Abstract
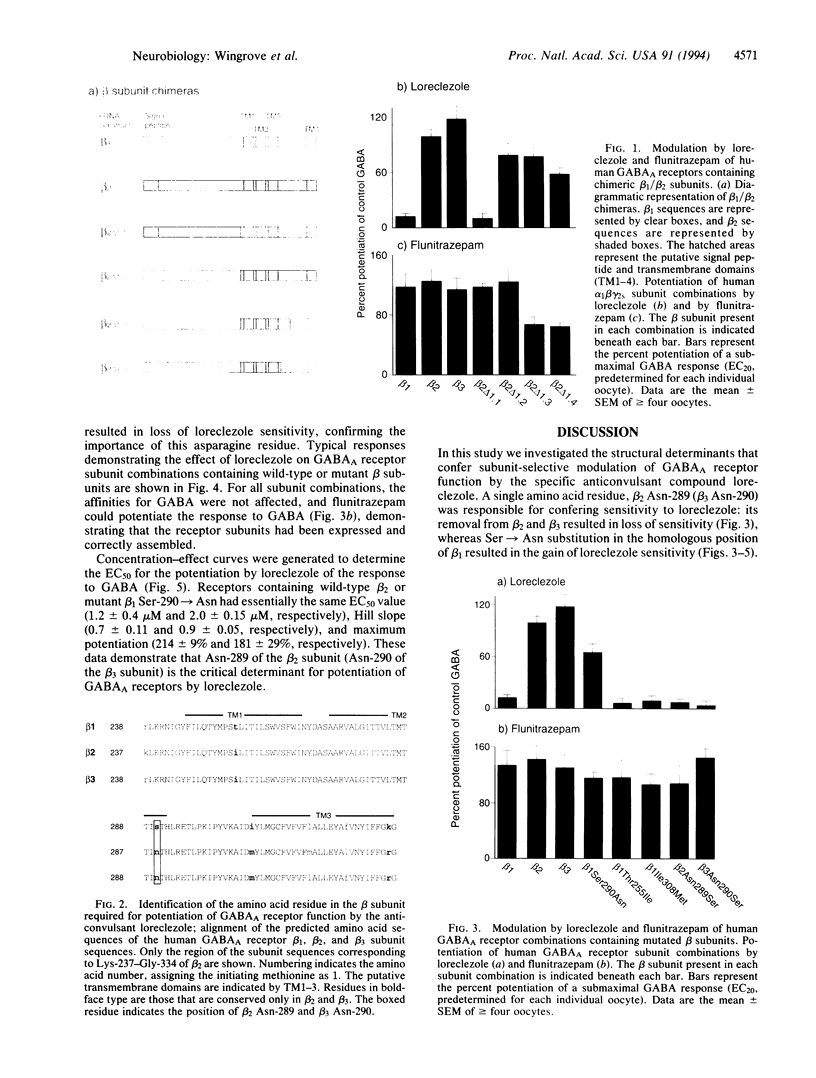
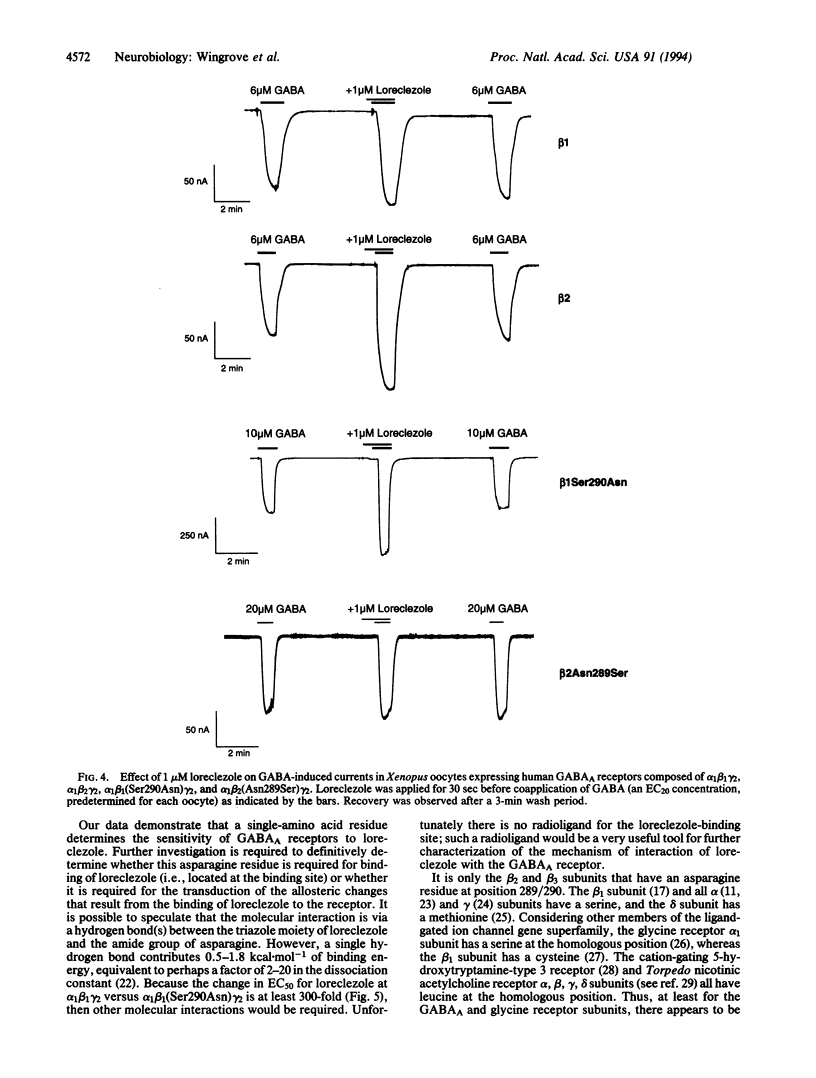
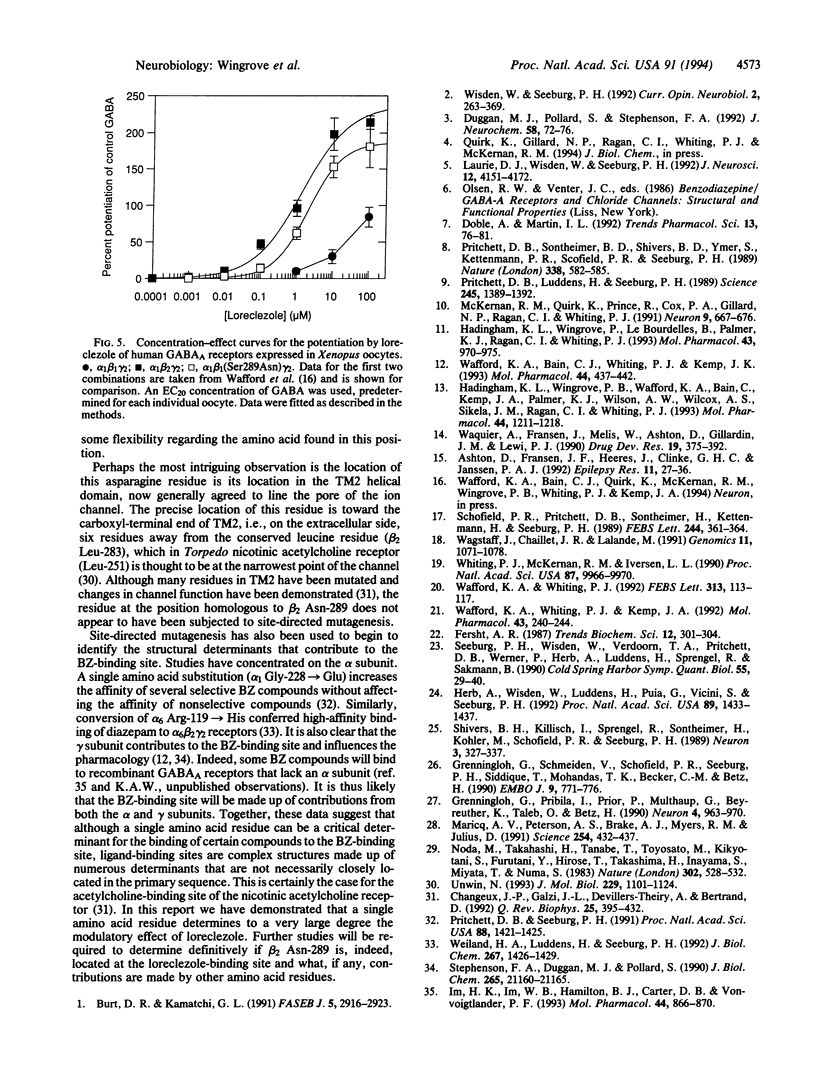
Type A gamma-aminobutyric acid (GABAA) receptors of the mammalian nervous system are a family of ligand-gated ion channels probably formed from the coassembly of different subunits (alpha 1-6, beta 1-3, gamma 1-3, delta) in the arrangement alpha beta gamma or alpha beta delta. The activation of these receptors by GABA can be modulated by a range of compounds acting at distinct allosteric sites. One such compound is the broad-spectrum anticonvulsant loreclezole, which we have recently shown to act via a specific modulatory site on the beta subunit of the GABAA receptor. The action of loreclezole depends on the type of beta subunit present in the receptor complex; receptors containing beta 2 or beta 3 subunits have > 300-fold higher affinity for loreclezole than receptors containing a beta 1 subunit. We have used this property to identify the amino acid residue in the beta subunit that determines the subunit selectivity of loreclezole. Chimeric beta 1/beta 2 human GABAA receptor subunits were constructed and coexpressed in Xenopus oocytes with human alpha 1 and gamma 2s subunits. The chimera beta 1/beta 2Lys237-Gly334 conferred sensitivity to 1 microM loreclezole. Within this region there are four amino acids that are conserved in beta 2 and beta 3 but differ in beta 1. By mutating single amino acids of the beta 1 subunit to the beta 2/beta 3 equivalent, only the beta 1 mutation of Ser-290-->Asn conferred potentiation by loreclezole. Similarly, mutation of the homologous residue in the beta 2 and beta 3 subunits to the beta 1 equivalent (Asn-->Ser) resulted in loss of sensitivity to loreclezole. The affinity for GABA and the potentiation by flunitrazepam were unchanged in receptors containing the mutated beta subunits. Thus, a single amino acid, beta 2 Asn-289 (beta 3 Asn-290), located at the carboxyl-terminal end of the putative channel-lining domain TM2, confers sensitivity to the modulatory effects of loreclezole.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton D., Fransen J., Heeres J., Clincke G. H., Janssen P. A. In vivo studies on the mechanism of action of the broad spectrum anticonvulsant loreclezole. Epilepsy Res. 1992 Mar;11(1):27–36. doi: 10.1016/0920-1211(92)90018-o. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Galzi J. L., Devillers-Thiéry A., Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992 Nov;25(4):395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- Doble A., Martin I. L. Multiple benzodiazepine receptors: no reason for anxiety. Trends Pharmacol Sci. 1992 Feb;13(2):76–81. doi: 10.1016/0165-6147(92)90027-4. [DOI] [PubMed] [Google Scholar]
- Duggan M. J., Pollard S., Stephenson F. A. Quantitative immunoprecipitation studies with anti-gamma-aminobutyric acidA receptor gamma 2 1-15 Cys antibodies. J Neurochem. 1992 Jan;58(1):72–77. doi: 10.1111/j.1471-4159.1992.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham K. L., Wingrove P. B., Wafford K. A., Bain C., Kemp J. A., Palmer K. J., Wilson A. W., Wilcox A. S., Sikela J. M., Ragan C. I. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993 Dec;44(6):1211–1218. [PubMed] [Google Scholar]
- Hadingham K. L., Wingrove P., Le Bourdelles B., Palmer K. J., Ragan C. I., Whiting P. J. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993 Jun;43(6):970–975. [PubMed] [Google Scholar]
- Herb A., Wisden W., Lüddens H., Puia G., Vicini S., Seeburg P. H. The third gamma subunit of the gamma-aminobutyric acid type A receptor family. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1433–1437. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H. K., Im W. B., Hamilton B. J., Carter D. B., Vonvoigtlander P. F. Potentiation of gamma-aminobutyric acid-induced chloride currents by various benzodiazepine site agonists with the alpha 1 gamma 2, beta 2 gamma 2 and alpha 1 beta 2 gamma 2 subtypes of cloned gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993 Oct;44(4):866–870. [PubMed] [Google Scholar]
- Laurie D. J., Wisden W., Seeburg P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992 Nov;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peterson A. S., Brake A. J., Myers R. M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991 Oct 18;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- McKernan R. M., Quirk K., Prince R., Cox P. A., Gillard N. P., Ragan C. I., Whiting P. GABAA receptor subtypes immunopurified from rat brain with alpha subunit-specific antibodies have unique pharmacological properties. Neuron. 1991 Oct;7(4):667–676. doi: 10.1016/0896-6273(91)90379-e. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Lüddens H., Seeburg P. H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989 Sep 22;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Seeburg P. H. gamma-Aminobutyric acid type A receptor point mutation increases the affinity of compounds for the benzodiazepine site. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1421–1425. doi: 10.1073/pnas.88.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Pritchett D. B., Sontheimer H., Kettenmann H., Seeburg P. H. Sequence and expression of human GABAA receptor alpha 1 and beta 1 subunits. FEBS Lett. 1989 Feb 27;244(2):361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Wisden W., Verdoorn T. A., Pritchett D. B., Werner P., Herb A., Lüddens H., Sprengel R., Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harb Symp Quant Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Stephenson F. A., Duggan M. J., Pollard S. The gamma 2 subunit is an integral component of the gamma-aminobutyric acidA receptor but the alpha 1 polypeptide is the principal site of the agonist benzodiazepine photoaffinity labeling reaction. J Biol Chem. 1990 Dec 5;265(34):21160–21165. [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Whiting P. J., Kemp J. A. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993 Aug;44(2):437–442. [PubMed] [Google Scholar]
- Wafford K. A., Whiting P. J. Ethanol potentiation of GABAA receptors requires phosphorylation of the alternatively spliced variant of the gamma 2 subunit. FEBS Lett. 1992 Nov 23;313(2):113–117. doi: 10.1016/0014-5793(92)81424-k. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Whiting P. J., Kemp J. A. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993 Feb;43(2):240–244. [PubMed] [Google Scholar]
- Wagstaff J., Chaillet J. R., Lalande M. The GABAA receptor beta 3 subunit gene: characterization of a human cDNA from chromosome 15q11q13 and mapping to a region of conserved synteny on mouse chromosome 7. Genomics. 1991 Dec;11(4):1071–1078. doi: 10.1016/0888-7543(91)90034-c. [DOI] [PubMed] [Google Scholar]
- Whiting P., McKernan R. M., Iversen L. L. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland H. A., Lüddens H., Seeburg P. H. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992 Jan 25;267(3):1426–1429. [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. GABAA receptor channels: from subunits to functional entities. Curr Opin Neurobiol. 1992 Jun;2(3):263–269. doi: 10.1016/0959-4388(92)90113-y. [DOI] [PubMed] [Google Scholar]




