Summary
Background
In the BRIM-3 trial, vemurafenib was associated with risk reduction versus dacarbazine of both death and progression in patients with advanced BRAFV600 mutation-positive melanoma. We present an extended follow-up analysis of the total population and in the BRAFV600E and BRAFV600K mutation subgroups.
Methods
Patients older than 18 years, with treatment-naive metastatic melanoma and whose tumour tissue was positive for BRAFV600 mutations were eligible. Patients also had to have a life expectancy of at least 3 months, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate haematological, hepatic, and renal function. Patients were randomly assigned by interactive voice recognition system to receive either vemurafenib (960 mg orally twice daily) or dacarbazine (1000 mg/m2 of body surface area intravenously every 3 weeks). Coprimary endpoints were overall survival and progression-free survival, analysed in the intention-to-treat population (n=675), with data censored at crossover. A sensitivity analysis was done. This trial is registered with ClinicalTrials.gov, NCT01006980.
Findings
675 eligible patients were enrolled from 104 centres in 12 countries between Jan 4, 2010, and Dec 16, 2010. 337 patients were randomly assigned to receive vemurafenib and 338 to receive dacarbazine. Median follow-up was 12·5 months (IQR 7·7–16·0) on vemurafenib and 9·5 months (3·1–14·7) on dacarbazine. 83 (25%) of the 338 patients initially randomly assigned to dacarbazine crossed over from dacarbazine to vemurafenib. Median overall survival was significantly longer in the vemurafenib group than in the dacarbazine group (13·6 months [95% CI 12·0–15·2] vs 9·7 months [7·9–12·8]; hazard ratio [HR] 0·70 [95% CI 0·57–0·87]; p=0·0008), as was median progression-free survival (6·9 months [95% CI 6·1–7·0] vs 1·6 months [1·6–2·1]; HR 0·38 [95% CI 0·32–0·46]; p<0·0001). For the 598 (91%) patients with BRAFV600E disease, median overall survival in the vemurafenib group was 13·3 months (95% CI 11·9–14·9) compared with 10·0 months (8·0–14·0) in the dacarbazine group (HR 0·75 [95% CI 0·60–0·93]; p=0·0085); median progression-free survival was 6·9 months (95% CI 6·2–7·0) and 1·6 months (1·6–2·1), respectively (HR 0·39 [95% CI 0·33–0·47]; p<0·0001). For the 57 (9%) patients with BRAFV600K disease, median overall survival in the vemurafenib group was 14·5 months (95% CI 11·2–not estimable) compared with 7·6 months (6·1–16·6) in the dacarbazine group (HR 0·43 [95% CI 0·21–0·90]; p=0·024); median progression-free survival was 5·9 months (95% CI 4·4–9·0) and 1·7 months (1·4–2·9), respectively (HR 0·30 [95% CI 0·16–0·56]; p<0·0001). The most frequent grade 3–4 events were cutaneous squamous-cell carcinoma (65 [19%] of 337 patients) and keratoacanthomas (34 [10%]), rash (30 [9%]), and abnormal liver function tests (38 [11%]) in the vemurafenib group and neutropenia (26 [9%] of 287 patients) in the dacarbazine group. Eight (2%) patients in the vemurafenib group and seven (2%) in the dacarbazine group had grade 5 events.
Interpretation
Inhibition of BRAF with vemurafenib improves survival in patients with the most common BRAFV600E mutation and in patients with the less common BRAFV600K mutation.
Funding
F Hoffmann-La Roche-Genentech.
Introduction
In the USA, more than 76 000 cases of melanoma are expected to be diagnosed in 2013, with 9180 deaths.1,2 Most are diagnosed at a localised stage with a 5-year overall survival of 91·2%.1 However, metastatic melanoma has a poor prognosis, and 5-year survival is 61·7% with regional stage disease and 15·2% with distant stage disease.1
Chemotherapy has limited success in metastatic melanoma, with responses noted in 6·3–12·1% of patients, and a median overall survival of 5·6–9·7 months in phase 3 trials of dacarbazine.3–6 Combinations of cytostatic drugs and cytokines have not improved survival.7,8 Highdose interleukin 2 can induce complete remission in some patients, which was the basis of its approval,9 but no predictive biomarkers for the patient’s response exist. In 2011, with the approval of the CTLA-4 antibody ipilimumab for all patients with advanced disease and of the BRAF inhibitor vemurafenib for BRAF-mutated disease, treatment for advanced disease finally improved.9–11 Dabrafenib, a BRAF inhibitor, and trametinib, a MEK inhibitor, have also been approved recently. Benefit from BRAF inhibition is consistent with the role of an activated RAS-RAF-MEK-ERK MAPK pathway as a major driver for transformation into malignant melanoma.12
The prevalence of BRAF codon 600 mutations in patients with melanoma ranges between 40% and 60%. The most prevalent mutations in melanoma are BRAFV600E (about 80%) and BRAFV600K (5–30%); other mutations are rare.12,13 In the phase 2 trial of vemurafenib,14 8% of patients had BRAFV600K melanoma; in a phase 2 trial15 of dabrafenib in patients with metastatic melanoma with brain metastases, 19% of patients had the BRAFV600K mutation. The ability to detect the BRAFV600K mutation also differs according to the methods used for mutation testing. The cobas 4800 BRAF V600 Mutation Test (cobas test; Roche Molecular Systems, Pleasanton, CA, USA), although specifically designed to detect the BRAFV600E mutation, detects 70% of BRAFV600K mutations, and is approved by the US Food and Drug Administration.16 By contrast with BRAFV600E, the frequency of BRAFV600K seems to increase with age.13 BRAFV600K mutation-positive melanoma is also associated with a higher degree of cumulative sun-induced damage, which might explain the variable frequency of BRAFV600K between studies in view of geographical variation in ultraviolet exposure.13 Data from a retrospective analysis of 80 patients with BRAF-mutated tumours, including 56 patients with melanoma, suggest that BRAFV600K mutations in metastatic melanoma might be associated with more frequent brain and lung metastases and a shorter time from diagnosis to metastasis and death than other BRAF mutations.17
BRIM-311 was a randomised phase 3 trial that compared vemurafenib with dacarbazine in patients with unresectable, previously untreated stage IIIc or IV melanoma that was positive for the BRAFV600 mutation. In the initial, prespecified analysis for the coprimary endpoints of overall survival and progression-free survival (Dec 30, 2010, cutoff), vemurafenib was associated with significant reductions in the risk for death and of either death or disease progression, compared with dacarbazine.11 Benefit with vemurafenib compared with dacarbazine was noted across patient subgroups, including those with stage IIIc and stage IV disease, and irrespective of lactate dehydrogenase concentrations.11
We present an update of safety and efficacy for the BRIM-3 study11 with extended follow-up for the entire population, and also analyse the efficacy and safety of vemurafenib versus dacarbazine in patients with BRAFV600E and BRAFV600K mutation-positive disease.
Methods
Study design and patients
Details of the BRIM-3 study have been reported elsewhere.11 Patients recruited from 104 centres in 12 countries worldwide with treatment-naive metastatic melanoma (unresectable stage IIIc or stage IV M1a, M1b, or M1c disease) were eligible if their tumour tissue was positive for the presence of BRAFV600 mutations by the cobas test. Additional key inclusion criteria included age of 18 years or older, a life expectancy of 3 months or longer, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate haematological, hepatic, and renal function. Patients were excluded if they had a history of cancer within the past 5 years (apart from basal-cell or squamous-cell carcinoma of the skin or carcinoma of the cervix) or metastases to the CNS that had progressed or required treatment in the previous 3 months. Patients were not permitted to receive concurrent anticancer therapy. The study protocol was approved by the institutional review board at every participating institution and was done in accordance with the ethics principles of the Declaration of Helsinki and within the Good Clinical Practice guidelines, as defined by the International Conference on Harmonisation. All patients provided written informed consent before enrolment.
Randomisation and masking
Patients were randomly assigned using an interactive voice recognition system supported by an independent vendor (in a 1:1 ratio) to receive either vemurafenib (960 mg twice daily orally) or dacarbazine (1000 mg/m2 of body surface area by intravenous infusion every 3 weeks). Patients were stratified according to American Joint Committee on Cancer stage (IIIc, M1a, M1b, or M1c), ECOG performance status (0 or 1), geographical region, and serum lactate dehydrogenase concentration (normal or raised). Patients and investigators were aware of treatment allocation.
Procedures
We prespecified dose reductions for both vemurafenib and dacarbazine for intolerable grade 2 or worse toxic effects. Treatment was discontinued on disease progression unless continued treatment was in the best interest of the patient, as judged by the investigator and sponsor. On Jan 14, 2011, after enrolment had reached its goal, and 13 months after the first patient was enrolled, the data and safety monitoring board recommended that patients in the dacarbazine group be allowed to cross over into the vemurafenib group, and the protocol was amended accordingly.
To identify the specific V600 mutation in tumours from patients of the BRIM-3 study,11 we did a combination of Sanger sequencing and higher sensitivity picotiter plate pyrosequencing (454 sequencing) on tumour DNA remaining after enrolment had closed. We sequenced all available baseline tumour DNA from enrolled patients using Sanger sequencing. Because this method can be less sensitive than the cobas test in detecting mutant alleles,16 we used 454 sequencing on samples that either gave no sequence or were mutation-negative by Sanger sequencing. We deemed samples that gave no sequence by one method and were mutation-negative by the other method to be indeterminate by sequencing. We also did retrospective Sanger sequencing on DNA available from all tumours that were screened by the cobas test before June, 2010, and identified 38 BRAFV600K samples, 25 of which had been identified as mutation-positive by the cobas test.16
Outcomes
Overall survival and progression-free survival were coprimary endpoints. Secondary endpoints included the proportion of patients with a confirmed response (ie, a complete response or partial response on RECIST version 1.1). Additional secondary endpoints were time to response, duration of response, time to treatment failure, the pharmacokinetic profile of vemurafenib, tolerability and safety of vemurafenib, and validation of the cobas test. Time to response and safety data are included in this report and other endpoints will be published elsewhere.
Statistical analysis
Overall survival and progression-free survival were co-primary endpoints for the trial. The trial was designed for 680 patients to be randomly assigned to receive either vemurafenib or dacarbazine. The trial had a power of 80% to detect a hazard ratio (HR) of 0·65 for overall survival with an α level of 0·045 (an increase in median survival from 8 months for dacarbazine to 12·3 months for vemurafenib) and a power of 90% to detect an HR of 0·55 for progression-free survival with an alpha level of 0·005 (an increase in median survival from 2·5 months for dacarbazine to 4·5 months for vemurafenib).
We estimated HRs for treatment with vemurafenib, as compared with dacarbazine, using unstratified Cox regression. We used the Kaplan-Meier method to estimate event-time distributions. All reported p values are two-sided, and CIs are at the 95% level. We used the software SAS 9.2 for all statistical analyses.
We included all patients randomly assigned to treatment in the efficacy analysis. We did the analyses in the whole population according to the treatment assigned at randomisation and by mutation subgroups (BRAFV600E and BRAFV600K) based on the clinical cutoff date of Feb 1, 2012, with censoring at the time when patients in the dacarbazine group crossed over to receive vemurafenib. Data without censoring event time at crossover are presented in the appendix. We used descriptive statistics for adverse events. We did the safety analysis in all patients who received a study drug and who had undergone at least one assessment during the study.
A sensitivity analysis was done to estimate the magnitude of the overall survival benefit of vemurafenib compared with dacarbazine, under assumptions of the effect of vemurafenib on overall survival after crossover. The sensitivity analysis examined five scenarios, with the treatment benefit of vemurafenib on overall survival after crossover assumed to reduce the risk for death by 20%, 30%, 40%, 50%, or 60%. For each of the five scenarios, survival after crossover was imputed to be the observed survival after crossover reduced by the assumed benefit in survival attributable to vemurafenib after crossover. We calculated total survival by adding the time from randomisation to crossover plus the imputed survival after crossover. Survival times were unchanged for patients receiving dacarbazine who did not cross over to receive vemurafenib and for patients in the vemurafenib group. Every patient’s survival status was also unchanged. We also did an analysis in which no benefit of vemurafenib after crossover is assumed (reduction in risk of 0%).
This trial is registered with ClinicalTrials.gov, NCT01006980.
Role of the funding source
This study was sponsored by F Hoffmann-La Roche Ltd and was designed by the academic investigators and representatives of the sponsor. All authors contributed to the interpretation of data and subsequent writing, reviewing, and finalisation of the report. Medical writing support was funded by the sponsor. All authors vouch for the completeness and veracity of the data and data analyses; all authors had access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
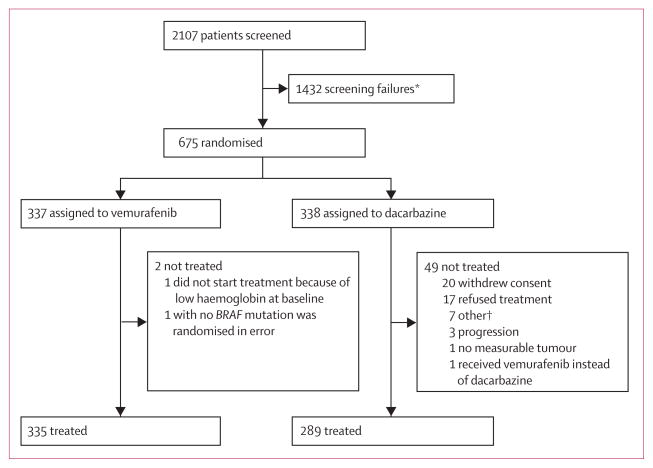
Of 2107 patients screened, we enrolled 675 eligible patients between Jan 4, 2010, and Dec 16, 2010. 337 patients were randomly assigned to receive vemurafenib and 338 to receive dacarbazine (figure 1). 399 (59%) patients had died by data cutoff (Feb 1, 2012). Five non-melanoma deaths occurred in the dacarbazine group and 11 in the vemurafenib group. The baseline characteristics of the intention-to-treat patient population have been described previously;11 the dacarbazine and vemurafenib groups were well balanced when examined as either a total randomised population (table 1) or as patients receiving randomised treatment (appendix).
Figure 1. Trial profile.
*Most common reason for screen failure included a negative cobas test for the BRAF mutation (1086 patients; 76%), brain metastases (110 patients; 8%), deterioration/ECOG status/death (46 patients; 3%). †Pulmonary embolism before treatment (one patient), not eligible per exclusion criteria (one patient), clinical deterioration (one patient), brain metastases (two patients), and two patients pending resolution on treatment status.
Table 1.
Patient characteristics by treatment group (randomised population)11
| Dacarbazine (n=338) | Vemurafenib (n=337) | |
|---|---|---|
| Median age (years) | 52·5 (43·0–62·0) | 56·0 (47·0–65·0) |
|
| ||
| Male sex | 181 (54%) | 200 (59%) |
|
| ||
| ECOG PS | ||
| 0 | 230 (68%) | 229 (68%) |
| 1 | 108 (32%) | 108 (32%) |
|
| ||
| Stage | ||
| Unresectable IIIc | 13 (4%) | 20 (6%) |
| M1a | 40 (12%) | 34 (10%) |
| M1b | 65 (19%) | 62 (18%) |
| M1c | 220 (65%) | 221 (66%) |
| LDH >ULN | 142 (42%) | 142 (42%) |
Data are number of patients (%) or median IQR. ECOG PS=Eastern Cooperative Oncology Group performance status. LDH=lactate dehydrogenase. ULN=upper limit of normal. Reprinted with permission from Massachusetts Medical Society, who own the copyright.
Median follow-up at the Feb 1, 2012, data cutoff was 12·5 months (IQR 7·7–16·0) for patients in the vemurafenib group and 9·5 months (IQR 3·1–14·7) for patients in the dacarbazine group. After initial treatment, some patients received additional anticancer therapies, including ipilimumab in 60 (18%) of 337 patients in the vemurafenib group and 73 (22%) of 338 patients in the dacarbazine group (table 2). 83 (25%) of 338 patients in the dacarbazine group crossed over to vemurafenib between January, 2011, and February, 2012.
Table 2.
Anticancer therapies received after initial treatment (randomised population)
| Dacarbazine (n=338) | Vemurafenib (n=337) | |
|---|---|---|
| Any | 149 (44%) | 122 (36%) |
| Ipilimumab* | 73 (22%) | 60 (18%) |
| Dabrafenib | 5 (1%) | 0 |
| Crossed over to vemurafenib | 83 (25%) | ·· |
Data are number of patients (%).
Eight patients received ipilimumab before commencing vemurafenib.
Because the cobas test is known to detect some cases with BRAFV600K mutations, we did DNA sequencing using the Sanger/454 methods retrospectively on DNA available from 673 of the 675 patients (336 patients in the vemurafenib group and 337 in the dacarbazine group) to differentiate BRAFV600E and BRAFV600K mutations. Of these 673 patients, Sanger/454 sequencing could not provide a valid result for 14 patients (six indeterminate results [three in each group]; eight with no sequence [four in each group]) and confirmed wild-type V600 for two patients whose tumours had been mutation-negative by the cobas test at screening (one in each group). Therefore, 657 (98%) of 671 patients had tumours that were cobas-positive at screening and for whom Sanger 454 sequencing results were available. Of these 657 tumours, 598 (91%) carried the BRAFV600E mutation (329 for dacarbazine, 328 for vemurafenib), 57 (9%) had the BRAFV600K mutation (24 for dacarbazine, 33 for vemurafenib), and two (<1%) had the BRAFV600D mutation (both in the dacarbazine group). Patient characteristics of the BRAFV600E and BRAFV600K populations confirm previous reports of older age in patients with the BRAFV600K mutation (table 3). One of the patients with a BRAFV600D mutation dropped out of the study before receiving study treatment.
Table 3.
Patient characteristics by mutation status (randomised population)11
| BRAFV600E (n=598) | BRAFV600K (n=57) | |
|---|---|---|
| Median age (years) | 53·0 (44·0–62·0) | 63·0 (47·0–65·0) |
|
| ||
| Geographical region | ||
| Australia/New Zealand | 72 (12%) | 5 (9%) |
| North America | 144 (24%) | 23 (40%) |
| Other | 18 (3%) | ·· |
| Western Europe | 364 (61%) | 29 (51%) |
|
| ||
| ECOG PS | ||
| 0 | 407 (68%) | 43 (75%) |
| 1 | 191 (32%) | 14 (25%) |
|
| ||
| Metastatic classification | ||
| M1a | 66 (11%) | 6 (11%) |
| M1b | 118 (20%) | 8 (14%) |
| M1c | 389 (65%) | 39 (68%) |
| Unresectable stage IIIc | 25 (4%) | 4 (7%) |
| Serum LDH elevated | 254 (42%) | 21 (37%) |
Data are number of patients (%), unless otherwise indicated. ECOG PS=Eastern Cooperative Oncology Group performance status. LDH=lactate dehydrogenase. Reprinted with permission from Massachusetts Medical Society, who own the copyright.
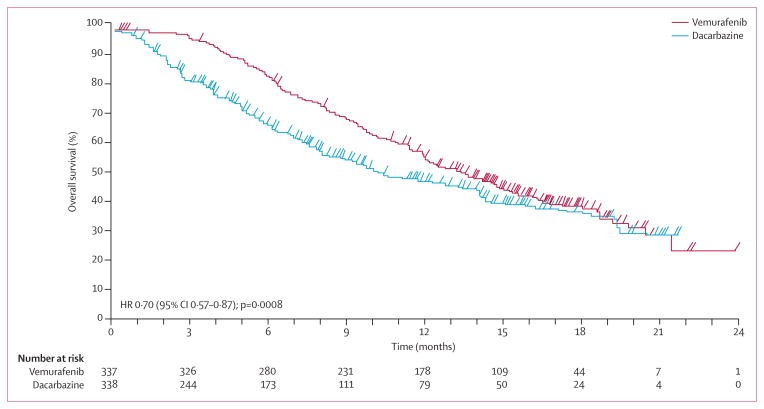
Median overall survival, censored at crossover, was 13·6 months (95% CI 12·0–15·2) in the vemurafenib group versus 9·7 months (7·9–12·8) in the dacarbazine group; 12 month overall survival was 56% (95% CI 50–61) for vemurafenib and 44% (38–51) for dacarbazine censored at crossover (figure 2). The HR for death in the vemurafenib group was 0·70 (95% CI 0·57–0·87; p=0·0008). Results from the sensitivity analysis to estimate the effect of crossover from dacarbazine to vemurafenib on HRs resulted in a range of HRs from 0·65 to 0·73 in favour of vemurafenib for all scenarios (appendix). Although the sensitivity analysis did not account for the effect of ipilimumab on the HR for death, it is notable that the use of ipilimumab after study treatments was similar in both groups. An intention-to-treat analysis of overall survival using the observed survival times, without regard to crossover, resulted in an HR of 0·76 (95% CI 0·63–0·93; p=0·0068; appendix). Results from stratified analyses were similar to the unstratified analyses (data not shown).
Figure 2.
Overall survival (randomised population; censored at crossover) for patients randomly assigned to vemurafenib or to dacarbazine (cutoff Feb 1, 2012)
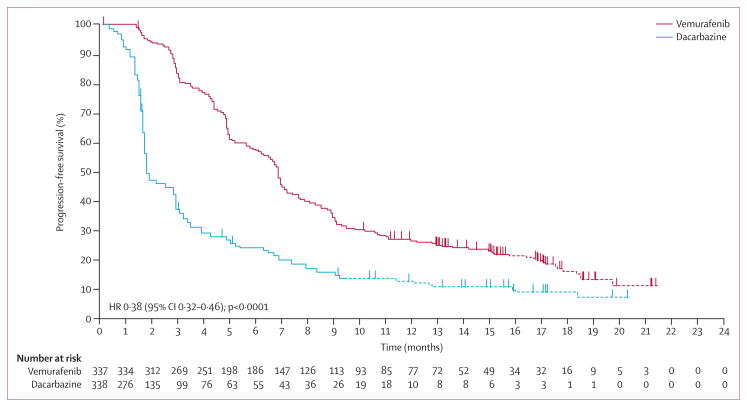
Median progression-free survival censored at crossover was also significantly longer in the vemurafenib group than in the dacarbazine group (6·9 months [95% CI 6·1–7·0] vs 1·6 months [1·6–2·1]), respectively; HR 0·38, 95% CI 0·32–0·46; log-rank p<0·0001; figure 3); this finding was noted in both patients with normal and raised lactate dehydrogenase concentration at baseline (appendix). Notably, both progression-free survival and overall survival were significantly shorter in patients with increased lactate dehydrogenase concentration at baseline in both groups of the study.
Figure 3.
Progression-free survival (rando mised population; censored at crossover) for patients randomly assigned to vemurafenib or to dacarbazine (cutoff Feb 1, 2012)
Kaplan-Meier curves of overall and progression-free survival without censoring at crossover are shown in the appendix (overall survival HR 0·76, 95% CI 0·63–0·93, p=0·0068). Without censoring for crossover, 18 month progression-free survival was 14% (95% CI 10–19) in the vemurafenib group and 6% (3–9) in the dacarbazine group; 18 month overall survival was 39% (95% CI 33–45) in the vemurafenib group and 34% (29–40) in the dacarbazine group.
Objective responses, confirmed by an independent review, were noted in 192 (57%) of 337 patients receiving vemurafenib and 29 (9%) of 338 patients treated with dacarbazine (appendix). Independently confirmed complete responses were attained by 19 (6%) patients in the vemurafenib group and four (1%) patients in the dacarbazine group.
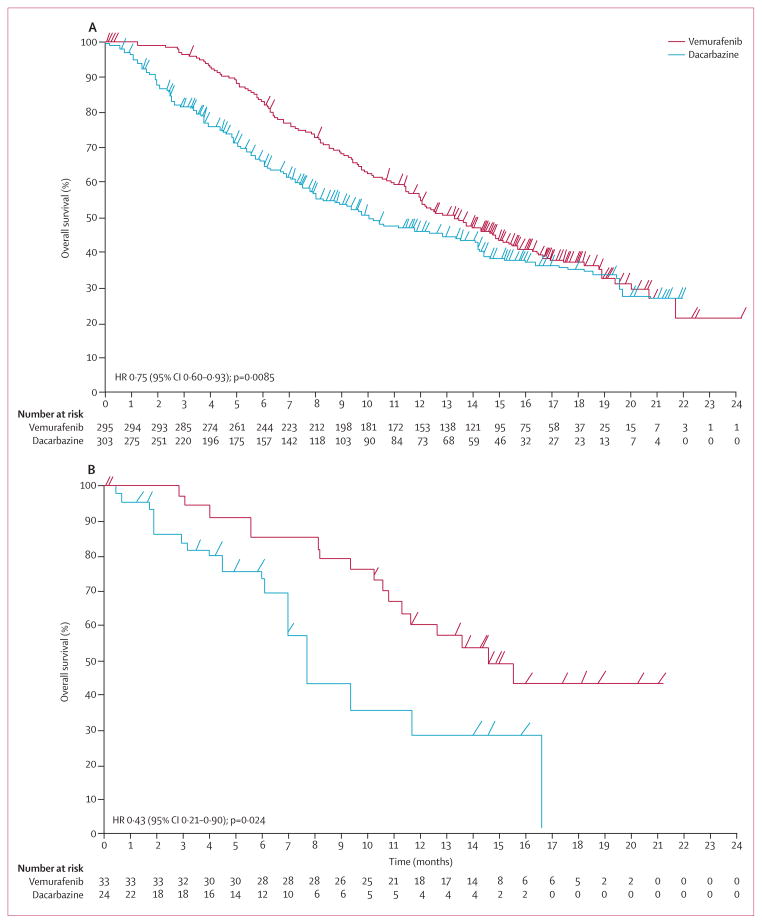
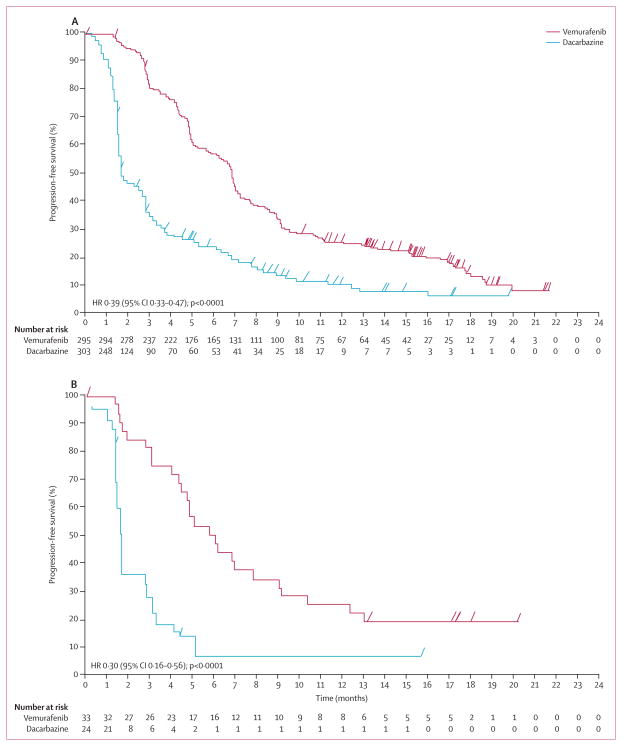
In a post-hoc analysis, median overall survival (censored at crossover) was significantly longer in the vemurafenib group than in the dacarbazine group for patients whose melanoma harboured either the BRAFV600E or the BRAFV600K mutation (figures 4A and 4B). For patients with BRAFV600E mutation-positive disease, median overall survival in the vemurafenib group was 13·3 months (95% CI 11·9–14·9) versus 10·0 months (8·0–14·0) in the dacarbazine group (HR 0·75, 95% CI 0·60–0·93; p=0·0085); for those with BRAFV600K mutation-positive disease it was 14·5 months (11·2–not estimable) in the vemurafenib group versus 7·6 months (6·1–16·6) in the dacarbazine group (HR 0·43, 95% CI 0·21–0·90; p=0·024). Progression-free survival was also significantly improved in the vemurafenib group compared with the dacarbazine group, irrespective of the mutation type (figures 5A and 5B). For patients with BRAFV600E mutation-positive disease, median progression-free survival in the vemurafenib group was 6·9 months (95% CI 6·2–7·0) versus 1·6 months (1·6–2·1) in the dacarbazine group (HR 0·39, 95% CI 0·33–0·47; p<0·0001); for those with BRAFV600K mutation-positive disease, it was 5·9 months (4·4–9·0) in the vemurafenib group versus 1·7 months (1·4–2·9) in the dacarbazine group (HR 0·30, 95% CI 0·16–0·56; p<0·0001).
Figure 4. Overall survival (randomised population; censored at crossover) for patients with BRAF mutations randomly assigned to vemurafenib or to dacarbazine (cutoff Feb 1, 2012).
(A) Patients with the BRAFV600E mutation. (B) Patients with the BRAFV600K mutation.
Figure 5. Progression-free survival (randomised population; censored at crossover) for patients with BRAF mutations randomly assigned to vemurafenib or to dacarbazine (cutoff Feb 1, 2012).
(A) Patients with the BRAFV600E mutation. (B) Patients with the BRAFV600K mutation.
The patient with BRAFV600D mutation-positive disease who received dacarbazine had a progression-free survival of 5 months, crossed over to vemurafenib at 6·4 months, and was alive at the study cutoff date with 14 months of follow-up. Of the 598 patients with BRAFV600E mutationpositive disease, 295 were randomly assigned to vemurafenib and 303 to dacarbazine. 173 (59%) had a response to vemurafenib, as did 34 (11%) to dacarbazine. Of the 57 patients with BRAFV600K mutation-positive disease, 33 were randomly assigned to vemurafenib and 24 to dacarbazine. 15 (45%) of those had a response to vemurafenib and one (4%) had a response to dacarbazine (appendix). The best objective response for the patient with the BRAFV600D mutation was stable disease. Median time to response to vemurafenib was 1·4 months (IQR 1·3–1·6) in patients with BRAFV600E mutation-positive disease and 1·4 months (IQR 1·4–2·6) in patients with BRAFV600K mutation-positive disease. Median time to response in patients crossing over to vemurafenib from dacarbazine was 3·0 months (IQR 2·7–6·3).
A summary of all adverse events is shown in the appendix. The most common grade 3 or 4 adverse events of interest in patients treated with vemurafenib were cutaneous squamous-cell carcinomas, increased liver function tests, keratoacanthomas, rash, and arthralgia (table 4). Grade 4 or worse adverse events occurred in 29 (8%) patients in the vemurafenib group (three [1%] increased liver function test, one [<1%] neutropenia), and 32 (11%) patients in the dacarbazine group (nine [3%] were neutropenia). Seven (2%) patients in the dacarbazine group and eight (2%) in the vemurafenib group had grade 5 events. Additionally, eight (2%) patients in the vemurafenib group reported new primary melanomas.
Table 4.
Summary of selected adverse events in treated patients (safety population)
| Dacarbazine (n=287)
|
Vemurafenib (n=337)
|
|||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Arthralgia | 8 (3%) | 3 (1%) | ·· | 169 (50%) | 20 (6%) | ·· |
|
| ||||||
| Rash | 6 (2%) | ·· | ·· | 108 (32%) | 30 (9%) | ·· |
|
| ||||||
| Fatigue | 94 (33%) | 6 (2%) | ·· | 146 (43%) | 10 (3%) | ·· |
|
| ||||||
| Photosensitivity | 13 (5%) | ·· | ·· | 124 (37%) | 13 (4%) | ·· |
|
| ||||||
| Increase in LFTs | 16 (6%) | 6 (2%) | ·· | 83 (25%) | 35 (10%) | 3 (1%) |
|
| ||||||
| Cutaneous squamous-cell carcinoma | ·· | 2 (<1%) | ·· | ·· | 65 (19%) | ·· |
|
| ||||||
| Keratoacanthoma | ·· | 2 (<1%) | ·· | 3 (<1%) | 34 (10%) | ·· |
|
| ||||||
| Skin papilloma | 1 (<1%) | ·· | ·· | 94 (28%) | 2 (<1%) | ·· |
|
| ||||||
| Nausea | 123 (43%) | 5 (2%) | ·· | 121 (36%) | 7 (2%) | ·· |
|
| ||||||
| Neutropenia | 8 (3%) | 17 (6%) | 9 (3%) | 1 (<1%) | ·· | 1 (<1%) |
|
| ||||||
| New primary melanoma | ·· | ·· | ·· | 2 (<1%) | 6 (2%) | ·· |
Data are number of patients (%). LFT=liver function test.
Treatment was discontinued because of adverse events in 24 (7%) patients receiving vemurafenib and six (2%) patients receiving dacarbazine. The incidence of keratoacanthoma, as defined by investigators, was higher in patients with BRAFV600K mutation-positive disease than in patients with BRAFV600E mutation-positive disease (table 5), whereas the incidence of squamous-cell carcinomas of the skin, as defined by investigators, did not differ by mutational status. The adverse event profile for vemurafenib was broadly similar for BRAFV600E and BRAFV600K groups, despite the age difference in the two populations.
Table 5.
Selected adverse events in patients treated with vemurafenib, by mutational status
| BRAFV600E (n=295) | BRAFV600K (n=33) | |
|---|---|---|
| Total patients with ≥1 adverse event | 294 (100%) | 33 (100%) |
| Arthralgia | 171 (58%) | 13 (39%) |
| Rash | 123 (42%) | 13 (39%) |
| Fatigue | 133 (45%) | 18 (55%) |
| Photosensitivity | 120 (41%) | 12 (36%) |
| Cutaneous squamous-cell carcinoma | 57 (19%) | 8 (24%) |
| Keratoacanthoma | 29 (10%) | 7 (21%) |
| Skin papilloma | 85 (29%) | 9 (27%) |
| Nausea | 117 (40%) | 8 (24%) |
Data are number of patients (%).
Discussion
This analysis, with a median follow-up of 12·5 months in the vemurafenib group and 9·5 months in the dacarbazine group, confirms the improved efficacy of vemurafenib versus dacarbazine for patients with BRAFV600 mutation-positive metastatic melanoma noted in the primary analysis of this study; the safety profile was also consistent with that in the primary analysis (panel).11
The HR for overall survival favouring vemurafenib in the present analysis is lower than that reported in the initial analysis (Dec 30, 2010, cutoff 11). Furthermore, the difference between the groups decreases when examining patients with longer follow-up, with similar overall survival at 18 months (figure 2). To understand the effect of crossover of 44% of patients to treatments known to improve overall survival, we did a sensitivity analysis, assuming a range of survival benefits (0–60% reduction in risk for death) after crossover. HRs for death while on vemurafenib ranged from 0·65 to 0·73, dependent on the assumed survival benefit after crossover. Other factors might also have affected the comparison of overall survival and progressionfree survival between the groups, including enrichment of uncensored patients with different prognosis in the intention-to-treat population, and development of resistance to vemurafenib. With or without censoring, the data still favoured the vemurafenib group.
Although BRAFV600E is the most common mutation in patients with metastatic melanoma, a substantial proportion of patients carry the BRAFV600K mutation (8·6% in the present study; up to 30% reported in specific populations),18 and data suggest that these patients might be at increased risk for brain and lung metastases and might have a shorter time from diagnosis to metastasis and death than patients with a BRAFV600E mutation.17 Consequently, it was important to assess the effects of vemurafenib in the BRAFV600K mutation-positive population. In-vitro data have shown activity of vemurafenib against activating mutant forms of BRAF including BRAFV600E, BRAFV600K, and BRAFV600R.19,20 57 patients in this analysis had BRAFV600K mutation-positive melanoma, and seems to be typical in terms of characteristics, compared with other studies.13 This group is the largest group of patients with this mutation in whom vemurafenib has been assessed, and the data indicate that BRAFV600K mutation-positive tumours are sensitive to vemurafenib; indeed, vemurafenib was associated with similar overall survival and progression-free survival outcomes, irrespective of the mutation. This finding is probably due to the equivalent effects of BRAFV600E and BRAFV600K on activation of the RAS-RAF-MEK-ERK pathway and the equally potent activity of vemurafenib against both forms of the mutant kinase, despite the potential for BRAFV600K mutations being associated with a higher rate of concomitant somatic genetic alterations.19,20
Secondary cutaneous squamous-cell carcinomas occurred in about a fifth of patients in the vemurafenib group, with similar rates in BRAFV600E and BRAFV600K mutation-positive disease (19% for BRAFV600E and 24% BRAFV600K). Keratoacanthoma was more common in BRAFV600K mutation-positive disease. Similar rates of secondary squamous-cell carcinoma have been reported in previous trials of vemurafenib,11,14,21 and dabrafenib.22,23 Secondary cutaneous neoplasia have also been reported in patients receiving the multikinase inhibitor sorafenib.24,25 Interestingly, eight vemurafenib-treated patients reported new primary melanomas. BRAF wild-type melanomas might develop during BRAF blockade as a result of BRAF inhibitor-induced tumour progression via the stimulation of MAPK signalling.26 Therefore, surveillance of melanocytic lesions in patients receiving BRAF inhibitors is warranted. Adverse event profiles for vemurafenib were broadly similar between BRAFV600E and BRAFV600K mutation-positive disease despite patients with BRAFV600K mutation-positive disease having a median age 10 years older than that of patients with the BRAFV600E mutation.
Panel: Research in context.
Systematic review
We did a systematic search of PubMed, Google Scholar, ClinicalTrials.gov, and meeting abstracts from the American Society of Clinical Oncology, and the European Society of Medical Oncology for 2012 and 2013 using the search terms “advanced melanoma”, “overall survival”, and “clinical trial”, or the same terms with the addition of “BRAF” or “mutation”, or both. The results of the search showed that until the publication of the randomised trial of ipilimumab compared with gp100 vaccine9 and the comparison of vemurafenib to dacarbazine,11 no randomised trial had shown an overall survival advantage for an investigational agent in advanced melanoma. The search showed that BRAFV600K mutations might be associated with worse survival17 and the absence of any data examining the effect on overall survival of inhibition of BRAF in patients with melanomas containing different BRAF mutations.
Interpretation
Based on these results, inhibition of BRAF significantly improves clinical outcome in patients with the two most common BRAF mutations. Adverse event profiles were similar to those previously reported, although eight patients reported new melanomas, suggesting that surveillance of melanocytic lesions is warranted in patients receiving BRAF inhibitors. Although the BRAFV600K mutation was known to activate the BRAF kinase and respond to BRAF inhibitors, the data showed for the first time, to our knowledge, improvements in overall survival in a less common mutational subset of melanoma.
In conclusion, our results show that vemurafenib continues, with longer follow-up, to be associated with improved efficacy compared with dacarbazine in patients with BRAFV600 mutation-positive metastatic melanoma. Our results also show that BRAFV600K mutation-positive melanoma is sensitive to vemurafenib, with safety and efficacy profiles similar to those noted in BRAFV600E mutation-positive disease.
Acknowledgments
We thank the patients who participated in this study. We also thank the clinical trial team for their support in the execution of the trial, and F Hoffmann-La Roche-Genentech for supporting the trial. Medical writing assistance was provided by David Gibson, PhD, of ApotheCom, San Francisco, CA, USA, and funded by F Hoffmann-La Roche. JL is funded by the National Institute for Health Research Biomedical Research Centre for cancer at Royal Marsden Hospital/Institute of Cancer Research. GAM is a practitioner fellow of National Health and Medical Research Council.
Footnotes
Contributors
GAM, PBC, CR, JL, JBH, RD, AR, DH, OH, PAA, CG, AT, MM, PL, CL, TJ, DS, SJO’D, JMK, AME, BD, JAS, KTF, and AH were involved in collection of the data and its interpretation; they also had access to the raw data. GAM, PBC, AR, KTF, MY, and AH designed the trial. MY, SC, IC, and KT contributed to the statistical analyses and generation of tables and figures. IC was the medical monitor for the study. All authors were involved in writing the report and reviewed and approved the final version of the report.
Conflicts of interest
GAM has received research funding from Novartis, Pfizer, and Millennium, and has provided uncompensated services for Roche- Genentech, Plexxicon, Novartis, Bristol-Myers Squibb, GlaxoSmithKline, Amgen, and Millennium. PBC has received research support and served on advisory boards for GlaxoSmithKline, and has been a consultant for Bristol-Myers Squibb. CR has served as a consultant for Roche, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Merck. JL has served on advisory boards for Roche-Genentech, Bristol-Myers Squibb, and GlaxoSmithKline. JBH has served on advisory boards. AR has received honoraria for advisory boards from Roche-Genentech, paid to his institution. OH has been a speaker and consultant for Genentech and has received research funding. PAA has been a consultant or had an advisory role for Bristol-Myers Squibb, Merck Sharp & Dohme, Roche-Genentech, GlaxoSmithKline, Celgen, Amgen, and Novartis, and has received honoraria from Bristol-Myers Squibb, Roche-Genentech, and GlaxoSmithKline. AME has served on advisory boards for Bristol-Myers Squibb, GlaxoSmithKline, MedImuune, and Merck. AT has a consultant and advisory board relationship with and has received honoraria from Bristol-Myers Squibb, GlaxoSmithKline, Igea, Roche, and Amgen, and he has received honoraria from Oncovision. MM has received honoraria for advisory boards, consultancy, and speakers bureaus from Bristol-Myers Squibb, Roche-Genentech, and MedImmune, and research funds from Bristol-Myers Squibb. CL has been a consultant or has had an advisory board relationship with Roche, GlaxoSmithKline, Novartis, Bristol-Myers Squibb, and Amgen. TJ has been a consultant or had an advisory role and received research funding from Bristol-Myers Squibb, Roche-Genentech, and GlaxoSmithKline. DS has received research funding from Merck, and has had consultancies or participated in advisory boards with GlaxoSmithKline, Roche, Bristol Myers-Squibb, Merck, Amgen, Delcath, and Novartis. SJO’D receives research funding from GlaxoSmithKline. JMK has been a consultant or had an advisory board relationship with Bristol-Myers Squibb, Merck, Celgene, and Ziopharm. RD receives research funding from AstraZeneca, Novartis, Cephalon, Merck Sharp & Dohme, Transgene, Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Bayer, and has a consultant or advisory board relationship with AstraZeneca, Novartis, Cephalon, Merck Sharp & Dohme, Transgene, Genta, Bayer, Roche, Bristol-Myers Squibb, GlaxoSmithKline, and Spirig. BD has served on advisory boards for Roche-Genentech, Bristol-Myers Squibb, and GlaxoSmithKline. JAS has served on advisory boards for Genentech. KTF is a consultant for Roche-Genentech. MY is an employee of Roche-Genentech, with company stock. IC is an employee of Roche- Genentech, is a stockholder, and has stock options. SC is employed by Roche Molecular Systems Inc. KT is an employee of F Hoffman-LaRoche, with stock ownership. AH has received honoraria for advisory boards, consultancy, and speakers bureaus, as well as research funding from Amgen, Bristol-Myers Squibb, Celgene, Eisai, GlaxoSmithKline, MedImmune, MetaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, and Roche Pharma. The other authors declare that they have no conflicts of interest.
Contributor Information
Prof. Grant A McArthur, Peter MacCallum Cancer Centre, East Melbourne, VIC, Australia.
Prof. Paul B Chapman, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Caroline Robert, Institut Gustave Roussy, Paris, France.
James Larkin, Royal Marsden Hospital, London, UK.
John B Haanen, The Netherlands Cancer Institute, Amsterdam, Netherlands.
Prof. Reinhard Dummer, University of Zurich, Zurich, Switzerland.
Prof. Antoni Ribas, Jonsson Comprehensive Cancer Center at University of California, Los Angeles, CA, USA.
Prof. David Hogg, Princess Margaret Hospital and University Health Network, Toronto, ON, Canada.
Omid Hamid, The Angeles Clinic and Research Institute, Los Angeles, CA, USA.
Paolo A Ascierto, Istituto Nazionale Tumori Fondazione Pascale, Naples, Italy.
Prof. Claus Garbe, The University of Tübingen, Tübingen, Germany.
Alessandro Testori, Istituto Europeo di Oncologia, Milan, Italy.
Michele Maio, University Hospital of Siena, Istituto Toscano Tumori, Siena, Italy.
Paul Lorigan, University of Manchester, Manchester, UK.
Prof. Celeste Lebbé, APHP Oncodermatology, Hôpital Saint Louis University, Paris, France.
Thomas Jouary, Saint André Hospital, Bordeaux, France.
Prof. Dirk Schadendorf, University Hospital Essen, Essen, Germany.
Stephen J O’Day, Beverly Hills Cancer Center, Beverly Hills, CA, USA.
Prof. John M. Kirkwood, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Prof. Alexander M Eggermont, Gustave-Roussy Cancer Center and University Paris-Sud, Villejuif/Paris Sud, France.
Prof. Brigitte Dréno, Nantes University Hospital, Nantes, France.
Prof. Jeffrey A Sosman, Vanderbilt University School of Medicine, Nashville, TN, USA.
Keith T Flaherty, Massachusetts General Hospital, Boston, MA, USA.
Ming Yin, Genentech Inc, San Francisco, CA, USA.
Ivor Caro, Genentech Inc, San Francisco, CA, USA.
Suzanne Cheng, Roche Molecular Systems Inc, Pleasanton, CA, USA.
Kerstin Trunzer, F Hoffmann-La Roche, Basel, Switzerland.
Prof. Axel Hauschild, University of Kiel, Kiel, Germany.
References
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–25. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 4.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–51. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 6.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 7.Huncharek M, Caubet JF, McGarry R. Single-agent DTIC versus combination chemotherapy with or without immunotherapy in metastatic melanoma: a meta-analysis of 3273 patients from 20 randomized trials. Melanoma Res. 2001;11:75–81. doi: 10.1097/00008390-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Sasse AD, Sasse EC, Clark LG, Ulloa L, Clark OA. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev. 2007;1:CD005413. doi: 10.1002/14651858.CD005413.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2012. Eur J Cancer. 2012;48:2375–90. doi: 10.1016/j.ejca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 13.Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012;18:3242–49. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- 14.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 16.Anderson S, Bloom KJ, Vallera DU, et al. Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed, paraffin-embedded tissue specimens of malignant melanoma. Arch Pathol Lab Med. 2012;136:1385–91. doi: 10.5858/arpa.2011-0505-OA. [DOI] [PubMed] [Google Scholar]
- 17.El Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS One. 2011;6:e25806. doi: 10.1371/journal.pone.0025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amanuel B, Grieu F, Kular J, Millward M, Iacopetta B. Incidence of BRAF p.Val600Glu and p. Val600Lys mutations in a consecutive series of 183 metastatic melanoma patients from a high incidence region. Pathology. 2012;44:357–9. doi: 10.1097/PAT.0b013e3283532565. [DOI] [PubMed] [Google Scholar]
- 19.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–27. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anforth RM, Blumetti TC, Kefford RF, et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol. 2012;167:1153–60. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 25.Arnault J-P, Mateus C, Escudier B, et al. Skin tumors induced by sorafenib; paradoxical RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53 and TGFBR1. Clin Cancer Res. 2012;18:263–72. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 26.Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375–83. doi: 10.1200/JCO.2011.41.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]