Abstract
The multicenter, single-arm BONAFIDE study characterized the skeletal response to cinacalcet in adult dialysis patients with plasma parathyroid hormone (PTH) levels of 300 pg/ml or more, serum calcium of 8.4 mg/dl or more, bone-specific alkaline phosphatase over 20.9 ng/ml and biopsy-proven high-turnover bone disease. Of 110 enrolled patients, 77 underwent a second bone biopsy with quantitative histomorphometry after 6–12 months of cinacalcet treatment. The median PTH decreased from 985 pg/ml at baseline to 480 pg/ml at the end of study (weeks 44–52). Bone formation rate/tissue area decreased from 728 to 336 μm2/mm2/day, osteoblast perimeter/osteoid perimeter decreased from 17.4 to 13.9%, and eroded perimeter/bone perimeter decreased from 12.7 to 8.3%. The number of patients with normal bone histology increased from none at baseline to 20 at 12 months. Two patients had adynamic bone at the end of study with a PTH under 150 pg/ml, and one patient with overt hypophosphatemia at baseline that reoccurred during follow-up developed osteomalacia. Thus, long-term treatment with cinacalcet substantially reduced PTH, diminished the elevated bone formation rate/tissue area, lowered several biochemical markers of high-turnover bone disease toward normal, and generally improved bone histology. Twenty patients had normal bone histology at follow-up, whereas most had mild hyperparathyroidism or mixed uremic osteodystrophy.
Keywords: bone, bone and mineral disorder, calcimimetic, dialysis, hyperparathyroidism
Bone disease is a major consequence of secondary hyperparathyroidism (sHPT) among patients with chronic kidney disease (CKD). It is an integral component of the syndrome of CKD–mineral and bone disorder affecting a substantial proportion of patients undergoing dialysis. The skeletal manifestations of sHPT arise largely from the ongoing release of excess amounts of parathyroid hormone (PTH) into the circulation by enlarged parathyroid glands.1 Accordingly, serum or plasma PTH levels are elevated, often markedly so. A key objective in the treatment of sHPT is to lower PTH levels toward recommended target ranges and to correct the histopathological changes of sHPT in bone.2
Vitamin D sterols and calcimimetic agents are the only two pharmacological interventions available currently to lower PTH levels among patients with sHPT managed with dialysis. Treatment with vitamin D sterols has been shown to reduce PTH, mostly in small studies lasting several weeks or only a few months.3, 4 The PTH-lowering effect of the calcimimetic agent cinacalcet (Sensipar/Mimpara) is recognized generally,5, 6, 7 and clinical outcomes after prolonged treatment were reported recently.8 Much less is known, however, about changes in bone histology after prolonged therapy with either vitamin D or cinacalcet.
Some improvements in bone histology have been described in small studies after treatment with daily oral doses or thrice weekly intravenous doses of calcitriol in adults with sHPT undergoing hemodialysis.9, 10 Similar findings were reported after treatment with oral or intraperitoneal doses of calcitriol or with oral doses of doxercalciferol in pediatric patients receiving peritoneal dialysis.11 With regard to cinacalcet, modest improvement in the histological features of sHPT and decreases in bone turnover were noted in one small study after 12 months of treatment,12 but the effect of cinacalcet on renal bone disease among patients undergoing dialysis has yet to be characterized adequately. Of note, the exploratory end point of fracture rate did not differ in unadjusted analyses in the EVOLVE outcomes study among subjects assigned randomly to treatment with cinacalcet or placebo.8
The purpose of the BONAFIDE study described herein was to further assess the skeletal response to cinacalcet treatment among patients with histological evidence of hyperparathyroid bone disease. Bone biopsy and quantitative bone histomorphometry were done before and after treatment. Various biochemical parameters of mineral metabolism were measured at baseline and at regular intervals during follow-up.
RESULTS
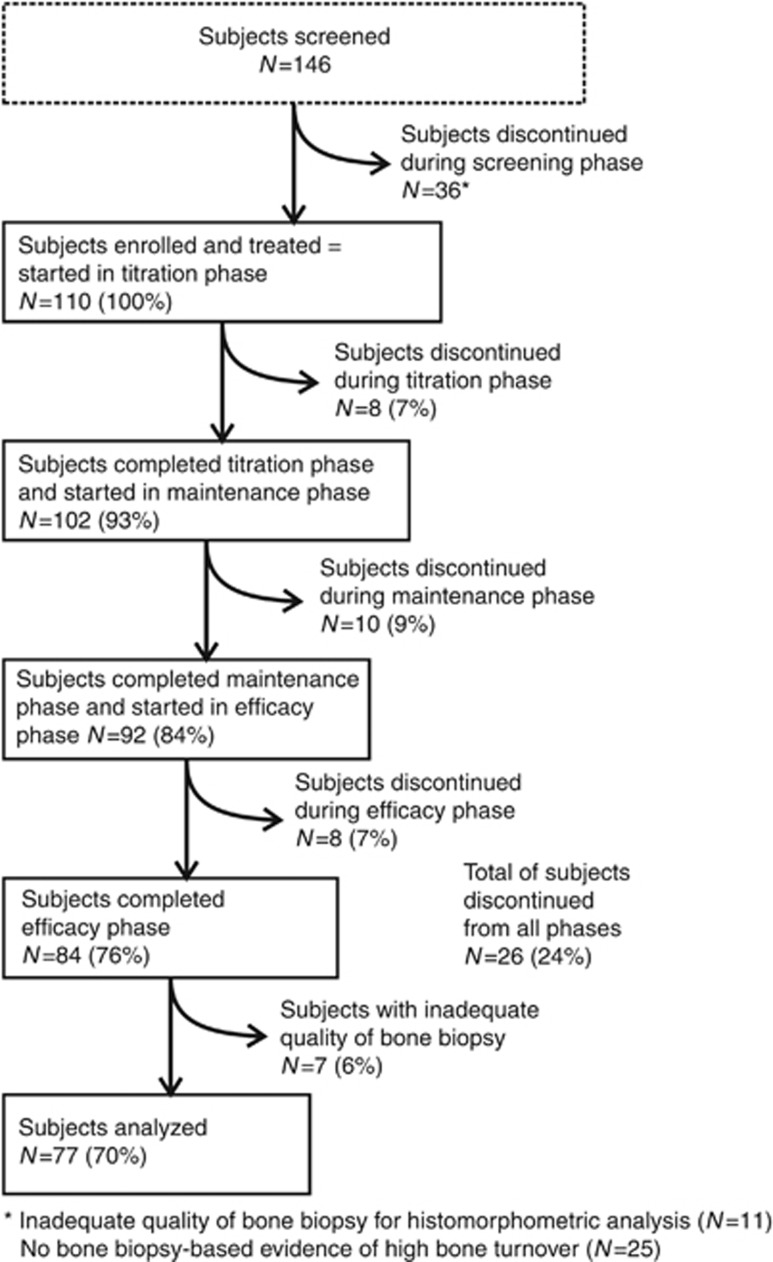
One hundred and forty-six (146) adult dialysis patients with plasma PTH (PTH) ⩾300 pg/ml, serum calcium ⩾8.4 mg/dl, and bone-specific alkaline phosphatase (BALP) >20.9 ng/ml underwent bone biopsy during screening (Figures 1 and 2). The biopsy sample was inadequate for histomorphometric evaluation in 11 cases, and 25 subjects were excluded from further study because they did not have biopsy findings consistent with hyperparathyroid bone disease as specified in the study protocol (Figure 2). Notably, 22 of these had normal bone histology and 3 had mixed lesions of renal osteodystrophy, whereas none had adynamic bone.
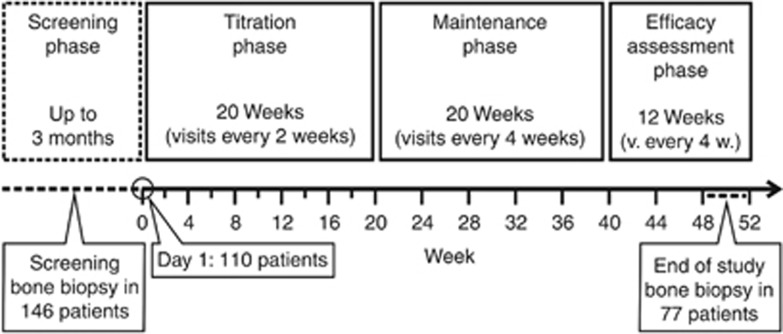
Figure 1.
Study design and treatment schema.
Figure 2.
Subject disposition by treatment phase.
Of 110 subjects who entered into the single-arm study and were treated with cinacalcet at a starting dose of 30 mg/day, 70 (64%) were men and 91 (83%) were Caucasian. The mean (s.d.) age was 55.2 (14.2) years, range 19–82 years, and the median (interquartile range; IQR) hemodialysis vintage was 43.0 (17.0, 89.0) months. At baseline, 59 subjects (54%) were receiving a vitamin D sterol and 92 (84%) were receiving a phosphate-binding agent and/or calcium supplement. None had been treated previously with cinacalcet.
Eighty-four subjects completed the study and 77 underwent a follow-up bone biopsy that provided tissue sufficient for quantitative histomorphometry (Figure 2). All but three subjects were biopsied after approximately 12 months of treatment with cinacalcet. In two subjects, treatment with cinacalcet was stopped after 6 months, and follow-up biopsies were obtained at month 7 in one and at month 8 in the other. A third subject was treated with cinacalcet for 3 months but did not have a follow-up biopsy until month 12. All final results are based upon the 77 subjects with bone biopsy data at baseline and follow-up (Tables 1 and 2). Demographic and biochemical results at baseline for the 33 subjects who did not have paired biopsy specimens before and after treatment also are provided for comparison (Table 3).
Table 1. Bone histomorphometrya before and after treatment with cinacalcet.
| Baseline, median (IQR) | End of study, median (IQR) | P-valueb | |
|---|---|---|---|
| B.Ar/T.Ar (%) [14.6–26.9] | 24.3 (20.2, 30.7) | 25.3 (20.2, 29.8) | 0.54 |
| O.Ar/B.Ar (%) [0.2–5.8] | 5.1 (3.5, 7.4) | 3.8 (2.4, 6.6) | 0.072 |
| O.Pm/B.Pm (%) [10.2–31.7] | 40.1 (30.3, 50.7) | 30.7 (21.4, 46.3) | 0.003 |
| O.Wi (μm) [4.1–13.1] | 9.6 (8.1, 12.3) | 10.3 (7.9, 14.0) | 0.17 |
| Ob.Pm/O.Pm (%) [1.8–58.3] | 17.4 (11.8, 26.0) | 13.9 (6.2, 25.0) | <0.001 |
| dL.Pm/B.Pm (%) [1.6–15.8] | 14.5 (9.7, 21.3) | 8.1 (4.3, 15.0) | <0.001 |
| E.Pm/B.Pm (%) [0.4–3.4] | 12.7 (9.9, 16.4) | 8.3 (6.2, 12.7) | <0.001 |
| Oc.Pm/E.Pm (%) [0.4–3.4] | 20.4 (13.2, 27.2) | 18.0 (13.9, 22.3) | 0.062 |
| Tb.Wi (μm) [90–175] | 145.5 (124.6, 168.8) | 153.6 (129.7, 174.6) | 0.25 |
| Tb.N (mm) [1.1–2.2] | 2.2 (1.8, 2.6) | 2.1 (1.8, 2.4) | 0.19 |
| BFR/T.Ar (μm2/mm2/day) [97–613] | 727.8 (545.9, 1052.6) | 336.1 (172.2, 641.8) | <0.0001 |
| Mlt (days) [2.4–63] | 17.8 (10.9, 26.3) | 21.4 (14.7, 44.0) | <0.001 |
| Fb.Ar/T.Ar | n (%) | n (%) | |
| 0% | 6 (8%) | 24 (31%) | |
| 1–5% | 50 (65%) | 42 (55%) | |
| 6–10% | 18 (23%) | 9 (12%) | |
| 11–25% | 1 (1%) | 2 (3%) | |
| >25% | 2 (3%) | 0 |
Abbreviations: B.Ar, bone area; B.Pm, bone perimeter; dL.Pm, double labeled perimeter; E.Pm, eroded perimeter; Fb.Ar, fibrosis area; Mlt, mineralization lag time; O.Ar, osteoid area; Ob.Pm, osteoblast perimeter; Oc.Pm, osteoclast perimeter; O.Pm, osteoid perimeter; O.Wi, osteoid width; T.Ar, tissue area; Tb.N, trabecular number; Tb.Wi, trabecular width.13, 14
Continuous variables are presented as median values with interquartile range (IQR). Numbers in [brackets] denote the lower and upper limit of values for subjects with normal kidney function.13, 14, 15
Results are formatted for two dimensions.
Descriptive P-values were obtained from the Wilcoxon signed-rank test.
Table 2. Biochemical measurements before and after treatment with cinacalcet.
| Baseline, median (IQR) | End of studya, median (IQR) | P-valueb | |
|---|---|---|---|
| PTH (pg/ml) c [10–65] | 985 (674, 1621) | 480 (268, 798) | <0.001 |
| Calcium (mg/dl) [8.4–10.3] | 9.9 (9.4, 10.3) | 9.1 (8.6, 9.7) | <0.001 |
| Phosphorus (mg/dl) [2.2–5.1] | 5.5 (4.7, 6.2) | 5.5 (4.9, 6.6) | 0.94 |
| Ca x P (mg2/dl2) [18.5–52.5] | 53.8 (46.6, 63.9) | 52.6 (43.2, 62.2) | 0.050 |
| BALP (ng/ml) [2.9–22.4] | 40.1 (27.3, 73.9) | 27.7 (18.8, 53.9) | 0.20 |
| N-telopeptide (nmol/l) [5.4–24.2] | 378 (169, 1304) | 249 (126, 425) | <0.001 |
| TRAP (U/l) [0.49–5.31] | 6.8 (5.1, 9.3) | 5.1 (3.8, 7.2) | 0.003 |
| Osteocalcin (ng/ml) [11–55.9] | 494 (245, 846) | 276 (151, 526.) | <0.001 |
Abbreviations: BALP, bone-specific alkaline phosphatase; IQR, interquartile range; PTH, parathyroid hormone; TRAP, tartrate-resistant acid phosphatase.
Numbers in [brackets] denote the lower and upper limit of values for subjects with normal kidney function.
The end of study value for biochemical parameters is defined as the mean value during the efficacy assessment phase (week 44–52).
Descriptive P-values were obtained from the Wilcoxon signed-rank test.
Values from 74 subjects with measurements both at baseline and at end of study.
Table 3. Demographic and biochemical characteristics at baseline for subjects with or without bone histomorphometry results after treatment with cinacalcet.
| Subjects included in final analysis (n=77) | Subjects excluded from final analysis (n=33) | |
|---|---|---|
| Male sex, n (%) | 53 (69) | 17 (52) |
| White race, n (%) | 63 (82) | 28 (85) |
| Mean (s.d.) age [range], years | 54.5 (13.6) [26, 82] | 56.8 (15.7) [19, 80] |
| Mean (s.d.) height, cm | 168 (10) | 167 (10) |
| Mean (s.d.) weight, kg | 73.7 (17.8) | 71.6 (18.6) |
| Dialysis duration, median (IQR) | ||
| Hemodialysis, months | 43 (17, 89) [n=73] | 43 (20, 117) |
| Peritoneal dialysis, months | 37 (16, 53) [n=4] | — |
| Use of phosphate binder/calcium supplement, n (%) | 65 (84) | 27 (82) |
| Lab values, median (IQR) | ||
| PTH, pg/ml | 997 (674, 1621) | 1322 (752, 2063) |
| Calcium, mg/dl | 9.9 (9.4, 10.3) | 10.1 (9.8, 10.5) |
| Phosphorus, mg/dl | 5.5 (4.7, 6.2) | 5.7 (5.0, 6.5) |
| Ca x P (mg2/dl2) | 53.8 (46.6, 63.9) | 58.6 (50.6, 65.0) |
| BALP, ng/ml | 40.1 (27.3, 73.9) | 47.5 (28.8, 74.1) |
| N-telopeptide, nmol/l | 378 (169, 1304) | 1041 (277, 1691) |
| TRAP, U/l | 6.8 (5.1, 9.3) | 6.9 (4.0, 10.9) |
| Osteocalcin, ng/ml | 494 (245, 846) | 671 (373, 1038) |
Abbreviations: BALP, bone-specific alkaline phosphatase; IQR, interquartile range; PTH, parathyroid hormone; s.d., standard deviation; TRAP, tartrate-resistant acid phosphatase.
The mean (s.d.) daily doses of cinacalcet among the 77 patients at the end of the titration (week 20), maintenance (week 40), and efficacy assessment phases (week 52) were 67.5 (40.7), 72.3 (46.6), and 73.2 (43.9) mg/day, respectively. Reasons for cinacalcet discontinuation varied as expected (Table 4). Of the 77 subjects, 39 (51%) were receiving vitamin D at baseline and 60 (78%) received vitamin D at some point during the study. The mean (s.d.) weekly dose of vitamin D ranged from 15.9 (10.1) to 21.8 (21.2) μg expressed in μg equivalents of paricalcitol. The types of vitamin D used included oral alfacalcidol only (n=3), oral calcitriol only (n=14), doxercalciferol only (n=4), paricalcitol only (n=21), and other/multiple vitamin D (n=14).
Table 4. Reasons for cinacalcet discontinuation.
| No. of subjects (%) | |
|---|---|
| Subjects enrolled | 110 |
| Subjects who received cinacalcet | 110 (100) |
| Subjects who completed the study | 84 (76) |
| Subjects who completed cinacalcet treatment | 69 (63) |
| Subjects who discontinued cinacalcet | 41 (37) |
| Adverse event | 2 (2) |
| Consent withdrawn | 4 (4) |
| Administrative decision | 1 (1) |
| Lost to follow-up | 2 (2) |
| Death | 5 (5) |
| Protocol-specified criteria | 13 (12) |
| Patient request | 1 (1) |
| Other reason | 13 (12) |
Percentages based on number of subjects enrolled.
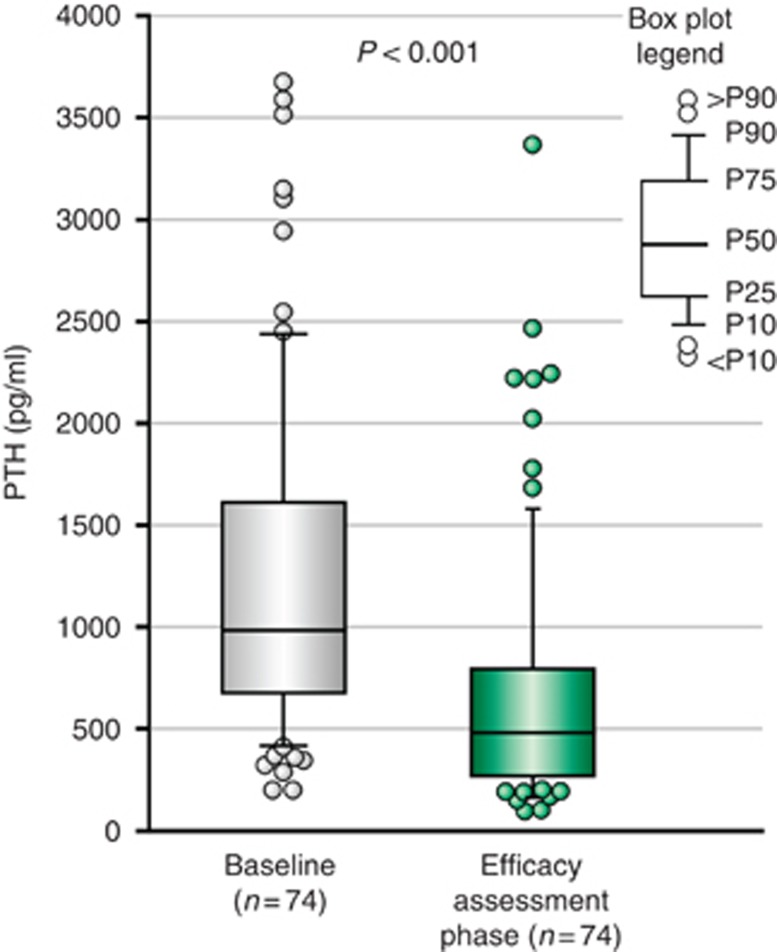
The median (IQR) PTH at baseline was 985 (674, 1621) pg/ml, and values decreased nominally to 480 (268, 798) pg/ml, or by a median (IQR) percent change of −48.3% (−68.8%, −26.5%), after treatment with cinacalcet, P<0.001 (Figure 3).
Figure 3.
Comparison of PTH level at baseline and efficacy assessment phase (median-IQR-range). P-value: Wilcoxon-signed rank test.
Bone histomorphometry
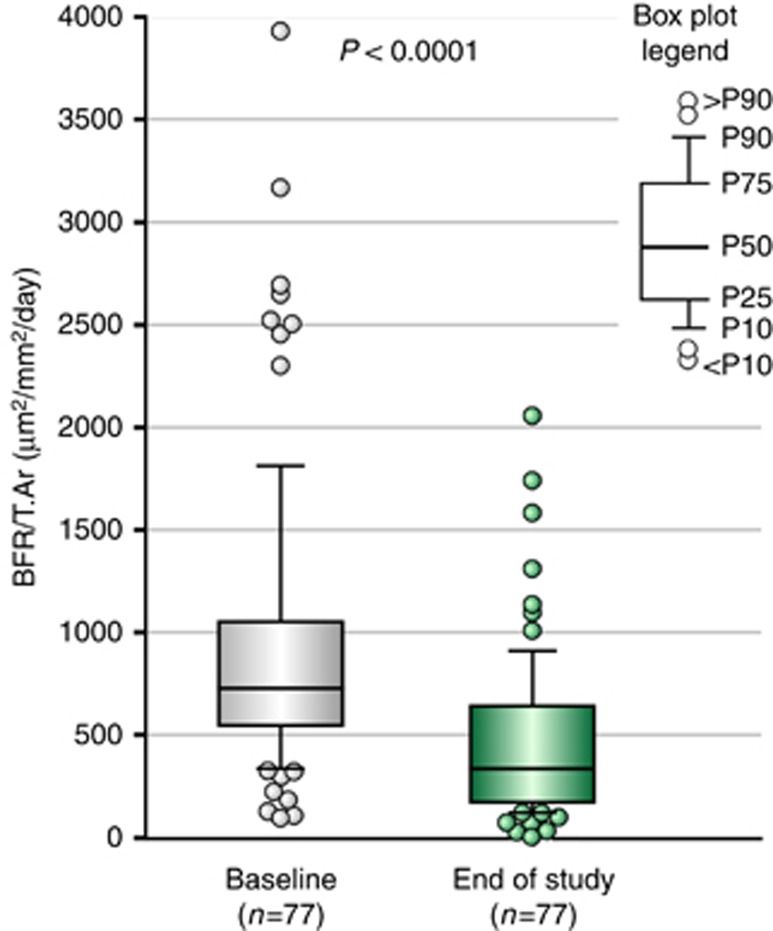
The primary study end point was the change in bone formation rate/tissue area (BFR/T.Ar) from baseline to follow-up during treatment with cinacalcet. The median (IQR) value for BFR/T.Ar at baseline exceeded the upper limit of the normal reference range (97–613 μm2/mm2/day),13 but it decreased from 727.8 (545.9, 1052.6) to 336.1 (172.2, 641.8) μm2/mm2/day at end of study (EOS), P<0.0001 (Figure 4; Table 1). The change in BFR/T.Ar during treatment was greater among subjects with baseline PTH concentrations >800 pg/ml (n=52) compared with those with results ⩽800 pg/ml (n=25); median (IQR) changes were −507 (−748.1, −276.1) μm2/mm2/day and −200 (−369.5, 26.1) μm2/mm2/day, respectively. Subjects who experienced reductions in BFR/T.Ar (n=65) during treatment had higher median (IQR) values at baseline, 816 (595, 1262) μm2/mm2/day, compared with the 12 subjects who did not have a decrease in BFR/T.Ar during follow-up, 425 (154, 592) μm2/mm2/day. The median (IQR) PTH level at baseline also was higher in subjects who had a decrease in BFR/T.Ar during treatment than in those who did not; values were 1113 (693, 1675) and 622 (454, 1110) pg/ml, respectively, at baseline and 536 (313, 959) and 352 (193, 917) pg/ml, respectively, at follow-up in these two groups.
Figure 4.
Primary efficacy end point: comparison of BFR/T.Ar (median-IQR-range) at baseline and end of study. P-value: Wilcoxon signed-rank test. BFR, bone formation rate; T.Ar, tissue area.
Several static histomorphometric measures of bone formation and bone resorption were elevated at baseline but decreased toward normal during treatment (Table 1). The median (IQR) change for osteoblast perimeter (Ob.Pm/O.Pm) was −3.8% (−9.7%, 1.7%), P<0.001, for osteoclast perimeter (Oc.Pm/E.Pm) was −2.1% (−10.6%, 5.8%), P=0.062, and for eroded perimeter (E.Pm/B.Pm) was −3.4% (−6.9%, 0.1%), P<0.001. Bone area/tissue area (B.Ar/T.Ar) did not differ at follow-up compared with baseline. The number of subjects with no peri-trabecular or marrow fibrosis increased from 6 (8%) at baseline to 24 (31%) at EOS (Table 1). Of 71 subjects (92%) with fibrosis at baseline, 21 (27%) had complete resolution at follow-up.
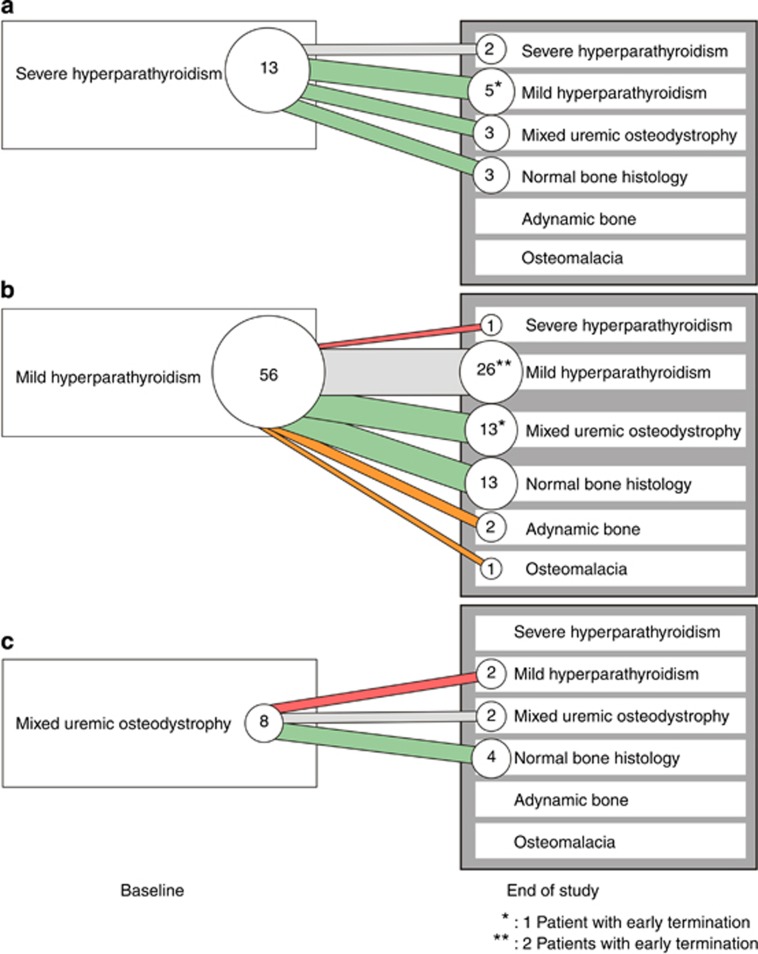
The distribution of the various types of renal osteodystrophy changed substantially during the study (Figure 5). At baseline, most subjects had either mild or severe hyperparathyroid bone disease. At EOS, the number of subjects with severe hyperparathyroid bone disease decreased substantially from 13 to 3. Normal bone histology was found in 20 subjects (26%) overall.
Figure 5.
Evolution of type of renal osteodystrophy after cinacalcet treatment.
The number of subjects with mixed uremic osteodystrophy increased from 8 at baseline to 18 at EOS. These subjects generally had a normal BFR/T.Ar with fibrosis but without evidence of impaired mineralization (type 2), which is generally associated with mixed lesions of type 1. Indeed, BFR/T.Ar at EOS was normal (combined with fibrosis) in 13 subjects with mixed lesions, of whom 10 showed an osteoid area <12%. Two subjects with mixed lesions at follow-up had BFR/T.Ar values below the lower limit of normal but also had fibrosis.
Two subjects had adynamic bone and one had osteomalacia on follow-up bone biopsy (vide infra). None of the subjects showed positive aluminum staining on bone biopsy.
Although biopsies were categorized into the various types of renal osteodystrophy using reference values for BFR/T.Ar obtained originally from children and adolescents, the results did not differ materially when assessed using published values for BFR/T.Ar from adults (Supplementary Figure S1 online).
Biochemistry
As stated previously, PTH levels decreased significantly during treatment with cinacalcet. The changes in PTH (Table 2, Figure 3) correlated positively with changes in BFR/T.Ar (rs: 0.344; P=0.003), E.Pm/B.Pm (rs: 0.310, P=0.007), and trabecular number (rs: 0.318; P=0.006). Serum calcium and Ca x P values were lower compared with baseline during the efficacy assessment phase of the study. The mean (s.e.) changes were −7% (1.1%) and −6.9% (3.1%), respectively. Serum phosphorus levels did not differ.
Values for two biochemical markers of resorption, N-telopeptide (NTx) and tartrate-resistant acid phosphatase (TRAP), and for the bone formation marker osteocalcin (OC) were elevated at baseline but decreased at follow-up (Table 2). The median (IQR) percent changes from baseline to week 52 were −39.1% (−62.5%, −13.9%), P<0.001, for OC, −31.6% (−56.0%, 4.1%), P<0.001, for NTx, and −19.9% (−39.7%, 10.0%), P=0.003, for TRAP. No notable change in BALP was observed.
In the subset of 20 subjects who had normal bone histology at follow-up, median (IQR) PTH values decreased from 884 (603, 1323) to 283 (164, 453) pg/ml (Table 5). The mean percentage changes in NTx, TRAP, and OC in these subjects were somewhat greater compared with the overall treatment group.
Table 5. Biochemical measurementsa in subjects who did or did not have normal bone histology at follow-up.
|
Normal histology (n=20) |
Abnormal histology (n=57) |
|||
|---|---|---|---|---|
| Baseline | End of study | Baseline | End of study | |
| PTH (pg/ml) [10–65] | 884 (603, 1323) | 283 (164, 453) | 1124 (681, 1716) | 606 (319, 1022) |
| BALP (ng/ml) [2.9–22.4] | 36.6 (27.6, 53.0) | 18.9 (13.7, 25.0) | 45.3 (27.3, 90.9) | 34.2 (20.6, 82.8) |
| N-telopeptide (nmol/l) [5.4–24.2] | 253 (166, 464) | 133 (60, 251) | 540 (169, 1364) | 303 (167, 762) |
| TRAP (U/l) [0.49–5.31] | 5.7 (4.1, 8.6) | 4.1 (3.0, 5.3) | 7.1 (5.2, 9.4) | 5.4 (4.1, 7.8) |
| Osteocalcin (ng/ml) [11–55.9] | 296 (215, 480) | 156 (107, 234) | 580 (303, 906) | 366 (195, 633) |
Abbreviations: BALP, bone-specific alkaline phosphatase; PTH, parathyroid hormone; TRAP, tartrate-resistant acid phosphatase.
Numbers in [brackets] denote the lower and upper limit of values for subjects with normal kidney function.
Data are presented as median (interquartile range).
Safety evaluation
A total of 110 subjects received more than one dose of cinacalcet and were included in the safety population. The mean (s.d.) daily dose of cinacalcet at the end of the titration, maintenance, and efficacy assessment phases was 74.1 (43.6), 75.2 (49.4), and 75.0 (45.5) mg/day, respectively.
The most common adverse events (AEs) were nausea (26%), vomiting (21%), diarrhea (20%), arthralgia (14%), hypocalcemia (12%), dyspnea (11%), and muscle spasms (11%). Treatment-related AEs were reported in 47 subjects (43%); these included nausea in 16 (15%), symptomatic hypocalcemia in 12 (11%), vomiting in 10 (9%), dyspepsia in 9 (8%), and diarrhea in 7 (6%). Three subjects had serious treatment-related AEs: symptomatic hypocalcemia, convulsion, and convulsion and symptomatic hypocalcemia, respectively. There were no deaths or life-threatening AEs that were judged to be related to cinacalcet. Only two subjects (2%) had AEs that led to discontinuation of cinacalcet and one subject had an AE that led to withdrawal from the study.
Overall, for the safety population, the mean (s.d.) serum calcium was 10.0 (0.7) mg/dl at baseline, 9.1 (0.9) mg/dl at week 20, and 9.2 (0.9) mg/dl at week 52. The mean (s.e.) percent change from baseline in serum calcium ranged from −11.1% (0.9%) to −5.7% (1.1%) over the course of the study.
Safety end points for bone mineralization were evaluated by comparing biopsy results at screening and at EOS. Mineralization lag time was highly skewed by extreme values resulting from sample testing, the median (IQR) mineralization lag time at baseline and at EOS was 18 (11, 26) and 21 (15, 44) days, respectively.
Double labeled perimeter/bone perimeter (dL.Pm/B.Pm) at baseline was within normal limits in 27 subjects (35%) and elevated in 50 subjects (65%). At EOS, values remained within the normal range in 22 subjects, decreased from elevated values to normal in 28 subjects, and remained elevated in 22 subjects. In the five remaining subjects with initially normal values for dL.Pm/B.Pm, results at follow-up were elevated in four and low in one. Overall, median (IQR) osteoid area/bone area (O.Ar/B.Ar) was unchanged from baseline to EOS with values of 5.1% (3.5%, 7.4%) and 3.8% (2.4%, 6.6%), respectively. This parameter was normal at baseline in 41 subjects (53%) and remained normal in 31 subjects (40%) at EOS. Values were normal initially and became elevated in 10 subjects (13%) and decreased from elevated values to normal in 20 subjects (26%), whereas O.Ar/B.Ar was elevated both at baseline and at EOS in 16 subjects (21%).
Two subjects developed adynamic bone as documented by EOS bone biopsy findings of O.Ar/B.Ar <12%, BFR/T.Ar <97 μm2/mm2/day, and no evidence of fibrosis. Both subjects had risk factors for adynamic bone that included a normal BFR/T.Ar at baseline with values of 385 and 398 μm2/mm2/day, respectively, and only minimal signs of fibrosis that supported a study diagnosis of hyperparathyroid bone disease. Both subjects received calcium-containing phosphate binders and had PTH values ⩽100 pg/ml during the study that required reductions in the dose of cinacalcet. At EOS, BFR/T.Ar was low but measureable with values of 25 and 32 μm2/mm2/day, respectively. One patient had a PTH of 331 pg/ml at screening that decreased to 198 pg/ml prior to the start of cinacalcet. Treatment was finally withdrawn at week 45 because PTH levels were persistently <150 pg/ml. BALP also decreased from 21.5 to 14.6 ng/ml during the study. Advanced age, type 2 diabetes, and treatment with a vitamin D sterol were additional potential contributors. In the other patient, PTH decreased from 711 to 236 pg/ml and BALP decreased from 63.2 to 9.3 ng/ml over the course of the study, but PTH was as low as 106 pg/ml during the titration phase.
An additional subject developed osteomalacia. At baseline, bone biopsy showed mild hyperparathyroid bone disease with BFR/T.Ar of 697 μm2/mm2/day, E.Pm/B.Pm of 11.5%, and some fibrosis (1–5%). At EOS, fibrosis area was unchanged, but BFR/T.Ar decreased to 71 μm2/mm2/day and E.Pm/B.Pm decreased to 2.4%. Mineralization lag time increased from 36 days at baseline to 894 days at follow-up, and O.Ar/B.Ar increased from 5.0% to 30.8%. Overt hypophosphatemia was present both at baseline and recurrently during follow-up.
DISCUSSION
Cinacalcet has been shown to lower plasma PTH levels in a number of clinical trials among patients undergoing dialysis,5, 6, 7 but the effects of treatment on the histological features of sHPT in bone have not been characterized adequately. The BONAFIDE study was designed to address this matter prospectively in a multicenter, single-arm, open-label trial. Treatment with cinacalcet for at least 6 months decreased the initially elevated mean value for BFR/T.Ar toward normal and reduced mean values for other parameters of bone histomorphometry that are typically abnormally high in patients with established sHPT. The observed changes in bone histology corresponded with interval reductions in PTH levels and with decreases in the serum concentrations of several biochemical markers of bone turnover.
Of 146 subjects with PTH ⩾300 pg/ml, BALP >20.9 ng/ml, and calcium ⩾8.4 mg/dl who underwent bone biopsy, 25 were excluded from study because they did not have histomorphometric evidence of high-turnover bone disease, a cardinal feature of sHPT. This finding is consistent with reports from others that have highlighted the limitations of using a single PTH measurement to differentiate between high-turnover and low-turnover skeletal lesions of renal osteodystrophy.14, 15 Indeed, reliance upon single PTH and BALP values rather than two or more measurements of each parameter on separate days to determine subject eligibility for the current study represents a potential shortcoming of the project. Nevertheless, the observation that no cases of adynamic bone were identified among subjects with PTH ⩾300 pg/ml and BALP >20.9 ng/ml in this large multicenter bone biopsy study suggests that adynamic renal osteodystrophy is highly unlikely among dialysis patients with this combination of biochemical findings.
During treatment with cinacalcet, there was a significant decrease in PTH from baseline, P<0.001, together with a substantial reduction in BFR/T.Ar. The changes in BFR/T.Ar were most pronounced among subjects with baseline PTH values >800 pg/ml. The strength of the relationship between changes in PTH and BFR/T.Ar during treatment was only modest as documented by the Spearman rank correlation coefficient of 0.344. Baseline median (IQR) values for BFR/T.Ar were higher among subjects who experienced a reduction in bone formation during treatment compared with those who did not, 816 (595, 1262) vs. 425 (154, 592) μm2/mm2/day. Such findings suggest that the likelihood of developing low-turnover skeletal lesions or adynamic bone is low among patients with CKD undergoing dialysis when cinacalcet is used to treat sHPT for as long as 12 months.
Of 71 subjects with evidence of peri-trabecular and/or marrow fibrosis at baseline, 21 had complete resolution of this finding at follow-up. Twenty subjects had normal bone histology at the follow-up biopsy. The median (IQR) PTH in these 20 subjects decreased from 884 (603, 1323) pg/ml at baseline to 283 (164, 453) pg/ml at follow-up, and median (IQR) BALP decreased from an initially elevated level of 36.6 (27.6, 53.0) ng/ml to 18.9 (13.7, 25.0) ng/ml, a value within normal limits. The serum concentrations of NTx, OC, and TRAP also decreased during treatment in this subgroup to levels that approached the normal range.
Bone mineralization was assessed by measuring mineralization lag time, O.Ar/B.Ar, and osteoid seam width. Values for mineralization lag time are high in patients with osteomalacia, in many cases of mixed uremic osteodystrophy, and in patients with adynamic bone.16 In the current study, median (IQR) mineralization lag time increased slightly from 18 (11, 26) days to 21 (15, 44) days, a change consistent with the observed reduction in BFR/T.Ar. Median values for O.Ar/B.Ar and for osteoid seam width did not increase during treatment. Accordingly, the use of cinacalcet to lower PTH levels in the current study reduced bone formation and turnover without adversely affecting skeletal mineralization.
The large number of subjects classified as having mixed uremic osteodystrophy reflects the relatively broad criteria used by the pathologist to define subjects with mixed lesions, i.e., subjects showing the classical picture of normal or high turnover in combination with a mineralization defect (type 1), as well as those showing normal bone turnover and fibrosis in the absence of a mineralization defect (type 2). Elevated values for dL.Pm/B.Pm, a finding consistent with high-turnover bone disease, were present in 65% of subjects at baseline but became normal in 56% (28/50) of these subjects at EOS. Such results are common among patients treated medically for sHPT. Changes in other secondary study end points that included several biochemical markers of bone turnover such as BALP, OC, NTx, and TRAP further suggest a favorable effect of cinacalcet therapy on bone formation and turnover among patients with sHPT.
Two subjects had BFR/T.Ar values below the lower limit of normal and other histomorphometric findings consistent with adynamic bone after 12 months of treatment with cinacalcet. Both had risk factors for adynamic bone at baseline; these included diabetes in one and advanced age exceeding 80 years in the other. Additional contributory factors in each case included concurrent treatment with calcium-containing phosphate-binding agents and vitamin D analogs. Notably, BFR/T.Ar was within the normal range at baseline in both subjects, and both developed PTH concentrations <150 pg/ml during the study. Very low PTH levels were seen on several occasions in one case, requiring reductions in the dose of cinacalcet. Cinacalcet therapy was withdrawn in the other subject because PTH values remained persistently below 150 pg/ml.
Such findings underscore the importance of regularly monitoring PTH levels and appropriately adjusting doses of cinacalcet, vitamin D, and/or calcium as needed to avoid lowering PTH levels excessively during the treatment of sHPT. Adynamic bone is seen most often among patients with CKD who have persistently low PTH levels, but the disorder can be corrected when measures that suppress parathyroid gland function are modified or withdrawn.17, 18 In this regard, the half-life of cinacalcet in serum is relatively short with an average of 32 hours, and plasma PTH levels increase within a few days after daily doses of cinacalcet are withheld.19 The capacity to correct parathyroid gland suppression and to allow PTH levels to rise after treatment with cinacalcet is interrupted thus diminishes the risk of developing adynamic bone when therapy is monitored appropriately. Apart from measurements of PTH,20, 21 low levels of BALP (⩽10 ng/ml) provide an additional biochemical marker of adynamic bone among patients undergoing hemodialysis.14, 15, 22
An additional subject in the current study developed osteomalacia during treatment with cinacalcet. Aluminum staining was negative on bone biopsy. For reasons that are unclear, serum phosphorus concentrations at baseline were low, and episodes of hypophosphatemia occurred repeatedly during follow-up, findings distinctly unusual in the setting of dialysis. Hypophosphatemia is a recognized cause of osteomalacia, and its presence should have precluded entry of this subject into the study.
Of the 110 subjects enrolled, 84 (76%) completed the study and 26 (24%) discontinued the study, most commonly (n=11) for protocol-specified criteria including parathyroidectomy, renal transplantation, AEs, or persistent hypocalcemia. Seventy-seven subjects successfully underwent a follow-up bone biopsy with quantitative histomorphometry after receiving at least 6, but no more than 12, months of cinacalcet treatment. The AEs reported in this study were generally mild or moderate and similar to those reported in other cinacalcet clinical trials in adult patients. Only 2 subjects (2%) had AEs that led to discontinuation of cinacalcet and 1 patient had an AE that led to withdrawal from the study.
A limitation of the current study is the inability to determine how much of the treatment effect was attributable to cinacalcet and how much to vitamin D. In randomized trials that have compared treatment with cinacalcet or vitamin D where most subjects were receiving vitamin D at baseline, the effect of treatment on biochemical parameters has been greater in the cinacalcet arm.7, 23, 24 In the current study, many subjects in the efficacy analysis set were receiving vitamin D at baseline and most received vitamin D during the study, so the primary change was the use of cinacalcet. This suggests that most of the observed effect on bone was due to cinacalcet, but the study was not designed to answer this question.
A second potential limitation of the study is the use of reference data for bone histomorphometry generated originally in biopsy specimens obtained from healthy children and adolescents to assess biopsies from older adult subjects with sHPT undergoing dialysis. Although the rates of bone formation and remodeling may be modestly higher in younger persons than in adults, there is considerable overlap among values as determined using the technique of double tetracycline-labeling in cancellous bone from the iliac crest.25, 26 In this regard, only modest differences in the distribution of subjects according to various types of renal bone disease were observed when published values for BFR/T.Ar in adults were used separately to categorize subjects in the current study both at baseline and at EOS. In addition, any purported age-related differences in bone formation would not account for the interval changes in bone formation and turnover observed in the current study during treatment with cinacalcet.
In conclusion, high rates of bone formation and several biochemical markers of high-turnover bone disease decreased toward normal as PTH was reduced during the treatment of sHPT with cinacalcet. Twenty-six percent of subjects had normal bone histology after 12 months of treatment, but histological abnormalities persisted in many subjects. As reported previously, adynamic bone can occur if PTH levels are lowered persistently to values below currently recommended ranges.
MATERIALS AND METHODS
Study subjects
Subjects were eligible for study if they were aged ⩾18 years, had CKD requiring dialysis, and were willing to undergo a bone biopsy procedure pending results of biochemical determinations obtained during screening (vide infra). Laboratory inclusion criteria were PTH ⩾300 pg/ml, BALP >20.9 ng/ml, and serum calcium ⩾8.4 mg/dl.
Candidates were excluded from study if they had an unstable medical condition, were pregnant or nursing, had undergone surgical parathyroidectomy within the previous 3 months, or had a change in the dose of vitamin D sterol within the past 30 days. Additional exclusion criteria included treatment with cinacalcet, bisphosphonate, or teriparatide within the previous 30 days.
Study design
This single-arm, multicenter study consisted of 4 phases: a screening phase lasting up to 3 months, a 20-week dose titration phase, a 20-week maintenance phase, and a 12-week efficacy assessment phase (Figure 1). Subjects entered into the study and were treated with cinacalcet if the bone biopsy obtained during screening showed changes consistent with hyperparathyroid bone disease using the following histomorphometric criteria: (1) O.Ar/B.Ar ⩽12% and BFR/T.Ar >613 μm2/mm2/day with no peri-trabecular or marrow fibrosis; (2) O.Ar/B.Ar ⩽12% and BFR/T.Ar >97 μm2/mm2/day with evidence of fibrosis; or (3) O.Ar/B.Ar >12% and BFR/T.Ar >97 μm2/mm2/day with or without fibrosis. A second bone biopsy was obtained after 48–52 weeks of treatment or after cinacalcet was withdrawn for subjects treated for less than 12 but more than 6 months.
Treatment
Treatment with cinacalcet was initiated at a dose of 30 mg once daily. Doses were titrated upwards sequentially at 4-week intervals as tolerated to 60, 90, or 120 mg/day or to a maximum of 180 mg/day if PTH values were ⩾300 pg/ml and serum calcium levels were ⩾8.0 mg/dl. The objective was to achieve and maintain PTH between >150 and <300 pg/ml. If PTH was <150 pg/ml, doses were reduced to the next lowest dose or withheld for subjects already receiving the lowest dose of cinacalcet. Cinacalcet also was withheld if serum calcium was <7.5 mg/dl or symptoms of hypocalcemia developed. Dosing was resumed when PTH levels again exceeded 300 pg/ml.
Concurrent treatment with vitamin D sterols was allowed throughout the study. Although not permitted during screening, adjustments to the dose of vitamin D could be made thereafter based upon measured values for PTH, serum calcium, phosphorus, and Ca x P. Changes to the doses of calcium supplements and/or phosphate-binding agents were made at the discretion of the investigator in accordance with existing standards of clinical practice.
The study was conducted at 30 sites located in the United States and Europe in compliance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee at each study site, and written informed consent obtained from all participants.
Bone biopsy and bone histomorphometry
Trans-iliac crest bone biopsies measuring approximately 1 cm in length and 4–7 mm in core diameter were obtained after double tetracycline labeling using a Bordier trephine in all study participants during screening. Biopsies were repeated at EOS for subjects who received at least 6 months of treatment with cinacalcet. Follow-up biopsy procedures were conducted between weeks 48 and 52 or as soon as possible after treatment was stopped for subjects who received cinacalcet for at least 6 months.
The method for quantitative histomorphometry of bone has been described elsewhere.15 Biopsy specimens were fixed in absolute ethanol and embedded in a methylmethacrylate-based resin. Undecalcified 5-μm thick sections were stained by the method of Goldner for quantitative histology to determine static bone parameters. Ten-μm thick sections were mounted unstained in 100% glycerol for fluorescence microscopy and visualization of tetracycline labels. The results were used to calculate dynamic parameters of bone turnover. All results are reported as measurements in two dimensions using nomenclature established by the American Society for Bone and Mineral Research.27, 28 The various types of renal osteodystrophy were categorized using histomorphometric criteria as reported previously.11, 13, 14, 15
Bone analysis was performed in the Laboratory of Pathophysiology of the University of Antwerp, Belgium, using the semi-automatic AxioVision system. Key parameters that were assessed included bone formation rate on tissue area (BFR/T.Ar) (μm2/mm2/day), mineralization lag time (days), perimeter of active osteoblasts on osteoid perimeter (Ob.Pm/O.Pm) (%), perimeter of active osteoclasts on eroded perimeter (Oc.Pm/E.Pm) (%), eroded perimeter on bone perimeter (E.Pm/B.Pm) (%), double tetracycline-labeled bone perimeter on bone perimeter (dL.Pm/B.Pm), fibrosis area on tissue area (Fb.Ar/T.Ar) (%), bone area on tissue area (B.Ar/T.Ar) (%), osteoid area on bone area (O.Ar/B.Ar) (%), osteoid width (μm), and aluminum stained perimeter on bone perimeter (%). Osteoid seams less than 2 μm in width were not included in primary measurements of osteoid width or area.
The various types of renal osteodystrophy were classified using prespecified criteria as reported elsewhere using several histomorphometric parameters including O.Ar/B.Ar, BFR/T.Ar, and Fb.Ar/T.Ar11, 13, 14, 15 as follows: normal bone histology: O.Ar/B.Ar ⩽12%, BFR/T.Ar ⩾97, and ⩽613 μm2/mm2/day, without fibrosis; mild hyperparathyroid bone disease: O.Ar/B.Ar ⩽12%, upper normal or increased BFR/T.Ar (>613 μm2/mm2/day), with 0–5% fibrosis; severe hyperparathyroid bone disease (osteitis fibrosa): O.Ar/B.Ar ⩽12%, BFR/T.Ar >613 μm2/mm2/day, with fibrosis >5% mixed uremic osteodystrophy: O.Ar/B.Ar >12%, BFR/T.Ar >97 μm2/mm2/day, with or without fibrosis (type 1) or O.Ar/B.Ar ⩽12%, BFR/T.Ar ⩽613 μm2/mm2/day, with fibrosis (type 2); adynamic bone disease: O.Ar/B.Ar ⩽12%, BFR/T.Ar <97 μm2/mm2/day, without fibrosis; and osteomalacia: O.Ar/B.Ar >12%, BFR/T.Ar <97 μm2/mm2/day, without fibrosis.
Study end points
Efficacy end points
The primary efficacy end point was the change from baseline in BFR/T.Ar. Secondary efficacy end points included (i) change from baseline in Ob.Pm/O.Pm and Oc.Pm/E.Pm, (ii) change from baseline in E.Pm/B.Pm and Fb.Ar/T.Ar, (iii) percent change from baseline in PTH, BALP, OC, NTx, and TRAP, and (iv) percent change from baseline in serum calcium, phosphorus, and Ca x P concentrations.
Safety end points
Safety end points included the following: (i) change from baseline in mineralization lag time, osteoid area/volume, and osteoid width/thickness; (ii) the occurrence of adynamic bone as defined by osteoid area ⩽12% and BFR/T.Ar <97 μm2/mm2/day with no evidence of fibrosis; (iii) the nature, frequency, severity and relationship to treatment of AEs; and (iv) changes in laboratory parameters.
Biochemical determinations
Plasma PTH (PTH) levels were measured using an immunometric assay (Advia Centaur) that detects both the full-length hormone and smaller peptide fragments. Serum calcium and phosphorus levels were measured by methods reported previously.7 Additional markers of bone turnover included total alkaline phosphatase (ALP, colorimetric assay), BALP (two-site Tandem-R-Ostase immunoradiometric assay), OC (electro-chemiluminescence immunoassay; ECLIA), NTx (enzyme-linked immunosorbent assay; ELISA), and TRAP5b (ELISA). TRAP5b was measured at Pacific Biometrics, Seattle, whereas the other biochemical analyses were performed at Covance central laboratories in Indianapolis and Geneva, Switzerland.
Statistical analysis
The focus of this study was to estimate the change in BFR/T.Ar after 1 year of cinacalcet treatment. All efficacy and bone safety end points were analyzed using the efficacy data set (subjects who had both screening and EOS bone biopsies). Biochemical parameters and baseline bone histomorphometry parameters were further analyzed using the safety data set (subjects who had received at least 1 dose of cinacalcet). Because of the small number of subjects enrolled per center, the analyses were based on pooled data from all study centers. All efficacy and safety end points were summarized using descriptive statistics such as n, mean, s.d., s.e., median, IQR, min, and max. Median and IQR are presented because data were not normally distributed. In exploratory analyses, a one-sample t-test was used to evaluate the mean change or mean percent change from baseline in bone histomorphometry and biochemical parameters. The Wilcoxon signed-rank test was used to compare median values at baseline and EOS. Because the study was not controlled and contained only one group, the probability values presented are solely descriptive in nature and should be considered nominal only. Spearman's correlation was used to examine the relationship between PTH and bone histomorphometric parameters. Statistical analyses were performed using SAS version 9.2.
Acknowledgments
Publication management support was provided by Caterina Hatzifoti and Lucy Hyatt of Amgen (Europe) GmbH. Editorial support was provided by Julia Balfour, Medical Writer/Consultant, Dundee, on behalf of Amgen (Europe) GmbH, and Lucy Hyatt of Amgen (Europe) GmbH. Our thanks to the many investigators who contributed biopsies to this study: Belgium: Pieter Evenepoel, Universitair Ziekenhuis Gasthuisberg, Leuven; Jean-Marie Krzesinski, Centre Hospitalier Universitaire de Liège, Liege; Christian Tielemans, Hôpital Erasme, Bruxelles. Czech Republic: Frantisek Svara, Interni oddeleni Strahov VFN, Prague; Sylvie Sulkova, Fakultni nemocnice Hradec Kralove, Hradec Kralove. Hungary: Gyorgy Deak, Semmelweis Egyetem I. sz., Budapest; Janos Szegedi, Josa Andras Oktatokorhaz Egeszsegugyi Szolgaltato Nonprofit Kft., Nyiregyhaza. Italy: Renato Lauro, Università Tor Vergata di Roma, Rome; Fabio Malberti, Istituti Ospedalieri, Cremona; Salvatore Di Giulio, Azienda Ospedaliera San Camillo Forlanini, Rome; Antonio Stingone, Ospedale G. Bernabeo, Ortona; Walter De Simone, Azienda Ospedaliera San Giuseppe Moscati, Avellino. Former Yugoslav Republic of Macedonia: Goce Spasovski, University of Skopje, Skopje. Poland: Wladyslaw Sulowicz, University Hospital of Krakow, Krakow; Michal Nowicki, Uniwersytecki Szpital Kliniczny nr 1 im. Norberta Barlickiego, Lodz; Marian Klinger, Akademicki Szpital Kliniczny im. Jana Mikulicza-Radeckiego we Wroclawiu, Wroclaw. Portugal: Joao Frazao, Diaverum Investimentos e Serviços Lda.– Unidade da Prelada, Porto; Jorge Baldaia, Centro de Hemodiálise de Guimarães (Uninefro, SA), Guimarães; Anibal Ferreira, Hemodial Centro de Diálise Renal Lda (FMC—Vila Franca de Xira), Vila Franca de Xira; Silvia Ribeiro, Hospital Particular De Almada, Almada; Antonio Sarmento, Centro Medico Doencas Renais (CMDR), Porto; Teresa Adragao, Nefroclinica—Centro De Hemodialise Do Estoril, Estoril. Spain: Ángel Luís Martín de Francisco, Hospital Universitario Marqués de Valdecilla, Santander; Higini Cao, Hospital del Mar, Barcelona. Switzerland: Rudolf Wuethrich, Universitätsspital Zurich, Zurich. UK: Alastair Hutchison, Manchester Royal Infirmary, Manchester USA: Chaim Charytan, Nephrology Associates, Private Corporation, Flushing, NY; Daniel Coyne, Washington University School of Medicine, St Louis, MO; Fred Husserl, Ochsner Health Systems, New Orleans, LA; David Spiegel, University of Colorado, Denver, CO; Azzour Hazzan, North Shore University Hospital, Great Neck, NY; Bharat Gupta, Horizon Institute For Clinical Research, Fort Lauderdale, FL; Fredric Finkelstein, Metabolism Associates, PC, New Haven, CT; Sungchun Lee Arizona Kidney Disease and Hypertension Center, Phoenix, AZ; Stuart Sprague, North Shore University Health System, Skokie Hospital, Evanston, IL; Mohamed Atta, Johns Hopkins University School of Medicine, Baltimore, MD; Louis Jan, Holy Name Hospital, Teaneck, NJ. Turkey: Mustafa Cirit, İzmir Atatürk Eğitim ve Araştırma Hastanesi, Izmir.
The BONAFIDE study was sponsored by Amgen Inc. LRS, WGG, and DMS are employees of, and may hold stock and/or stock options in, Amgen Inc., USA. PCD'H has received research grants from Amgen, Vifor Pharma, Novartis, Fresenius Medical Care, Diasorin, Shire Pharmaceuticals, Baxter Healthcare, Sanifit, Janssen Pharmaceutica. All the remaining authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. Distribution of the various types of renal osteodystrophy at baseline (left panel) and at the end of study (right panel) after treatment with cinacalcet. Lesions were categorized using reference values for bone formation (BFR/T.Ar) as determined from healthy children and adolescents or using published values for BFR/T.Ar from normal adults.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group KDIGO Clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76 (Suppl 113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SM, Saifullah A, LaClair RE, et al. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:299–306. doi: 10.2215/CJN.07131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WG, Hladik GA, Turner SA, et al. The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol. 2002;13:1017–1024. doi: 10.1681/ASN.V1341017. [DOI] [PubMed] [Google Scholar]
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- EVOLVE Trial Investigators. Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- Brickman AS, Sherrard DJ, Jowsey J, et al. 1,25-dihydroxycholecalciferol. Effect on skeletal lesions and plasma parathyroid hormone levels in uremic osteodystrophy. Arch Intern Med. 1974;134:883–888. doi: 10.1001/archinte.134.5.883. [DOI] [PubMed] [Google Scholar]
- Andress DL, Norris KC, Coburn JW, et al. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989;321:274–279. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- Salusky IB, Kuizon BD, Belin TR, et al. Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int. 1998;54:907–914. doi: 10.1046/j.1523-1755.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Monier-Faugere M-C, Wang G, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin.Nephrol. 2008;69:269–278. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- Sanchez CP, Salusky IB, Kuizon BD, et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998;53:1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- Couttenye MM, D'Haese PC, Van Hoof VO, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1065–1072. [PubMed] [Google Scholar]
- Bervoets AR, Spasovski GB, Behets GJ, et al. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am J Kidney Dis. 2003;41:997–1007. doi: 10.1016/s0272-6386(03)00197-5. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Curr Opin Nephrol Hypertens. 2003;12:387–403. doi: 10.1097/00041552-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Montenegro J, Saracho R, González O, et al. Reversibility of parathyroid gland suppression in CAPD patients with low i-PTH levels. Clin Nephrol. 1997;48:359–363. [PubMed] [Google Scholar]
- Haris A, Sherrard DJ, Hercz G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006;70:931–937. doi: 10.1038/sj.ki.5001666. [DOI] [PubMed] [Google Scholar]
- Arenas MD, de la Fuente V, Delgado P, et al. Pharmacodynamics of cinacalcet over 48 hours in patients with controlled secondary hyperparathyroidism: useful data in clinical practice. J Clin Endocrinol Metab. 2013;98:1718–1725. doi: 10.1210/jc.2012-4003. [DOI] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Moyses RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- Garrett G, Sardiwal S, Lamb EJ, et al. PTH—a particularly tricky hormone: why measure it at all in kidney patients. Clin J Am Soc Nephrol. 2013;8:299–312. doi: 10.2215/CJN.09580911. [DOI] [PubMed] [Google Scholar]
- Urena P, Hruby M, Ferreira A, et al. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol. 1996;7:506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- Messa P, Macario F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña-Torres P, Bridges I, Christiano C, et al. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol Dial Transplant. 2013;28:1241–1254. doi: 10.1093/ndt/gfs568. [DOI] [PubMed] [Google Scholar]
- Han ZH, Palnitkar S, Rao DS, et al. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12:498–508. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Travers R, Rauch F, et al. Structural and cellular changes during bone growth in healthy children. Bone. 2000;27:487–494. doi: 10.1016/s8756-3282(00)00353-7. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Mineral Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Mineral Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.