic asthma. During the workshop, a number of priority areas were identified. These included increased focus on the human disease and subphenotyping (through the use of biobanks, experimental medicine, and biomarkers), and the need for the development of disease-related in vitro human models to test novel mechanisms and targets of disease and therapeutics. The findings of the workshop directed future investment and, with support from the charity Asthma UK, a £1 million (approximately USD$1.5 million) strategic research call subsequently was launched by the NC3Rs to support some of the research and development ideas coming from the workshop (see following section). The consensus and expert view on the future direction of the field was published as a Keynote Review article in Drug Discovery Today.20
The NC3Rs funding for novel research in asthma.
The NC3Rs’ total investment in asthma research now stands at £1.5 million (approximately USD$2.3 million) and has resulted in several peer-reviewed publications (for example, reference 39); for more detailed information, see www.nc3rs.org.uk/asthma-models. The funded work includes 2 strategic awards and a project grant for research into modeling the human asthmatic airway through 3D tissue engineering techniques. Cultures of cells taken directly from asthmatic patients and healthy volunteers are used in these models, providing potentially greater relevance to the human disease. In addition, one of the inaugural NC3Rs David Sainsbury fellowships, aimed at outstanding early-career researchers, was awarded for research in this area. The Centre recently announced a new strategic funding call to support the development of nonmammalian models (such as C. elegans, Dictyostelium, and zebrafish) for asthma research. It is anticipated that these, and subsequent, models will provide the asthma research community with additional tools to better understand the human disease and for the development of more efficacious therapies with reduced reliance on mammalian animals.
Continuing the NC3Rs activities in the asthma field.
Beyond funding of basic research, the NC3Rs has brought together a strong 3Rs asthma research community to work together on expanding the toolkit of approaches available to asthma researchers, including human tissue, nonmammalian models, and mathematical modeling. In collaboration with the Maths in Medicine Study Group (funded by the Engineering and Physical Sciences Research Council), asthma researchers from the University of Nottingham and Imperial College London now have access to expert mathematicians. These collaborations are helping to transform the science and reduce reliance on animals to study airway smooth muscle turnover in asthma (www.maths-in-medicine.org/uk/2011/asthma/report.pdf) and steroid responsiveness in severe asthma and chronic obstructive pulmonary disease (www.maths-in-medicine.org/uk/2013-nc3rs/steroids/). In addition, the NC3Rs has been working with the UK Human Tissue Authority to better understand the real and perceived barriers to wider adoption of human tissue for asthma research. In 2012 an expert group of asthma researchers was convened by the NC3Rs from across industry and academia to explore ongoing opportunities for the 3Rs. This group continues to identify and influence asthma research priorities, stimulate greater cross-sector collaborations, and raise the profile of 3Rs activities. Furthermore, this work has extended beyond the asthma field to other respiratory diseases—for example, the NC3Rs has now funded 2 project grants applying the 3Rs to research in fibrotic lung disease and influenza.
A timeline demonstrating the progress and various strategies associated with the asthma project can be seen in Figure 4A.
CRACK IT Challenge: Rodent Big Brother
What is the Rodent Big Brother Challenge?
One of the first CRACK IT Challenges to be launched was Rodent Big Brother. This Challenge originated from an initial idea by AstraZeneca scientists working in safety assessment who wished to obtain more information from animals that were already being used in drug development. The Challenge is that currently much data from rodent studies is lost because it is not recorded or observed. To capture this information, rodents have to be removed from their home cage and singly housed, which has animal welfare implications.
Improving the scientific quality of the data by providing the most stress-free environment could lead to better prediction of clinical outcomes, the reduction and refinement of animal use, and potentially reduce drug attrition. Therefore the Challenge was set for the research community to develop an integrated system for the nonsurgical, automated recording of rat activity and temperature in the home cage of freely moving animals for at least a 24-h period. Such activity measurements will provide valuable information in studies for both basic research and drug discovery and development. The use of the home cage would avoid the need for single housing (a requirement of previous videotracking systems) and would enable the incorporation of these measurements into existing studies, reducing the number of separate experiments needed.
Challenge requirements.
For Rodent Big Brother, the unique performance requirements of the technology defined by AstraZeneca included: no complex wiring to or from cages; wireless transmission to a receiver that collates the data; mechanisms in place to automatically log the precise timing of the light–dark cycle and when technicians are in the room; and the capacity to measure the time spent at each end of the cage, as well as rearing behavior. In addition, the system has to be able to distinguish between different types of motor activity and specific movements such as eating, drinking, tremor, and convulsion; quick and simple to set up and start recording; Good Laboratory Practice-compliant; and capable of recognizing erroneous data. The successful development of such a system will represent a significant technologic advance in the activity monitoring field. AstraZeneca provided evaluation, optimization, and validation of the equipment in their laboratories, using their expertise in the running and handling of data from safety and toxicology studies.
Cracking the Challenge.
Professor Douglas Armstrong from Actual Analytics led the contract research team responsible for delivery of the end-product. The system, which consists of videorecording combined with state-of-the-art base plates and radiofrequency identification chips, has been fully developed and has undergone final technical validation at AstraZeneca and the University of Strathclyde (Figure 4 B). One essential part of solving this Challenge was training the software to automatically recognize and distinguish the location and behavior of individual animals from a combination of video footage and base-plate data when animals are housed together. The new technology has engaged researchers at both validation centers, because new behaviors in rats were observed during product testing. The final product was showcased at Eurotox in September 201434 and is now available to companies and researchers who are interested in translating the technology into their own laboratory.
Consolidating and maximizing CRACK IT investment.
Recognizing the potential for the technology developed for Rodent Big Brother to be applied to other species and research areas, the NC3Rs worked with another Sponsor, MRC Harwell, on a follow-up CRACK IT Challenge known as Rodent Little Brother. In the face of strong competition, Actual Analytics, in collaboration with Wideblue and the University of Strathclyde, secured the contract for this Challenge.
MRC Harwell is a world-leading center in mouse genetics, carrying out an extensive portfolio of research in the discovery and characterization of mouse models of neurologic, behavioral, and sensory disorders (see www.har.mrc.ac.uk/services/phenotyping). The Challenge is to develop a monitoring system specifically for use in the mouse to identify behavioral, locomotor, and social deficits that may be associated with mouse models of nervous system disorders to better translate these findings to human disease. The complexity of behaviors that will need to be monitored combined with the small size of the mouse make this challenge particularly ambitious.
The current systems used to phenotype locomotor and learning behaviors involve moving individual animals to different environments where they are singly housed. Typical mouse behaviors such as socialization and nesting are highly sensitive to variations in the environment; social interactions such as grooming, play, and nest building can be affected by placing the animals in unfamiliar cages in different rooms, with different enrichment regimes and handlers, particularly if the mice are singly housed. Home-cage phenotyping will have a number of scientific and welfare benefits such as the ability to detect more subtle phenotypes relevant to human disease earlier and to avoid singly housing animals. In addition, the expected reduction in data variability will lead to an overall reduction in the numbers of animals used to obtain high-quality data. As with Rodent Big Brother, a key component of solving the Rodent Little Brother Challenge is the development of advanced software systems and algorithms for data interpretation. Once the system is launched and validated by MRC Harwell, the ultimate aim is to introduce operant tasks in the home-cage set-up; Harwell will also take the lead in scaling up the solution and disseminating it to other users who would benefit from the technology.
CRACK IT Solution: an early screen for emetic liability of novel chemical entities by using the amoeba Dictyostelium
In 2012, Professor Robin Williams from Royal Holloway, University of London, submitted his new model for detecting the emetic liability of novel chemical entities to the CRACK IT Solutions technology-partnering scheme. The scheme provided him with a mechanism to identify partners that could provide advice and expertise on the application of the assay in a pharmaceutical setting and to supply compounds for which preclinical and clinical data on nausea and vomiting are already available.
Why invest in an alternative screen for emetic liability?
Emetic side effects of drugs are extremely common, often negatively affecting their efficacy and patient compliance. The liability of a new chemical entity to cause nausea and vomiting often is not realized until the late stages of preclinical development, such as during toxicologic analysis (where there can be significant suffering and distress as a result of emetic effects), and by which point many animals may have been used for proof-of-concept studies. Because rodents are unable to vomit, surrogate endpoints (such as measurements of food intake) are often used, or tests have to be conducted in other species, such as ferrets, dogs, and occasionally nonhuman primates, if the drug target is considered to have a high emetic risk. Compound development often is halted when there are unacceptable levels of nausea and vomiting during costly first-in-human studies, and therefore the use of nonanimal methodologies to predict potentially emetic compound classes could be invaluable for future compound development and optimization. Such an approach could save companies time, money, and animals early on, as well as reduce the levels of attrition in later stages of development.19
Dictyostelium as a model organism.
Professor Williams has demonstrated that the nonsentient amoeba Dictyostelium could be a useful tool for the early identification of possible emetic or aversive compounds. The ‘emetic’ endpoint for this assay is the blockade of Dictyostelium cell movement, which so far has been predictive with known emetic compounds, such as curcumin analogs. Dictyostelium is already used widely across a range of biomedical research areas, such as in the study of WBC movement and neuronal pharmacology. Although this model will not necessarily reveal the mechanisms underlying the emetic effects of compounds, it has the potential to identify characteristics related to their emetic potential. In addition, the assay had garnered interest from chemical companies with a different application—the development of chemicals that are aversive, so that animals or humans do not attempt to ingest them.
Technology partnering works for CRACK IT Solutions.
Within 1 mo of the Solution being showcased on the CRACK IT website, discussions began between Professor Williams and GlaxoSmithKline on a potential collaboration to assess the utility of the Dictyostelium model for emetic liability and drug palatability studies. GlaxoSmithKline provided bitter compounds for screening with Dictyostelium and historical data for comparison, and the NC3Rs provided an initial 6 mo of funding through the CRACK IT Solutions funding scheme for proof-of-concept studies. This proof-of-concept study has provided GlaxoSmithKline with sufficient confidence in the potential utility of this model for assessing palatability issues that they are now supporting the further development of the model through a GlaxoSmithKline-funded doctoral fellowship.
Developing practical guidance to minimize the use of nonhuman primates in the development of monoclonal antibodies.
Why invest in monoclonal antibody testing alternatives?
There is increasing interest in using monoclonal antibodies (mAbs) as therapeutics, particularly for the treatment of cancer and autoimmune diseases. mAbs tend to have high target and species specificity, and often a nonhuman primate is the only relevant species for preclinical studies. The large number of mAbs research and development programs thus is driving an increase in the use of nonhuman primates. Close consultation with the pharmaceutical and biotechnology industry identified this area as a priority for the 3Rs and therefore the NC3Rs. As a result, during the last 8 y, the Centre has worked with pharmaceutical and biotechnology companies, as well as contract research organizations and regulators, to assess the use of nonhuman primates in mAbs development.
Initiation of the NC3Rs mAbs project.
The project was initiated in 2006 with an international workshop hosted by the NC3Rs in collaboration with the Association of the British Pharmaceutical Industry. Delegates were set an intellectual challenge that nonhuman primate use was not an option in drug development, either due to a disease outbreak, legislative changes, or supply problems. It is noteworthy that this scenario was not entirely hypothetical, given that many mAbs at the time did not have any relevant preclinical species and only showed potency in humans. The objective of the workshop was to explore alternative approaches, such as the use of in vitro methodologies, surrogate antibodies, and transgenic mice as well as the initiation of clinical trials in the absence of any preclinical toxicology data, and to assess the benefits and limitations of each approach. The output of this workshop was published in a perspectives article in Nature Reviews Drug Discovery,5 which described a future vision and strategy of how these issues could be addressed.
The NC3Rs approach to tackling the 3Rs challenges in mAbs development.
After the workshop, an expert working group was established to determine the best strategy to implement and integrate potential alternatives into current practice and study design. This working group consists of 23 companies and regulatory bodies, with half of members based in the United States and the others representing organizations based throughout Europe. Through a large data-sharing exercise, the working group used data from preclinical safety studies for more than 54 mAbs as an evidence base to design scientifically robust alternative preclinical development pathways that could replace or reduce the use of nonhuman primates. This analysis revealed that the use of rodents may be possible in some cases, as well as the use of fewer dose or recovery groups, the combining of studies, or the reuse of nonhuman primates. The knowledge was developed into practical guidelines on how to minimize primate use in monoclonal antibody development by assessing relevance over other species and applying good study design.4 If the recommendations are put into practice, there is the potential to reduce the number of nonhuman primates used from 144 to 52 per mAbs in some cases. In addition, there are opportunities to reduce the number of reproductive toxicology studies that are completed in nonhuman primates, for example, if the mAbs is not intended for use in women of child-bearing age or is designed for the treatment of a life-threatening disease. Data have subsequently been analyzed from an additional 86 mAbs in development, and this additional information used to further improve the practical guidance and provide the evidence base for implementation.7 This guidance is now applied internally by many of the international companies who were involved in this initiative.
This program of work has helped to influence regulatory change at a global level. For example, in 2011 an addendum to the ICH S6 guideline (Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals) was published and has enabled the NC3Rs working group recommendations to be put into practice. A timeline demonstrating the progress of the mAbs project can be seen in Figure 4 C.
Current and future NC3Rs activities in mAbs development.
The NC3Rs has held 5 international workshops in the United Kingdom and United States and published 5 papers on this work (Figure 3). A workshop in 2013, in collaboration with Charles River Laboratories, titled “Debunking the Urban Myth: Rodents Don't Support Biotech Programs,” demonstrated the opportunities for the added value of the rodent in mAbs development programs. The output from this workshop will be used to support companies in putting the ICH S6 addendum into practice. For example, the use of a single species (which could be a rodent) for chronic toxicology studies might be adequate if the toxicology profiles in 2 species are the same in the short-term studies.
The NC3Rs continues to work closely with international pharmaceutical, biotechnology, and contract research organizations as well as regulators in this field, and the mAbs expert working group convenes regularly to identify additional 3Rs opportunities. Most recently, in June 2014, a workshop was held in Washington, DC, to identify opportunities for greater use of emerging in vitro and systems biology approaches. Workshop participants explored how these approaches could be used to optimize prediction of human safety by better understanding of target pharmacology and to gain more value from fewer in vivo studies.
The Centre is currently exploring 3 main areas of interest: the reduction of nonhuman primates in the testing of biosimilars, microsampling for mAbs, and a vision for protein-based biopharmaceuticals in the future, with a focus on in vitro technologies.
Strategic CRACK IT Challenge: improving the predictive capacity of in vitro cytokine release assays to reduce animal use and drug attrition.
In 2011 a CRACK IT Challenge was launched to develop in vitro human cell-based models for the testing of protein-based biopharmaceuticals (which include mAbs) that will allow the prediction of human cytokine release and to develop a parallel assay that uses nonhuman primate cells for prediction of cytokine release in preclinical safety assessment.
The use of such assays to accurately predict immune responses, including the likelihood of a cytokine storm, could reduce the number of nonhuman primates used in current assessments, by halting the progression of potentially unsafe and inefficacious compounds into preclinical studies at early stages. In addition, these screens may provide information on the mechanism of action or potency of candidates, thus informing the design of subsequent studies and possibly helping to avoid the administration of later doses that result in significant adverse events or mortality. Sponsored by Huntingdon Life Sciences, who is providing clinical antibodies, multiplex analysis platforms, automation, and tissue-banking facilities, the contract was awarded to Professor Martin Glennie from the University of Southampton, along with collaborators from the University of York. The team currently is developing a multitier assay system that uses peripheral blood mononuclear cells from healthy volunteers to look at the mAbs-stimulated cytokine release in soluble, cellular, and tissue-based assays. The team has presented its work at the ILSI Health and Environmental Sciences Institute Workshop on Cytokine Release, in Washington in October 2013, to leading scientists within the industry.
Looking to the Future
Perception of the 3Rs has changed significantly over the last 10 y. Scientists at all levels across the biosciences are becoming involved in 3Rs activities, and we see engagement from many key organizations in the public and private sectors in the United Kingdom, increased investment in the 3Rs, and many more cross-sector and cross-discipline collaborations. In addition, the NC3Rs has shown that delivering measureable 3Rs effects can support new scientific discoveries, technologic advances, and commercial opportunities.
The NC3Rs recently published its vision for the next 10 y (www.nc3rs.org.uk/news/minimizing-and-improving-animal-use-next-decade). In this timeframe, it will be critically important to anticipate and respond to any shifts in future research investment that may affect the use of animals and to address the challenges that remain in the current environment. We view these challenges within 5 interrelated areas: practice, procedures, people, places, and policy. The NC3Rs’ future strategy will build on the previous 10 y of experience and is based on collaboration, international outreach, and a back-to-basics approach. Areas that the NC3Rs has already identified for program development in the short-term include the use of adverse outcome pathways as a predictive tool in safety assessment and disease modeling, and the evaluation of animal models of efficacy for therapeutic areas such as oncology.
The NC3Rs will also continue to provide new resources to scientists and institutions and relevant training on how to use them. The evidence showing that publicly funded in vivo research is poorly reported, which triggered the development of the ARRIVE guidelines, also suggested that in vivo experiments were suboptimally designed. Our Experimental Design Assistant, to be launched in 2015, will guide researchers through the design of their experiments, helping to ensure that they use the fewest animals consistent with their scientific objectives, methods to reduce subjective bias, and appropriate statistical analysis. The NC3Rs will focus on supporting scientists who use the Experimental Design Assistant to plan their experiments and on facilitating its dissemination internationally, given that this resource will be globally applicable. From a regulatory perspective, the requirement to collaborate at a global level has arguably never been greater. Our plan is to maintain and increase international outreach through project areas such as the use of the Fixed Concentration Procedure for acute inhalation toxicology, microsampling, and the development of biosimilar products.
Innovate UK, in collaboration with the NC3Rs and with the support of the Engineering and Physical Sciences Research Council, BBSRC, and MRC, will publish a UK Roadmap for Nonanimal Technologies in 2015. This effort accompanies increased investment from the NC3Rs, Innovate UK, and other research councils into this area (£4 million [approximately USD$6million). This roadmap will describe a strategy for fast-tracking the commercialization of technologies and that is focused on human relevance for efficacy, safety, and toxicity to ensure that validation and uptake is achieved as quickly as possible.
We hope that the next generation of researchers will be better equipped to advance the 3Rs. If progress continues at a similar rate, the scientific landscape could be transformed in the next 10 y. However, as more scientists seek to address the 3Rs, more resources will be required to support them. Continuing to equip scientists with the right tools will help to overcome challenges and limitations of emerging 3Rs innovations and benefit the whole bioscience community.
Acknowledgments
We thank Dr Cathy Vickers and Dr Anthony Holmes of the NC3Rs for their help in showcasing some of their work as example case studies.
References
- 1.Beaumont K, Gardner I, Chapman K, Hall M, Rowland M. 2011. Toward an integrated human clearance prediction strategy that minimizes animal use. J Pharm Sci 100:4518–4535. [DOI] [PubMed] [Google Scholar]
- 2.Buckley LA, Chapman K, Burns-Naas LA, Todd MD, Martin PL, Lansita JA. 2011. Considerations regarding nonhuman primate use in safety assessment of biopharmaceuticals. Int J Toxicol 30:583–590. [DOI] [PubMed] [Google Scholar]
- 3.Chapman K, Chivers S, Gliddon D, Mitchell D, Robinson S, Sangster T, Sparrow S, Spooner N, Wilson A. 2014. Overcoming the barriers to the uptake of nonclinical microsampling in regulatory safety studies. Drug Discov Today 19:528–32. [DOI] [PubMed] [Google Scholar]
- 4.Chapman K, Pullen N, Coney L, Dempster M, Andrews L, Bajramovic J, Baldrick P, Buckley L, Jacobs A, Hale G, Green C, Ragan I, Robinson V. 2009. Preclinical development of monoclonal antibodies: considerations for the use of nonhuman primates. MAbs 1:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman K, Pullen N, Graham M, Ragan I. 2007. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov 6:120–126. [DOI] [PubMed] [Google Scholar]
- 6.Chapman K, Sewell F, Allais L, Delongeas JL, Donald E, Festag M, Kervyn S, Ockert D, Nogues V, Palmer H, Popovic M, Roosen W, Schoenmakers A, Somers K, Stark C, Stei P, Robinson S. 2013. A global pharmaceutical company initiative: an evidence-based approach to define the upper limit of body weight loss in short-term toxicity studies. Regul Toxicol Pharmacol 67:27–38. [DOI] [PubMed] [Google Scholar]
- 7.Chapman KL, Andrews L, Bajramovic JJ, Baldrick P, Black LE, Bowman CJ, Buckley LA, Coney LA, Couch J, Maggie Dempster A, de Haan L, Jones K, Pullen N, de Boer AS, Sims J, Ian Ragan C. 2012. The design of chronic toxicology studies of monoclonal antibodies: implications for the reduction in use of nonhuman primates. Regul Toxicol Pharmacol 62:347–354. [DOI] [PubMed] [Google Scholar]
- 8.Chapman KL, Holzgrefe H, Black LE, Brown M, Chellman G, Copeman C, Couch J, Creton S, Gehen S, Hoberman A, Kinter LB, Madden S, Mattis C, Stemple HA, Wilson S. 2013. Pharmaceutical toxicology: designing studies to reduce animal use while maximizing human translation. Regul Toxicol Pharmacol 66:88–103. [DOI] [PubMed] [Google Scholar]
- 9.Chapman KL, Pullen N, Andrews L, Ragan I. 2010. The future of nonhuman primate use in mAbs development. Drug Discov Today 15:235–242. [DOI] [PubMed] [Google Scholar]
- 10.Creton S, Aardema MJ, Carmichael PL, Harvey JS, Martin FL, Newbold RF, O'Donovan MR, Pant K, Poth A, Sakai A, Sasaki K, Scott AD, Schechtman LM, Shen RR, Tanaka N, Yasaei H. 2012. Cell transformation assays for prediction of carcinogenic potential: state of the science and future research needs. Mutagenesis 27:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creton S, Billington R, Davies W, Dent MP, Hawksworth GM, Parry S, Travis KZ. 2009. Application of toxicokinetics to improve chemical risk assessment: implications for the use of animals. Regul Toxicol Pharmacol 55:291–299. [DOI] [PubMed] [Google Scholar]
- 12.Creton S, Clook M, Wheeler JR. 2014. Application of the threshold approach for acute fish toxicity testing to plant protection products: a proposed framework. Chemosphere 96:195–200. [DOI] [PubMed] [Google Scholar]
- 13.Creton S, Dewhurst IC, Earl LK, Gehen SC, Guest RL, Hotchkiss JA, Indans I, Woolhiser MR, Billington R. 2010. Acute toxicity testing of chemicals—opportunities to avoid redundant testing and use alternative approaches. Crit Rev Toxicol 40:50–83. [DOI] [PubMed] [Google Scholar]
- 14.Creton S, Douglas M, Wheeler JR, Hutchinson TH. 2010. Challenging the requirement for chronic fish toxicity studies on formulated plant protection products. Toxicol Lett 199:111–114. [DOI] [PubMed] [Google Scholar]
- 15.Creton S, Saghir SA, Bartels MJ, Billington R, Bus JS, Davies W, Dent MP, Hawksworth GM, Parry S, Travis KZ. 2012. Use of toxicokinetics to support chemical evaluation: informing high-dose selection and study interpretation. Regul Toxicol Pharmacol 62:241–247. [DOI] [PubMed] [Google Scholar]
- 16.Creton S, Weltje L, Hobson H, Wheeler JR. 2013. Reducing the number of fish in bioconcentration studies for plant protection products by reducing the number of test concentrations. Chemosphere 90:1300–1304. [DOI] [PubMed] [Google Scholar]
- 17.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. 2014. Clinical development success rates for investigational drugs. Nat Biotechnol 32:40–51. [DOI] [PubMed] [Google Scholar]
- 18.Holmes A, Brown R, Shakesheff K. 2009. Engineering tissue alternatives to animals: applying tissue engineering to basic research and safety testing. Regen Med 4:579–592. [DOI] [PubMed] [Google Scholar]
- 19.Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PL. 2009. Opportunities for the replacement of animals in the study of nausea and vomiting. Br J Pharmacol 157:865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes AM, Solari R, Holgate ST. 2011. Animal models of asthma: value, limitations, and opportunities for alternative approaches. Drug Discov Today 16:659–670. [DOI] [PubMed] [Google Scholar]
- 21.Jennings M, Prescott MJ, Buchanan-Smith HM, Gamble MR, Gore M, Hawkins P, Hubrecht R, Hudson S, Keeley JR, Morris K, Morton DB, Owen S, Pearce PC, Robb D, Rumble RJ, Wolfensohn S, Buist D. 2009. Refinements in husbandry, care, and common procedures for nonhuman primates: 9th report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim 43 Suppl 1:1–47. [DOI] [PubMed] [Google Scholar]
- 22.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kola I, Landis J. 2004. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3:711–715. [DOI] [PubMed] [Google Scholar]
- 24.Lave T, Chapman K, Goldsmith P, Rowland M. 2009. Human clearance prediction: shifting the paradigm. Expert Opin Drug Metab Toxicol 5:1039–1048. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor EC, Chapman K, Butler P, Mead AN. 2011. The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938. [DOI] [PubMed] [Google Scholar]
- 26.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. 2010. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 9:203–214. [DOI] [PubMed] [Google Scholar]
- 27.Percie du Sert N. 2011. Improving the reporting of animal research: when will we ARRIVE? Dis Model Mech 4:281–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Percie du Sert N. 2011. Systematic review and meta-analysis of preclinical research: the need for reporting guidelines. Eur Heart J 32:2340. [PMC free article] [PubMed] [Google Scholar]
- 29.Percie du Sert N. 2012. Maximising the output of osteoarthritis research: the ARRIVE guidelines. Osteoarthritis Cartilage 20:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percie du Sert N, Holmes AM, Wallis R, Andrews PL. 2012. Predicting the emetic liability of novel chemical entities: a comparative study. Br J Pharmacol 165:1848–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott MJ, Brown VJ, Flecknell PA, Gaffan D, Garrod K, Lemon RN, Parker AJ, Ryder K, Schultz W, Scott L, Watson J, Whitfield L. 2010. Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: report of a Working Group of the The NC3Rs. J Neurosci Methods 193:167–188. [DOI] [PubMed] [Google Scholar]
- 32.Prescott MJ, Buchanan-Smith HM. 2003. Training nonhuman primates using positive reinforcement techniques. J Appl Anim Welf Sci 6:157–161. [DOI] [PubMed] [Google Scholar]
- 33.Price C, Stallard N, Creton S, Indans I, Guest R, Griffiths D, Edwards P. 2011. A statistical evaluation of the effects of gender differences in assessment of acute inhalation toxicity. Hum Exp Toxicol 30:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redfern WS, Armstrong JD, Heward J, Allison B, Lukins T, Grant C, Leslie L, Craig DJ, Vickers C, Champman K. 2014. Rodent Big Brother: development and validation of a home cage automated behavioural monitoring system for use in repeat-dose toxicity studies in rats. Toxicol Lett 229:S47–S48. [Google Scholar]
- 35.Robinson S, Delongeas JL, Donald E, Dreher D, Festag M, Kervyn S, Lampo A, Nahas K, Nogues V, Ockert D, Quinn K, Old S, Pickersgill N, Somers K, Stark C, Stei P, Waterson L, Chapman K. 2008. A European pharmaceutical company initiative challenging the regulatory requirement for acute toxicity studies in pharmaceutical drug development. RTP 50:345–352. [DOI] [PubMed] [Google Scholar]
- 36.Robinson V. 2009. Less is more: reducing the reliance on animal models for nausea and vomiting research. Br J Pharmacol 157:863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sewell F, Chapman K, Baldrick P, Brewster D, Broadmeadow A, Brown P, Burns-Naas LA, Clarke J, Constan A, Couch J, Czupalla O, Danks A, DeGeorge J, de Haan L, Hettinger K, Hill M, Festag M, Jacobs A, Jacobson-Kram D, Kopytek S, Lorenz H, Moesgaard SG, Moore E, Pasanen M, Perry R, Ragan I, Robinson S, Schmitt PM, Short B, Lima BS, Smith D, Sparrow S, van Bekkum Y, Jones D. 2014. Recommendations from a global cross-company data sharing initiative on the incorporation of recovery phase animals in safety assessment studies to support first-in-human clinical trials. Regul Toxicol Pharmacol 70:413–429. [DOI] [PubMed] [Google Scholar]
- 38.Stallard N, Price C, Creton S, Indans I, Guest R, Griffiths D, Edwards P. 2011. A new sighting study for the fixed concentration procedure to allow for gender differences. Hum Exp Toxicol 30:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun T, Swindle EJ, Collins JE, Holloway JA, Davies DE, Morgan H. 2010. On-chip epithelial barrier function assays using electrical impedance spectroscopy. Lab Chip 10:1611–1617. [DOI] [PubMed] [Google Scholar]
- 40.Swallow J, Anderson D, Buckwell AC, Harris T, Hawkins P, Kirkwood J, Lomas M, Meacham S, Peters A, Prescott M, Owen S, Quest R, Sutcliffe R, Thompson K. 2005. Guidance on the transport of laboratory animals. Lab Anim 39:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westmoreland C, Holmes AM. 2009. Assuring consumer safety without animals: applications for tissue engineering. Organogenesis 5:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]