Abstract
Here in we introduce the European Partnership for Alternative Approaches to Animal Testing (EPAA) and its activities, which are focused on international cooperation toward alternative methods. The EPAA is one of the leading organizations in Europe for the promotion of alternative approaches to animal testing. Its innovative public–private partnership structure enables a consensus-driven dialogue across 7 industry sectors to facilitate interaction between regulators and regulated stakeholders. Through a brief description of EPAA's activities and organizational structure, we first articulate the value of this collaboration; we then focus on 2 key projects driven by EPAA. The first project aims to address research gaps on stem cells for safety testing, whereas the second project strives for an approach toward demonstration of consistency in vaccine batch release testing. We highlight the growing need for harmonization of international acceptance and implementation of alternative approaches and for increased international collaboration to foster progress on nonanimal alternatives.
Abbreviations: EC, European Commission; EDQM, European Directorate for the Quality of Medicines and Healthcare; EPAA, European Partnership for Alternative Approaches to Animal Testing; EURL–ECVAM, European Union Reference Laboratory for Validation of Alternatives to Animal Testing; REACH, Registration, Evaluation, Authorisation, and Restriction of Chemicals; PPP, public–private partnership
The European Union is a leading body for the promotion of alternative approaches to animal testing. In accordance to the 3Rs principle (reduction, refinement and replacement of laboratory animal use) proposed in 1959 by WMS Russell and RL Burch,13 the European law has progressed in regulating animal welfare since 1986, when the European Council adopted Directive 86/609/EEC. Since then, several directives defined standards for housing and care of animals as well as for the training of personnel handling animals and performing the experiments. It is clear that replacement, reduction and refinement of animal testing (3Rs) has been a priority since then. To continue promoting the 3Rs, the European Commission (EC) created its own center for the validation of alternative methods throughout Europe in 1991. This center, now known as the European Union Reference Laboratory for Validation of Alternatives to Animal Testing (EURL–ECVAM), is a unit of the Joint Research Centre Directorate General of the European Commission.
In addition, the EC developed collaboration with industry stakeholders. In November 2005, European Commissioners Günther Verheugen and Janez Potocnik launched the formation of a public–private consortium that comprised 5 Directorate Generals and industry representatives from Europe and beyond (including 7 sectors and their respective trade associations; http://ec.europa.eu/enterprise/epaa/about/index_en.htm). In this context, the European Partnership for Alternative Approaches to Animal Testing (EPAA) was born as a unique and innovative public–private partnership (PPP). The major goal of the organization is the promotion of 3Rs to meet regulatory requirements based on better and more predictive science. Since its creation, EPAA has facilitated the dialogue between industry partners that need to comply with regulatory safety requirements and the branches of the EC that envisage the development and implementation of alternatives.
A Transparent Partnership across 7 Industry Sectors
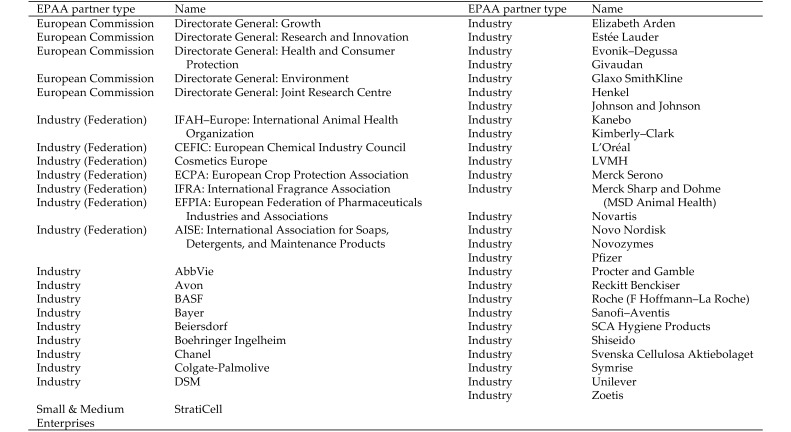
As of January 2014, the EPAA gathers 36 corporate members from 7 industry sectors and 5 Directorates General of the European Commission (Figure 1; the updated list is found at http://ec.europa.eu/enterprise/epaa/partners/index_en.htm). The sectors represented are agrochemicals, animal health, chemicals, cosmetics, fragrances, pharmaceuticals, and soaps and detergents. With the broad representation of several industrial sectors, EPAA's remit encompasses various domains of European law, such as Directive 2010/63/EU on the protection of animals used for scientific purposes, REACH (one component of which is the promotion of alternative methods for the hazard assessment of substances to reduce the number of tests on animals), and the recent marketing ban on animal-tested cosmetics (http://ec.europa.eu/consumers/sectors/cosmetics/animal-testing/index_en.htm). Directive 2010/63/EU is the horizontal legislation for the protection of animals used for scientific purposes, whereas specific legislation covers sectorial areas (for example, REACH for the chemical sector). There are many incentives to promote the 3Rs in every possible sector. Belong to EPAA, stakeholders have the privilege to interact with each other and initiate common cross-sector projects in direct collaboration with regulators, including dedicated agencies like the European Chemicals Agency and the European Medicines Agency. Another essential structure of EPAA is the Mirror Group, gathering expert stake holders from academia, civil animal welfare organizations, who also provide advice toward EPAA's projects. (Figure 1). The EPAA Mirror Group is a consultation forum with an advisory capability to the EPAA Steering Committee. The Mirror Group gathers third-party (that is, nonEC, nonindustry) representatives to provide perspectives from Civil society, academia, nongovernmental organizations, and other regulators on the development, acceptance and validation of alternatives (http://ec.europa.eu/enterprise/epaa/about/mirror-group_en.htm). Since EPAA's inception, its existence and success have relied on transparency for decision-making, consensus among its members, and mutual trust for each side of the PPP.
Figure 1.
EPAA partners (November 2014)
The broad spectra to which EPAA members belong enables a constructive cross-sector dialogue between decision-makers focused on 2 aspects: 1) the scientific aspect through the prioritization, promotion, and implementation of research projects dedicated to the application of the 3Rs and 2) the regulatory aspect through dialogue with EC partners to better understand and facilitate the implementation of 3Rs principles.
As a logical consequence of its PPP structure, EPAA is staffed by a permanent EC–Industry shared secretariat. The EPAA Secretariat is formally hosted by the EC's Directorate General Enterprise Internal Market, Industry, Entrepreneurship and SMEs (DG GROWTH). The secretariat's tasks are to coordinate and facilitate the communication within its platforms to ensure a balanced collaboration among its members. Both industry and EC partners are committed to support EPAA activities and provide experts on a voluntary basis to run the projects. Each side of the PPP appoints a cochair to steer the actions of EPAA; current cochairs are Gwenole Cozigou for the EC and Dr Tzutzuy Ramirez-Hernandez from the industry side. Although project operational costs are covered by industry membership fees (currently €10,000 annually for corporate members; €15,000 annually for federations), the EC hosts the Steering Committee, Mirror Group meetings, and the Annual Conference.
Development and Acceptance of Alternatives: 2 Examples of the Importance for Internationalization
Since the beginning of EPAA operations in 2006, its partners have worked on various projects because EPAA is mindful of the need to consider innovation and advances in technology not only within its membership but also to cultivate international initiatives with other prominent stakeholders. In this regard, EPAA has opened international dialogue with various federal agencies, including the US Food and Drug Administration. In 2012, the EPAA identified the need to reinforce international collaboration on the 3Rs, and to put emphasis on this challenge, EPAA members decided to launch this collaboration as the leading focus of 2012–2013. To this end, EPAA invited members from international agencies, US-based animal welfare nongovernmental organizations, India-based vaccine producers, and academics from many regions outside Europe to participate in EPAA projects that could be enriched through this internationalization. This dynamic was echoed in the creation of the International Cooperation on Alternative test Methods, comprising EURL–ECVAM, the Interagency Coordinating Committee on the Validation of Alternative Methods, and other validation laboratories throughout the world. This initiative has 2 main thrusts: 1) the development of more predictive science and 2) the harmonization worldwide of the implementation and acceptance of alternative methods. In the following section, we provide brief insights into 2 projects that are good examples of EPAA activities that embrace innovative technologies in 3Rs and international collaborations. The first project is being led by members of the platform on science, and it strives for the sharing of knowledge in the use of nonembryonic stem cells for potential use in toxicologic research safety and its prospective future application under the regulatory context. The second project, led by members of the platform on regulation, aims to implement the vaccines consistency approach into the regulatory framework in accordance with 3Rs principles.
The use of stem cells and their derivatives in toxicologic research programs and as potential regulatory tool.
Since the end of 2008 and according to the recommendation of the experts participating in the workshop “New Perspectives on Safety”10, EPAA has agreed to explore the opportunities that stem cells might offer in the development of novel approaches for the characterization of the potential hazard of new products. Fundamental research on stem cells is performed intensively worldwide, but nonacademic applications still need to be explored. The pharmaceutical sector is particularly interested in better understanding how this knowledge might be integrated into an overall strategy for safety assessment to potentially reduce the use of animals. In this context, this EPAA stem cells project strives for steering stem cells research toward industry needs for the implementation of 3Rs principles and practices in safety assessment.
In the opinion of EPAA project leaders, current research on the application of stem cells is not oriented toward developing novel testing strategies for safety assessment. For this reason, one of the working group's objectives is to identify gaps between basic and applied research on stem cells. Only with this information will it be possible to propose relevant models and the most appropriate readouts to design reliable in vitro stem-cell-based methods to contribute to improving the conduct of science and the application of 3Rs principles. Although much research and a better understanding of its potential applications are still needed, EPAA is facilitating the dialogue among experts from academia and industry and by this means is contributing to advancing this field.
This project is subdivided in 2 major areas, fundamental research on stem cells and communication between international research consortia on stem cells. Regarding fundamental research, the aim is to address the main challenges of the application of cells derived from induced pluripotent stem cells for safety testing. Induced pluripotent stem cells can be derived from human adult tissue and reprogrammed to their embryonic stage. In this sense, theoretically these cells can give rise to copious numbers of any kind of cell of the body, thus they represent an attractive alternative method for generating specific models that can be applied to drug discovery assays, drug development, toxicologic purposes, and so forth. Experimental protocols using induced pluripotent stem cells are complex and still under development, consequently it is difficult to prove their reliability and the biological relevance of the models derived from them. Therefore, additional work is needed to identify the robustness of this technology for its further use in alternative methods. The EPAA experts on the field have agreed that several important questions regarding reliability, relevance, and robustness need to be carefully kept in mind before they could be implemented into the nonregulatory research. And even more when thinking in the future potential application in the regulatory framework.
Regarding communication on stem cells, EPAA created a forum in 2013, the aim is to facilitate dialogue among stem cells experts worldwide. This forum could greatly contribute to build synergies and allow pooling of resources on stem cells research. The forum gathers not only EPAA members, but also representatives of foreign regulators, academia researchers, representatives of various stem cells research consortia. This forum has noncommercial purposes and is intended to share good practices and advice on stem cells research. The forum is intended to operate on a permanent basis to help to speed up the better understanding of their potential application and in the long term the potential future adoption of stem cells in many areas of research, such as toxicological testing. EPAA member AbbVie has committed support to the work of the Forum and provides the leader for this project. Five key issues have been identified as priorities for the Forum:
1) relevance of standardization of protocols and test methods,
2) confirmation of the maturity of cell phenotype before any potential application,
3) definition of criteria for validation and acceptance of novel models,
4) confirmation of biologic relevance (that is, through a set of reference test substances appropriate to the developed model), and
5) investigation of the epigenetic status of the mature cells.
The EPAA intends to support this Forum to continue promoting international collaboration regarding the 3Rs in the area of stem cells. For this reason, a second workshop on the topic will take place during 2014: “Benchmarking of Stem Cell Assays in Safety Assessment across International Consortia.” This workshop is expected to focus on 2 relevant cellular models for safety assessment in the pharmaceutical industry, cardiomyocytes and hepatocytes.
Application of 3Rs through the Vaccines Consistency Approach in regulatory testing.
Another ambitious project is the EPAA Vaccines Consistency Approach project. This project started in 2011, involving stakeholders coming from as far as New Zealand, and is showing real promise regarding the incorporation of 3Rs principles in animal testing. Human and animal vaccines worldwide require batch-related quality control to ensure their safety and potency. Part of this quality control, particularly of the final product, involves tests requiring laboratory animals; consequently, the legislatively mandated use of laboratory animals is extensive. Of the approximately 100 million laboratory animals that are used each year in laboratories throughout the world, 10 to 15 million are used for vaccine batch testing. European Directives 2001/82/EC6 and 2003/63/EC,5 relating to veterinary and human medicinal products respectively, require quality-control tests to be conducted to ensure batch-to-batch consistency.
As described by Hendriksen and colleagues,11 the consistency approach is based upon thorough characterization of the vaccine during development and the principle that the quality of subsequent lots is guaranteed by the strict application of a quality system and of a demonstrated consistent production of batches identical to reference lots of known potency and safety. The consistency approach is already used for recently registered vaccines, whereas many established vaccines (that is, vaccines produced through inactivation or attenuation of the virulent microorganism or the toxin thereof) continue to rely on tests in laboratory animals for confirming the quality of each batch.
The replacement of animal testing by in vitro methods for in-process and final batch testing of vaccines might be hampered by unresolved scientific and technical questions and a reluctance to accept that in vitro methods can provide adequate assurance of safety and potency. The consistency approach provides a framework for resolving these issues and implementing the 3Rs but requires radical rethinking regarding the current practice for established vaccines.
One of the flagship projects of the EPAA, the Vaccines Consistency Approach project has the active support of industry and commission partners of the EPAA, as well as national authorities and academicians, including Professor Hendriksen, who is one of the project leaders and collaborates with the EPAA. Considerable time and resources are being invested to promote the implementation of the Vaccines Consistency Approach more widely. Several past workshops (2010 to 2013) have provided a solid basis for an improved dialogue with stakeholders from EU and beyond and addressed with priority 4 types of vaccines through different workstreams on human and veterinary rabies, clostridial vaccines, and diptheria–tetanus–acellular pertussis vaccines.15
These priorities were acknowledged as those with the most pressing animal welfare concerns. Of the 4 areas, the clostridial vaccine project is the most advanced to date. To summarize, EPAA member MSD Animal Health, with the financial support of the United Kingdom's National Centre for the 3Rs, has developed cell culture tests as alternatives to 2 animal methods used as in-process controls for the manufacture of a vaccine against Clostridium septicum. These tests measure the toxicity of the toxin, the residual toxicity of the inactivated toxin (toxoid), and the antigenicity of the toxoid. All of these tests rely on the measurement of toxin neutralization, and whereas this testing conventionally has relied on mice, the new tests aim to replace these conventional testing with cell cultures.
The aim of the project is to bring together an expert group of manufacturers, regulatory entities, and standards bodies to design and carry out a collaborative study, which the European Directorate for the Quality of Medicines and Healthcare (EDQM) will oversee. Successful collaborative studies run under the Biologic Standardization Program of the EDQM will lead to the validation of new methods and their inclusion in the European Pharmacopoeia as approved alternatives. The expert group consists of scientists from laboratories in the United States, New Zealand, Turkey, Spain, Hungary, Germany, Switzerland, France, and the United Kingdom, showing that ambitious improvements on 3Rs should involve an audience as large as possible. Eleven laboratories from the 9 previously mentioned countries will be involved in the study. To ensure the validity of the study, each contributing lab must adhere strictly to the agreed-on experimental protocols for carrying out and reporting the work. Six toxins and 6 toxoids donated by the participants will be coded to ensure that no one knows their origin and will be distributed to the various labs, together with a reference toxin and antitoxin, so that results from different labs can be normalized to these standards. Each lab will undertake to test the toxicity of the toxins and the antigenicity of the toxoids in vivo or in vitro (or both).
The data will then be collated and analyzed by an EDQM statistician to assess the performance of the tests in different hands and to compare in vivo and in vitro results. The data, still coded, will be shared with the participants through teleconferences and a final workshop targeted for 2015. The workshop will outline the study report to be published in the EDQM journal and in a peer-reviewed journal. The eventual inclusion of these tests in the European Pharmacopoeia may be a milestone for the entire EPAA Vaccines Consistency Approach project. In addition to looking for means to extend the methodology to other clostridial species, the group intends to encourage manufacturers to consider how they could adopt the general methodology to their current manufacturing quality control for clostridial vaccines.
The previous examples of EPAA projects illustrate that long-term and concerted efforts are needed to further promote the development and international acceptance of alternative approaches. Thanks to its multisectoral composition, the EPAA fosters international and interdisciplinary collaboration as the most efficient way to meet the scientific challenges and progressively reduce the need for animal testing. Ten years after the creation of EPAA, its work is now acknowledged by industry, European Commission, and third-party stakeholders as essential for the promotion of alternative methods in Europe. As a political PPP, EPAA intends to keep promoting the development of novel alternatives for regulatory safety testing. The EPAA partners also call for a reinforced international collaboration with European regulatory agencies and international regulators, such as the Organisation for Economic Cooperation and Development, to accelerate the validation of alternatives. Europe has been one of the first areas where 3Rs were promoted, and the European Union's legislation has inspired that of several countries (for example, India, Israel, Brazil) throughout the world through a ‘spill-over’ effect. This last outcome is why EPAA shall continue its efforts toward better science, safer testing, and less animal testing.
REFERENCES
- 1.Annys E, Billington R, Clayto R, Bremm KD, Graziano M, McKelvie J, Ragan I, Schwarz M, van der Laan JW, Wood C, Öberg M, Wester P, Woodward KN. 2014. Advancing the 3Rs in regulatory toxicology–Carcinogenicity testing: scope for harmonization and advancing the 3Rs in regulated sectors of the European Union. Regul Toxicol Pharmacol 69:234–242 [DOI] [PubMed] [Google Scholar]
- 2.Basketter D, Alépée N, Casati S, Crozier J, Eigler D, Griem P, Hubesch R, de Knecht J, Landsiedel R, Louekari K, Manou I, Maxwell G, Mehling A, Netzeva T, Petry T, Rossi L. 2013. Skin sensitization—moving forward with nonanimal testing strategies for regulatory purposes in the EU. Regul Toxicol Pharmacol 67:531–535. [DOI] [PubMed] [Google Scholar]
- 3.Bessems JG, Loizou G, Krishnan K, Clewell III HJ, Bernasconi C, Bois F, Coecke S, Collnot E, Diembeck W, Farcal LR, Geraets L, Gundert-Remy U, Kramer N, Küsters G, Leite S, Pelkonen O, Schröder K, Testai E, Wilk-Zasadna I, Zaldívar-Comenges JM. 2014. PBTK modelling platforms and parameter estimation tools to enable animal-free risk assessment: recommendations from a joint EPAA–EURL ECVAM ADME workshop. Regul Toxicol Pharmacol 68:119–139. [DOI] [PubMed] [Google Scholar]
- 4.Council of the European Communities 1986. Council Directive 86/609/EEC on the approximation of laws, regulations, and administrative provisions of the member states regarding the protection of animals used for experimental and other scientific purposes. OJEC L358:1–29. [PubMed] [Google Scholar]
- 5.European Commission. [Internet] 2003. Commission Directive 2003/63/EC amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use. [Cited on date]. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003L0063:en:NOT.
- 6.European Parliament and European Council. [Internet] 2001. Directive 2001/82/EC on the community code relating to veterinary medicinal products. [Cited on 10 January 2014]. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0082:en:NOT.
- 7.European Parliament and European Council 2006. European regulation concerning the registration, evaluation, authorisation, and restriction of chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Official Journal of European Council L396. [Cited on date] . Available at: http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm.
- 8.European Parliament and European Council 2010. Directive 2010/63/EU on the protection of animals used for scientific purposes. OJEC L276:33–79. [Google Scholar]
- 9.European Partnership for Alternative Approaches to Animal Testing. [Internet] 2005. The 3Rs declaration. [Cited 30 November 2006]. Available at: https://circabc.europa.eu/sd/d/3a0533fa-cfbf-4536-a7ca-f9c7c10f3eae/3rs-declaration.pdf
- 10.European Partnership for Alternative Approaches to Animal Testing. [Internet] 2008. New perspectives on safety: workshop report, p 1–16. [Cited 29 April 2008] Available at: https://circabc.europa.eu/sd/d/82d88bee-c433-4470-bdc0-65e266ca8a8d/new-perspectives.pdf
- 11.Hendriksen C, Arciniega JL, Chevalier M, Coppens E, Descamps J, Duchene M, Dusek DM, Halder M, Kreeftenberg H, Maes A, Redhead K, Ravetkar SD, Spieser JM, Swam H. 2008. The consistency approach for the quality control of vaccines. Biologicals 36:73–77 Available at http://dspace.library.uu.nl/handle/1874/32024. [DOI] [PubMed] [Google Scholar]
- 12.Moore NP, Andrew DJ, Bjerke DL, Creton S, Dreher D, Holmes T, Prieto P, Seidle T, Rowan T. 2013. Can acute dermal systemic toxicity tests be replaced with oral tests? A comparision of route-specific systemic toxicity and hazard classifications under the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Regul Toxicol Pharmacol 66:30–37. [DOI] [PubMed] [Google Scholar]
- 13.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. Methuen, London. [Google Scholar]
- 14.Schroeder K, Bremm KD, Alépée N, Bessems JGM, Blaauboer B, Boehn SN, Burek C, Coecke S, Gombau L, Hewitt NJ, Heylings J, Huwyer J, Jaeger M, Jagelavicius M, Jarrett N, Ketelslegers H, Kocina I, Koester J, Kreysa J, Note R, Poth A, Radtke M, Rogiers V, Scheel J, Schulz T, Steinkellner H, Toeroek M, Whelan M, Winkler P, Diembeck W. 2011. Report from the EPAA workshop: in vitro ADME safety testing used by EPAA industry sectors. Toxicol In Vitro 25:589–604. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization 2013, Geneva manual for quality control of diphtheria, tetanus, and pertussis vaccines. [Cited date]. Available at: http://apps.who.int/iris/bitstream/10665/80681/1/WHO_IVB_11.11_eng.pdf