Abstract
Objective: The first international summit on anastomotic leak was held in Chicago in October, 2012 to assess current knowledge in the field and develop novel lines of inquiry. The following report is a summary of the proceedings with commentaries and future prospects for clinical trials and laboratory investigations.
Background: Anastomotic leakage remains a devastating problem for the patient, and a continuing challenge to the surgeon operating on high-risk areas of the gastrointestinal tract such as the esophagus and rectum. Despite the traditional wisdom that anastomotic leak is because of technique, evidence to support this is weak-to-non-existent. Outcome data continue to demonstrate that expert high-volume surgeons working in high-volume centers continue to experience anastomotic leaks and that surgeons cannot predict reliably which patients will leak.
Methods: A one and one-half day summit was held and a small working group assembled to review current practices, opinions, scientific evidence, and potential paths forward to understand and decrease the incidence of anastomotic leak.
Results: Results of a survey of the opinions of the group demonstrated that the majority of participants believe that anastomotic leak is a complicated biologic problem whose pathogenesis remains ill-defined. The group opined that anastomotic leak is underreported clinically, it is not because of technique except when there is gross inattention to it, and that results from animal models are mostly irrelevant to the human condition.
Conclusions: A fresh and unbiased examination of the causes and strategies for prevention of anastomotic leak needs to be addressed by a continuous working group of surgeons, basic scientists, and clinical trialists to realize a real and significant reduction in its incidence and morbidity. Such a path forward is discussed.
Despite new antibiotics and improvements in surgical technique, anastomotic leaks persist and remain a feared and disabling complication following gastrointestinal surgery. The diagnosis of anastomotic leak has been facilitated greatly by advances in imaging and today is often managed non-operatively using percutaneous abscess drainage and antibiotics. Yet in this era of non-operative management, short hospital stays, and liberal use of the diverting ileostomy in cases of colorectal surgery, deaths because of anastomotic leaks persist, in part, because of the inability to predict in which patients a leak will occur and when. Although many attempts have been made over the years to understand the pathogenesis of anastomotic leak at a fundamental biologic level, little-to-none of the generated body of knowledge has shed much meaningful insight to change clinical practice leading to a decrease in leakage.
The current report is a communication of the proceedings of the first international summit on anastomotic leak held in Chicago, Illinois on October 4–5, 2012. It was co-hosted by the University of Chicago and the University of Munich. There were 40 international participants from Europe and the U.S. with a total of nine speakers. The idea was to assemble clinical academic surgeons, surgeon-scientists, and industry researchers to discuss intestinal anastomotic leak openly and evaluate the current state of research progress. The meeting format did not allow every participant to present a lecture, but rather was designed to drive discourse in a way that would expose the clinical mythology and mis-directed research on anastomotic leak in an attempt to move the field forward. The summit directors, Doctor John C. Alverdy and Doctor Han Schardey, selected participants who they believed would offer a critical and unbiased approach to the topic, and therefore meeting participation was by invitation only. Although the meeting was supported by equal contributions from Covidien (Mansfield, MA) and Ethicon (Somerville, NJ), there were neither industry speakers nor input from the industry sponsors regarding the speakers, content, or conduct of the program.
The meeting proceeded as follows: Doctor Jeffery Matthews, Dallas B. Phemister Professor of Surgery and Chairman of Surgery at the University of Chicago, delivered the opening address. He discussed potential pitfalls of dogmatic reasoning, syllogistic logic, and the trappings of confirmation bias. He discussed the distinction of that which is actually true (truth) from what is believed to be true (truthiness). Next, Doctor Schardey, Professor of Surgery from the University of Munich, and Doctor James Fleshman, Professor of Surgery from Washington University, presented their interpretations of actual leak rates for gastrointestinal surgery in Europe and the US. Doctor Hans Jeekel, Professor of Surgery Emeritus from the University of Rotterdam, Netherlands, declared that the failure of 20 years of research to significantly decrease or eliminate anastomotic leaks was disappointing. Professors of Surgery, Garcia-Granero from the University of Valencia in Spain and Harry Van Goor from Radboud University Nijmegen Medical Center in Nijmegen, Netherlands, presented translational perspectives on new approaches to understand anastomotic leak. The Alverdy laboratory proposed that anastomotic leak is an infectious disease caused by luminal microbes. Doctor E. Patchen Dellinger from the University of Washington reviewed the pitfalls of antibiotic practices in the US and Europe to eliminate these causative microbes. The first day session concluded with a presentation by Doctor Donald Fry who asserted that we have ignored the history of intestinal antisepsis and worse yet, have created our own revisionist history on the subject. Doctor Fry argued that this misdirection has led to our current dismissal of the important role of microbes in bowel surgery in general and more specifically in the pathogenesis of anastomotic leak. The next day began with Doctor Jens Hoeppner from the Department of Surgery, University of Freiberg, Germany, who discussed potential flaws of animal models as applied to anastomotic leak. The next session was a molecular “tour de force” that discussed the use of confocal laser endomicroscopy, Raman spectroscopy, and metagenomic techniques that are available now and can elucidate the pathobiology of anastomotic leak in patients. The summit was concluded by Doctor Gary An from the University of Chicago, a general surgeon and computational biologist who reviewed the fundamentals of agent-based modeling techniques as a tool to disentangle complex problems in biology and medicine. He provided the view that in order to understand a complex pathophysiologic problem such as anastomotic leak, modeling of all of the potential biological elements, their pathways, and their nodes of interactions is needed. Key points of insight were provided in discussions by Doctor Philip S. Barie (Cornell University), Doctor Naji Abumrad (Vanderbilt University), Doctor Karl Jauch (University of Munich Ludwig Maximillians University), and Doctor Adrian Barbul (Johns Hopkins University). The entire list of participants can be found in Table 1. The summary below is a synopsis of each presentation and a commentary on the relevance and importance of each to the field by the co-director, Doctor John Alverdy.
Table 1.
List of All Summit Participants
| Name | Affiliation |
|---|---|
| Naji N. Abumrad, MD | Vanderbuilt University, USA |
| John C. Alverdy, MD (co-director) | University of Chicago, USA |
| Gary C. An, MD | University of Chicago, USA |
| Gregory W. Auner, PhD | Wright State University, USA |
| Adrian Barbul, MD | Johns Hopkins University, USA |
| Philip S. Barie, MD, MBA | Weill Cornell Medical College, USA |
| E. Patchen Dellinger, MD | University of Washington, USA |
| James W. Fleshman, Jr., MD | Baylor College of Medicine, USA |
| Blas Flor-Lorente, MD | Hospital Universitario la Fe de Valencia, Spain |
| Donald E. Fry, MD | Northwestern University Feinberg School of Medicine, USA |
| Jack A. Gilbert, PhD | University of Chicago, USA |
| Eduardo Garcia-Granero, MD, PhD | University of Valencia, Spain |
| Harry van Goor, MD, PhD | Radboud University Nijmegen Medical Center, Netherlands |
| Jens Hoeppner, MD | University of Freiberg, Germany |
| Karl-Walter Jauch, MD | University of Munich, Germany |
| Hans Jeekel, MD, PhD | University of Rotterdam, Netherlands |
| Michael S. Kasparek, MD | University of Munich, Germany |
| Vani Konda, MD | University of Chicago, USA |
| Jeffrey B. Matthews, MD | University of Chicago, USA |
| Hans M. Schardey, MD, PhD (co-director) | University of Munich, Germany |
| Benjamin D. Shogan, MD | University of Chicago, USA |
| Konstantin Umanskiy, MD | University of Chicago, USA |
| Industry Sponsors | |
| Covidien | Ethicon |
| Michael J. Bettuchi | Gary Knight |
| Dwight Bronson, MS | Rosa Kwak |
| Scott DePierro | Kurt Matheson |
| Eligio Floscoli | Allison Mooney |
| Marisha Godek, PhD | Michelle O'Connell |
| Laura Lassandro | Jerome Riebman, MD |
| Patrick Mozdzierz | David Stoloff, DVM |
| David Racenet | Suzanne Thompson DVM, MS |
| Ashish Sharma, PhD | Jennifer Yohrling, PhD |
| Joe Taylor | Andrew Yoo, MD |
| Jennifer Whiffen | |
Doctor Jeffrey B. Matthews Presentation
Truth and truthiness in surgery
Doctor Matthews began the conference by discussing the actual evidence that surgeons use to form opinions and the influence that personal bias plays in clinical decisions. First, he presented data showing that despite the growing focus on the importance of evidence-based practices, in the “real world” only ∼55% of surgeons comply with current guidelines for a given practice [1]. As an editor and editorial board member of several major surgical journals, he presented data suggesting that when strong treatment effects are published, less than 50% of the time are they ever confirmed subsequently and less that 30% of the time are they never challenged subsequently [2]. This disturbing statistic was then followed by a discussion on the well-known publication bias against negative studies. He then presented several examples of dogma accepted as fact questioning, “Is evidence-based medicine evidence-based?” In this context, cognitive distortion was discussed including the bandwagon (groupthink) effect, “deformation professionnelle’ ” (seeing only through the lens of our own profession), focusing effect-myopia leading to prediction bias, framing effect (conclusions depend on how data are presented), and confirmation bias (confirming our preconceptions). He suggested that current surgical practice is “an accumulated wisdom, mixing fact, opinion, and magical thinking in unknown proportions.” Finally he concluded that (1) “truth” is fluid and complex, and “truthiness” is pervasive in current surgical practice, (2) an irreducible degree of uncertainty and complexity must be accepted in clinical care and research, and (3) tacit knowledge and evidence-based medicine are both relevant to good clinical judgment.
Commentary
The hope of this first lecture was to encourage participants to be mindful, in the subsequent discourse, of the difficulty of discussing low-incidence complications in surgery where bias and opinion can overtake scientific evidence. Clinical anastomotic leaks are often only discussed at surgical morbidity and mortality conferences and often deemed to be some type of breach in technique. The group recognized the pitfalls in thinking discussed by Doctor Matthews as they pertain to anastomotic leak and agreed, in general, that they remain major obstacles in enhancing our understanding of the complication.
Doctor Konstantin Umanskiy Presentation
What do we all really believe are the causes and consequences of anastomotic leak?
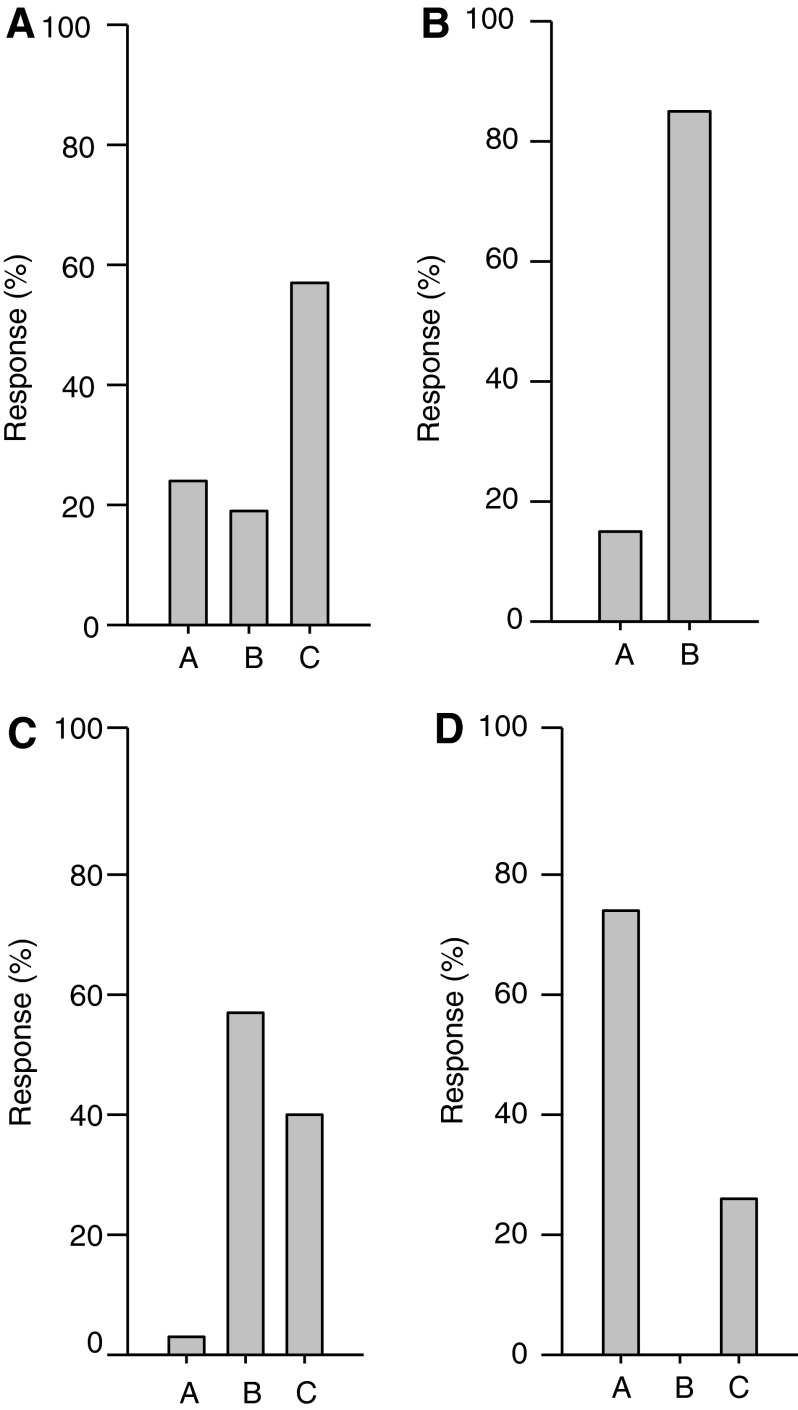
In order to obtain an anonymous view of an individual's opinion regarding the causes of anastomotic leak, Doctor Umanskiy provided surgeons in the audience with an electronic response remote control and posited a series of 32 multiple-choice questions on anastomotic leak. The electronic system allowed for immediate visualization of how individuals in the audience responded to the questions by projecting the results on the overhead screen in bar graph form. (Fig. 1 provides a sample of the questions and answers.) The entire set of questions and their answers can be found in Table 2.
FIG. 1.
Sample questions and responses of participants. (1A) When an intestinal anastomosis is performed by a highly qualified, high-volume surgeon, the most common cause of a leak is: A. Technical, B. Patient factors, or C. Unknown; (1B) When an intestinal anastomosis is performed by a highly qualified, high-volume surgeon, an anastomotic leak is most often: A. Predictable or B. Not predictable; (1C) When an anastomotic leak occurs, a detailed analysis of the precise cause of leakage is able to be determined: A. Most of the time (>50%), B. Sometimes (<50%), or C. Never; (1D) The true incidence of anastomotic leak is: A. Higher than reported, B. Lower than reported, or C. Same as reported; n=35.
Table 2.
Questions and Answers From Electronic Audience Response System
| Question | Answer Choice | Distribution of Responses (n=35 total respondents) |
|---|---|---|
| The Role of Technique | ||
| When an intestinal anastomosis is performed by a highly qualified, high-volume surgeon, the most common cause of a leak is? | A. Technical | 9 |
| B. Patient factors | 6 | |
| C. Unknown | 20 | |
| Considering all the elements of technique that might contribute to an anastomotic leak, which of the following is most important? | A. Blood supply | 25 |
| B. Method of construction | 4 | |
| C. Tension | 6 | |
| When an intestinal anastomosis is performed by a highly qualified, high-volume surgeon, an anastomotic leak is most often: | A. Predictable | 4 |
| B. Not predictable | 31 | |
| When an intestinal anastomosis is performed by a highly qualified, high-volume surgeon in a healthy patient under ideal conditions, and intra-operatively appears technically acceptable it: | A. Will never leak | 2 |
| B. Still can leak | 33 | |
| You are a surgeon with a 7% leak rate for colorectal anastomoses and have taken videos of 100 of your operations. You then submit the videos for evaluation by a panel of 10 expert surgeons. By watching the videos, the panel will be able to determine which patient will have an anastomotic leak: | A. Yes | 7 |
| B. No | 28 | |
| When an anastomotic leak occurs, a detailed analysis of the precise cause of leakage is able to be determined: | A. Most of the time (>50%) | 1 |
| B. Sometimes (<50%) | 20 | |
| C. Never | 14 | |
| Definition and Incidence | ||
| The surgical literature reports that the incidence of anastomotic leak is ∼10% for esophago-gastric and ∼5–10% for colo-rectal anastomosis. The true incidence of anastomotic leak is: | A. Higher than reported | 26 |
| B. Lower than reported | 0 | |
| C. Similar to reported | 9 | |
| Over the last decade, the incidence of anastomotic leaks in high-risk areas is: | A. Unchanged | 18 |
| B. Decreased significantly | 9 | |
| C. Increased significantly | 1 | |
| D. Unknown | 7 | |
| The morbidity after anastomotic leak is: | A. Insignificant: Most patients can be managed without surgery | 1 |
| B. Significant: Leak results in delay in chemotherapy, incontinence, re-operation, permanent stoma | 34 | |
| A patient has an infected fluid collection adjacent to a new anastomosis. The barium enema is negative. The fluid collection is: | A. Most likely not an anastomotic leak | 10 |
| B. Most likely an anastomotic leak | 25 | |
| Can an asymptomatic patient with a perfectly healed anastomosis at two weeks (i.e., normal endoscopy/barium enema/computed tomography (CT) scan) develop a delayed leak: | A. Yes | 23 |
| B. No | 12 | |
| Current Research | ||
| Experimental animal research on anastomotic leak over the last decade has: | A. Improved our understanding and clinical management of anastomotic leaks | 1 |
| B. Provided little insight into the real biologic mechanisms of clinical leaks | 9 | |
| C. Provided some new insight but it has not changed the management of clinical leaks | 15 | |
| Current experimental animal models of anastomotic leak: | A. Are useful and should continue | 26 |
| B. Are useful but more large animals models (e.g., dogs, pigs, monkeys) should be used | 4 | |
| C. Are useless because they do not reflect the clinical circumstances of leaks | 5 | |
| Research into the pathogenesis of anastomotic leak could be advanced markedly by: | A. Focusing on devices (i.e., sutures, stapler, stents) | 0 |
| B. Developing more appropriate animal models that mimic clinical leakage | 3 | |
| C. Performing more analysis (i.e., oxygen, pH, collagen) on human anastomotic tissues during and after surgery | 32 | |
| Intestinal microbes as initiating and causative agents in anastomotic leak: | A. Have been investigated sufficiently, but likely only play a secondary role in leak | 2 |
| B. Have been investigated sufficiently, and likely play an important and causative role in leak | 6 | |
| C. Have been investigated insufficiently and requires further study | 27 | |
| Future Directions | ||
| Over the last decade, industry research and product development has contributed significantly to reducing the incidence of anastomotic leak: | A. Agree | 14 |
| B. Disagree | 21 | |
| Current industry research and product development (e.g., improved staplers, stents, glues) will have a significant impact on anastomotic leak rates in the near future: | A. Agree | 12 |
| B. Disagree | 23 | |
| If you were given $5 million to develop and execute research on intestinal anastomotic leak, you would: | A. Study the biology of anastomotic healing in large animals and develop biologic agents (e.g. growth factors, stem cells, angiogenesis) to prevent leakage | 11 |
| B. Perform clinical studies and analyze anastomotic tissues directly to define and characterize the biologic variables that are associated with anastomotic healing versus leakage. | 24 | |
| C. Develop novel devices to reduce leakage (e.g. stents, antibiotic-coated suture, absorbable staples, new staplers) | 0 | |
| With properly funded and properly focused research performed over the next three years, the incidence of anastomotic leaks in high-risk areas can be decreased by >50%: | A. Agree | 11 |
| B. Disagree | 24 | |
| If we could measure blood flow, oxygen status, microbial content, and collagen synthesis at anastomotic sites, the biggest impact on anastomotic leak would occur by: | A. Measuring parameters immediately after an anastomosis is created allowing surgeons to refine our surgical technique | 10 |
| B. Serially measuring parameters days and weeks after an anastomosis is created, allowing surgeons to understand why a given anastomosis heals or leaks | 25 | |
Commentary
Results indicated clearly that the majority of respondents opined that anastomotic leak is rarely because of a technical error, it cannot be predicted for a given case by the surgeon, it is rarely if ever subjected to a root cause analysis by concrete clinical evidence, and it is under-reported. The group also conceded that because clinical circumstances that are relevant to high-risk patients cannot be recreated in animal models, human studies are needed to move the field forward. It was concluded that the clinical and scientific basis upon which an anastomotic leak is referred to invariably during morbidity and mortality conference as a “technical error” and thus “preventable” does not exist.
Doctor Hans Schardey and Doctor James Fleshman Presentation
Anastomotic leak in Europe and the US: The problem persists and the consequences are real
Doctor Schardey reviewed the surgical problem of anastomotic leak. He began by discussing how comparison between investigations is difficult because no universal definition exists of anastomotic leak [3]. He then presented data on the incidence of anastomotic leak, which in the European literature ranges from zero to 50%, with the highest incidence occurring after esophagectomy or anterior rectal resection [4,5]. After plotting the published leak rate of 174 consecutive studies from 1975 versus time, he showed that over the last 25 years, the anastomotic leak rate has not changed significantly. This was followed by a discussion regarding the consequences of leakage, which include a decrease in overall survival and a possible decrease in cancer-free survival. Also discussed, was a Swedish nationwide multicenter randomized control trial (RCT) showing that 56% of patients with symptomatic anastomotic leakage received a permanent stoma [6].
Doctor Fleshman followed by discussing the American anastomotic leak experience. He reaffirmed a wide range of published leak rate incidences, ranging between 2%–20%, and discussed their distribution across procedures occurring most commonly after colorectal (5–10%) or coloanal (10–17%) anastomoses [7–9]. He then reviewed the known and controversial risk factors for anastomotic leak. He presented data from a prospective study assessing whether surgeons, at the time of anastomotic construction, can predict which patients will leak [10]. Results demonstrated that surgeons cannot predict which patients will leak. After a review of multiple retrospective and prospective studies, he concluded that evidence supports associated risk factors such as hypoalbuminemia, long operative time, and multiple co-morbidities. Other potential risk factors include rectal cancer within 5 cm of the anal verge, diverticulitis, Crohn disease, and radiation enteritis [11,12].
Commentary
Discussion following these presentations centered on the limitations of retrospective risk analysis and the need for more clinical data that leverages emerging technology. Although risk indices demonstrate that sicker patients are more likely to leak, it was impossible to answer why, within the higher-risk patients, still most do not leak. Despite prevailing opinions to the contrary, there was unanimous agreement that serial endoscopy early in the post-operative course to predict who might leak would be safe and is the only way to gather the key biological information necessary to advance our understanding of why one anastomosis heals whereas another is destined to leak.
Doctor Hans Jeekel, Doctor Eduardo Garcia-Granero, and Doctor Harry von Goor Presentation
Why basic research in anastomotic leak has failed to move the field forward
Professor Jeekel began his presentation with the declaration that the persistence of anastomotic leaks in clinical practice represents an abject failure of surgical research and practice. He discussed the emotional devastation of an anastomotic leak to both the patient and surgeon. He reflected on the intrinsic flaws of research in the field. These flaws ranged from the known resistance of animal models to develop spontaneous leakage and peritonitis as is observed in humans, to inadequate analyses of human tissues from patients with leaks. He discussed the resistance of rodents to develop peritonitis even when the anastomosis is grossly misconstructed (e.g., suturing closed only half of the intestinal circumference) and how despite this, the rodent model continues to be used.
Professor Garcia-Granero presented the clinical path forward to understanding anastomotic leak by using mucosal pH as a proxy for anastomotic blood flow and healing. He presented one of the largest series of dynamic tracking of mucosal pH in patients to predict anastomotic leak [13]. He discussed the potential use of pre- and post-operative hyperoxia to prevent alterations in mucosal pH [14]. Finally, he suggested that tracking subtle markers of inflammation such as procalcitonin and C-reactive protein in patients may signal surgeons that leakage may be developing [15].
Professor Harry van Goor reviewed the real and present danger of non-steroidal anti-inflammatory drug (NSAID) use during gastrointestinal surgery as agents that affect anastomotic healing. The clinical evidence presented was compelling and confirmatory animal models were presented based on work from his laboratory showing that NSAIDs play a contributory role in altering the natural history of anastomotic leakage [16]. Novel animal models and unpublished data show real promise of this line of inquiry. Doctor van Goor concluded that if animal models are to continue to be part of anastomotic leak research, additional elements of the post-operative management of surgical patients must be included in the models.
Commentary
Although this series of presentation pointed out the potential limitations of animal studies, it did not rule out completely their usefulness. Professor Garcia-Granero provided a thoughtful approach to obtaining clinical data at the anastomosis by measuring real physiologic parameters directly using advanced instrumentation. As before, a discussion followed regarding the safety, possibilities, and procedural hurdles in tracking anastomotic healing dynamically in patients over the course of days to weeks to obtain images, physiologic parameters, microbiologic data, and even tissue biopsies. The group concluded that the execution of such a study is possible and would be an unprecedented move to advance the field. Doctor van Goor's work on NSAIDs is ongoing and currently being followed up with clinical studies in multiple European centers.
Doctor John C. Alverdy and Doctor Benjamin D. Shogan Presentation
Anastomotic leak as an infectious disease
Doctor Alverdy introduced the concept that intestinal microbes play a role in anastomotic leak. Over the course of his presentation he reviewed investigations thoroughly that show clearly that luminal bacteria are the initiating cause and driving force behind the pathogenesis of anastomotic leak. Doctor Alverdy began by presenting a study from 1954 by Isidore Cohn to illustrate that this hypothesis is now nearly 60 years old [17]. By infusing tetracycline directing into a colon anastomosis via an indwelling tube in dogs, Cohn et al. demonstrated that an ischemic devascularized segment of anastomosed colon heals completely and re-vascularizes without dehiscence when exposed to luminally antibiotics. In 1985, Steven Cohn performed a similar study in rats showing that oral antibiotics prevented anastomotic leaks completely in devascularized colon segments with complete healing and resolution of ischemia [18]. A seminal study reconfirming the role of microbes as causative agents was then performed in 1994 when Schardey et al. demonstrated that introduction of pathogenic bacteria to a newly formed rat anastomosis directly causes anastomotic leak [19]. In a clinical followup, his group then performed a multi-center placebo-controlled, blinded prospective randomized clinical trial (RCT) in 1996 demonstrating that oral decontamination prevent anastomotic leak following gastro-esophageal surgery [20]. In both Europe and the US, the emergence and convenience of systemic broad-spectrum antibiotics began to replace oral antibiotics as surgeons assumed mechanical cleansing with purgatives was sufficient to clear the luminal microbes that were missed by systemic antibiotics. Doctor Alverdy discussed how surgeons around the world were eliminating oral antibiotics based on essentially no confirmatory evidence that intravenous antibiotics were as effective.
Next, Doctor Shogan, a research fellow in Doctor Alverdy's lab, presented his results on the microbial pathogenesis of anastomotic leak using an animal model where a technically well-constructed anastomosis develops spontaneous leakage. He presented results that confirmed that intestinal bacteria are required in order for anastomotic leaks to occur. He presented the molecular details and pathways that regulate in vivo expression of microbial genes among gut pathogens that lead to the secretion of potent tissue-destroying proteases that cause spontaneous leakage in a technically intact and well-constructed anastomosis. The power and promise of microbial sequencing technology to unravel the mechanisms by which microbes cause anastomotic leak was presented.
Commentary
There was substantial audience acceptance of the microbial pathogenesis theory of anastomotic leak. Discussion centered on missed opportunities, lack of followup on significant studies, and the need to pursue this line of inquiry with newly available tools to confirm the animal studies in human beings.
Doctor E. Patchen Dellinger Presentation
Role of antibiotics in preventing anastomotic leak: What are we doing right and what are we doing wrong?
Doctor Dellinger presented and reinforced the seminal work of Isidore Cohn as outlined above and restated that we have overlooked the crucial contribution of the intestinal microflora on anastomotic leak. He reviewed a study in which patients were given oral polymyxin B, tobramycin, and amphotericin B for two days preoperatively and three days postoperatively, and cefoxitin and metronidazole intravenously during the time of surgery [21]. Patients receiving the antibiotic treatment had a three-fold lower intestinal anastomotic leak rate compared with patients who did not receive antibiotics, but it failed to reach significance (6.3% vs. 15.1%; p=0.16). He reviewed the scientific rationale behind the use of oral antibiotics, their bioavailability to anastomotic tissues, and the progression to use intravenous antibiotics without first establishing that they decontaminated anastomotic tissues equivalent to oral antibiotics. Doctor Dellinger acknowledged the need for more clinical science in the field, a better definition of the microbial content that colonizes anastomotic tissue in high risk patients, and more standardized approaches to the use of antibiotics following gastrointestinal surgery that extend beyond their effect on the incidence of surgical site infection.
Doctor Donald Fry Presentation
Preparing the colon: Is getting rid of waste a waste of time?
Doctor Fry concluded the first day with a powerful presentation to explain how a complete misinterpretation of the history of intestinal antisepsis has led to the chaos and complacency currently surrounding bowel preparation for surgery. He pointed out that as early as the 1930s it had been known that mechanical bowel preparation did not reduce the concentration of bacteria in the colon lumen, much less the mucosa [22]. He cited the many publications by E.J. Poth introducing the idea that sulfa-based antibiotics were necessary to reduce colon bacteria yet were limited by their lack of effect on anaerobic bacteria [23–25]. Even when Nichols and Condon began their seminal work in the field by developing tactics to decrease anaerobic bacteria, they re-confirmed that mechanical cleansing had no effect on bacterial concentrations in the colon lumen [26]. Yet despite this ample and growing body of knowledge, including more recent reports showing that bowel cleansing has an adverse effect on the intestinal mucosa, surgeons continued to be seduced into abandoning oral antibiotics in favor of systemic antibiotics alone. However, in the late 1990's, mechanical bowel preparation was not holding up as a necessary element to prepare a patient for colon surgery based on rates of anastomotic leak and surgical site infections. In fact, Doctor Fry presented some comparative studies that showed a worse outcome with the use of mechanical bowel preparation on both endpoints [27]. Randomized prospective trials and meta-analyses comparing mechanical bowel cleansing with purgatives to no mechanical cleansing filled the pages of surgical journals around the world with little attention to history. Doctor Fry presented the results of several studies demonstrating oral antibiotics with mechanical bowel preparation, in addition to systemic antibiotics, reduce rates of both anastomotic leak and surgical site infection [28,29]. Doctor Fry commented that we have created our own revisionist history on the rationale and efficacy of bowel preparation prior to gastrointestinal surgery and as such, have assumed mistakenly we have reached equipoise over which regimens (bowel prep, no bowel prep, oral antibiotics, no oral antibiotics) are best.
Commentary
Both Doctor Dellinger and Doctor Fry reviewed current practices to prepare the bowel for surgery, their historic rationale, and the need for more high-resolution microbiological information to lead us forward. Re-emphasizing that purgative bowel preparations do not change intestinal bacterial counts, that oral antibiotics have important effects on the intestinal microflora and anastomotic leak rates over IV antibiotics, and when added to IV antibiotic regimens was crucial. These lectures and the discussion that followed left the impression that much remains to be learned about how bowel preparations were designed in the first place, and how they were changed rapidly to exclude mechanical cleansing and oral antibiotics without testing the effect of such changes on the intestinal microflora itself.
Doctor Jens Hoeppner Presentation
Technique and ischemia play a minor role in anastomotic leak: Results and limitations of animal models and the need for human studies
Frequent criticisms of animal models to recapitulate anastomotic leak in human beings have centered on the overuse of rodents, the lack of studies in larger animals, and the general resistance of animals to develop spontaneous anastomotic leak. Doctor Hoeppner reviewed his work of a pig model where three groups of 12 German domestic pigs were assigned to the following experimental groups: Group A underwent a technically inadequate anastomosis leaving a 18 mm dehisced section, Group B was treated similar to group A but devascularized by vessel ligation for a 5 cm segment adjacent to the anastomosis, and Group C was treated similar to A and B but a 10 cm segment of devascularization was created [30]. Among the 12 pigs, only two developed leakage and surprisingly none of the animals in group C developed leakage, although bowel obturation by necrotic parts of the bowel wall was observed. All pigs were sacrificed and no gross evidence of ischemia was observed. Images of colon segments were observed at sacrifice and the mucosal blood supply appeared normal.
Commentary
This well-executed study reinforces the experience of many master surgeons, which is that the healing potential of the gastrointestinal tract is robust, and even when the blood supply is limited, anastomotic leaks are rare. This type of study provides compelling evidence that ischemia, often identified as the main causal event in anastomotic dehiscence, probably plays a much less important role in anastomotic leak than is often publicized. There was consensus among the group that animal models may not always recapitulate, in the aggregate, all of the various factors that predispose to anastomotic leakage (e.g., age, smoking, radiation exposure, blood loss). It was agreed, though, that animal models do not need to be relevant clinically to be useful to advance biology, and are indeed helpful, but new lines of inquiry must be developed to move beyond the notions of tension and ischemia. In addition, for animal models to be useful, the group concluded that they must develop spontaneous leaks, as in humans, after a technically adequate anastomosis has been created.
Doctor Vani Konda, Doctor Greg Auner, and Doctor Jack Gilbert Presentation
How to design a clinical trial to get at the most important issues surrounding anastomotic leak and its prevention
Doctor Vani Konda discussed confocal laser endomicroscopy as a methodology to provide visualization with microscopic detail of mucosal architecture [32], vascular patterns, and intramucosal bacteria [33]. She first highlighted the updated capabilities on newer endoscopes with high resolution, zoom magnification, and narrow-band imaging as ways to visualize surface mucosal patterns and superficial vascular patterns. A published proposed approach described how narrow-band imaging may be a way to evaluate the surgical anastomosis [31]. She demonstrated work on polarized gated spectroscopy as a way to measure superficial hemoglobin concentration and oxygenation in tissue, and low-coherence enhance backscattering to provide quantitative assessments on tissue microarchitecture in the colon performed by her collaborators Hemant Roy and Vadim Backman [34].
Doctor Greg Auner discussed the use of Raman spectroscopy as a tool capable of providing unprecedented molecular and functional detail of anastomotic tissues. He described how Raman spectroscopy is a vibrational spectroscopy based upon the frequency difference between the sample and scattered light. He further described how the intensity of the band (i.e., Raman shift) is proportional to the composition of the sample in which is being analyzed, therefore providing a “fingerprint” allowing for identification of molecular events in tissues. Doctor Auner cited much of his own breakthrough work, describing how Raman spectroscopy can differentiate between malignant tumors from normal tissues in various models of breast, kidney, lung, and liver neoplasia [35]. He then focused on a more recent study in which Raman spectroscopy could differentiate between different antibiotic-resistant strains of Staphylococcus aureus with up to 96% accuracy (unpublished). He concluded by pondering the potential further uses that Raman spectroscopy may have in the clinical diagnosis of anastomotic leak. This technique has been functionalized on endoscopes and can be readily available clinically.
Doctor Jack Gilbert concluded this segment with an overview of the power of metagenomics to interrogate the microbiome associated with anastomotic tissues. He outlined his ongoing work to characterize the diversity and spatiotemporal dynamics of microbes in various settings, specifically detailing the Earth Microbiome Project (www.earthmicrobiome.org), which is handling >20,000 environmental samples from hundreds of ecosystems around the world. Sequenced microbes isolated from patients (i.e., from anastomotic tissues) can now be cross-referenced with these rapidly accumulating databases. Analogously, Doctor Gilbert also highlighted work in the Home Microbiome Project (www.homemicrobiome.com) and Hospital Microbiome Project (www.hospitalmicrobiome.com) that are using metagenomic sequencing to uncover the microbial diversity of indoor ecosystems. Finally, Doctor Gilbert highlighted the importance of niche-specific and envelope-based modeling to create a predictive understanding of the influence of microbial community structure dynamics (both taxonomic and functional) in problems where microbes contribute to a localized disease process such as anastomotic leakage. These new and emerging concepts suggest that microbial community structure and function are highly dependent on both spatial construct and local ecology and require sampling techniques take place at the precise site of pathology to provide contextual meaning and invoke causality.
Commentary
The power and promise of metagenomics to sequence microbes and identify microbial genes and gene products associated with disease processes such as anastomotic leak have the potential to unleash an unprecedented amount of information about the composition and function of microbes at anastomotic tissues. This same technology can also be used to determine host genes and gene products that are present or absent at anastomotic tissues. Combining confocal endomicroscopy and Raman spectroscopy with sampling at anastomotic tissues can answer important questions such as: Do patients who smoke or who have recently received antibiotics or chemotherapy harbor more pathogenic bacteria at anastomotic tissues? Do these bacteria produce more tissue destroying enzymes? Is blood supply impaired because of these pathogenic bacteria? Are there ultrastructural defects that occur as a result of these complex and dynamic interactions? Does loss of probiotic bacteria at anastomotic sites weaken tissues? When do these changes occur, in the operating room, on post-operative day three, or later?
Doctor Gary An Presentation
Use of agent-based modeling (ABM) to unravel the complex interactions between the host genes and microbial genes to understand anastomotic leak and formulate strategies to prevent it
As an appropriate concluding presentation to the summit on anastomotic leak, Doctor Gary placed the challenge of preventing anastomotic leak within the overall context of the current translational dilemma facing the general biomedical community: the challenge to integrate, translate and effectively utilize the ever-increasing volume of cellular and molecular data into a coherent and useful structure to potentially improve human health. He recounted the seemingly pessimistic divergent trend between increasing expenditures on basic biomedical research and the decreasing success rate of bringing new therapies to market, and applied a diagnostic approach that is to the general causes of this failure familiar to any clinician. In doing so, he highlighted some existing insights, that is, the fact that highly complex and interconnected systems (such as are involved in anastomotic leak) are characterized insufficiently using traditional reductionist science, but also proposed solutions to scientific situations that have often been described as intractable. Given the complexity of pathophysiologic processes such as anastomotic leak, integrating the vast extent of data available overwhelms the ability of the human brain to organize and exercise their intuition. Computational modeling methods, such as agent-based modeling, could play a vital role in providing dynamic knowledge representation of complex biological systems to facilitate hypothesis visualization and allow for the execution of “thought experiments” by biomedical researchers.
Conclusions
Highly experienced and super-specialized gastrointestinal surgeons working in high volume centers continue to experience anastomotic leak. Although the mortality from leakage appears to have decreased over the last 10 years, the incidence of leakage remains unchanged. A clearly defined path to reduce anastomotic leak does not exist because of multiple biases in the field and the individual perception that patient factors involving lifestyle and genetic predisposition are beyond the reach of current medical practice. Technology can now move the field forward to define, in high-resolution molecular detail, the natural history of healing versus non-healing at anastomotic tissue sites in patients via serial endoscopic surveillance (SES) and sampling. However, for this to occur, anastomotic tissues must be examined over the entire course of healing which may continue for several weeks. This paper calls for an international and focused effort to develop a working group of investigators who can move the field forward and realize a measureable and significant decrease in the incidence of anastomotic leak by application of currently available technology to determine the biologic basis of anastomotic leaks by visual and analytical tracking of human anastomoses, in real time over the entire course of healing.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Slim K, Panis Y, Chipponi J. Half of the currecnt practice of gastrointestinal surgery is against the evidence: A survey of the French Society of Digestive Surgery. J Gastrointest Surg 2004;8:1079–1082 [DOI] [PubMed] [Google Scholar]
- 2.Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005;294:218–228 [DOI] [PubMed] [Google Scholar]
- 3.Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Brit Journal Surg 2001;88:1157–1168 [DOI] [PubMed] [Google Scholar]
- 4.Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587–595 [DOI] [PubMed] [Google Scholar]
- 5.Kube R, Mroczkowski P, Steinert R, et al. [Anastomotic leakage following bowel resections for colon cancer: Multivariate analysis of risk factors]. Chirurg 2009;80:1153–1159 [DOI] [PubMed] [Google Scholar]
- 6.Lindgren R, Hallbook O, Rutegard J, et al. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum 2011;54:41–47 [DOI] [PubMed] [Google Scholar]
- 7.Elton C, Makin G, Hitos K, et al. Mortality, morbidity and functional outcome after ileorectal anastomosis. Br J Surg 2003;90:59–65 [DOI] [PubMed] [Google Scholar]
- 8.Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–270 [DOI] [PubMed] [Google Scholar]
- 9.Telem DA, Chin EH, Nguyen SQ, et al. Risk factors for anastomotic leak following colorectal surgery: A case-control study. Arch Surg 2010;145:371–376 [DOI] [PubMed] [Google Scholar]
- 10.Karliczek A, Harlaar NJ, Zeebregts CJ, et al. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 2009;24:569–576 [DOI] [PubMed] [Google Scholar]
- 11.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998;85:355–358 [DOI] [PubMed] [Google Scholar]
- 12.Salem L, Flum DR. Primary anastomosis or Hartmann's procedure for patients with diverticular peritonitis? A systematic review. Dis Colon Rectum 2004;47:1953–1964 [DOI] [PubMed] [Google Scholar]
- 13.Millan M, Garcia-Granero E, Flor B, et al. Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum 2006;49:595–601 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Botello SA, Garcia-Granero E, Lillo R, et al. Randomized clinical trial to evaluate the effects of perioperative supplemental oxygen administration on the colorectal anastomosis. Brit Jour Surg 2006;93:698–706 [DOI] [PubMed] [Google Scholar]
- 15.Komen N, de Bruin RW, Kleinrensink GJ, et al. Anastomotic leakage, the search for a reliable biomarker. A review of the literature. Association of Coloproctology of Great Britain and Ireland 2008;10:109–115 [DOI] [PubMed] [Google Scholar]
- 16.de Hingh IH, van Goor H, de Man BM, et al. Selective cyclo-oxygenase 2 inhibition affects ileal but not colonic anastomotic healing in the early postoperative period. Br J Surg 2006;93:489–497 [DOI] [PubMed] [Google Scholar]
- 17.Cohn I, Jr., Rives JD. Antibiotic protection of colon anastomoses. Ann Surg 1955;141:707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SR, Cornell CN, Collins MH, et al. Healing of ischemic colonic anastomoses in the rat: Role of antibiotic preparation. Surg 1985;97:443–446 [PubMed] [Google Scholar]
- 19.Schardey HM, Kamps T, Rau HG, et al. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother 1994;38:2564–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schardey HM, Joosten U, Finke U, et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 1997;225:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos D, Dijksman LM, Oudemans-van Straaten HM, et al. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg 2011;98:1365–1372 [DOI] [PubMed] [Google Scholar]
- 22.Nichols RL, Gorbach SL, Condon RE. Alteration of intestinal microflora following preoperative mechanical preparation of the colon. Dis Colon Rectum 1971;14:123–127 [DOI] [PubMed] [Google Scholar]
- 23.Poth EJ. A clean intestinal anastomosis: An experimental study. Arch Surg 1934;28:1087–1094 [Google Scholar]
- 24.Poth EJ. A clean intestinal anastomosis, II: An experimental study. Arch Surg 1935;31:579–586 [Google Scholar]
- 25.Poth EJ. Succinylsulfathiazole: An adjuvent in surgery of the large bowel. J Am Med Assoc 1942;120:265–269 [Google Scholar]
- 26.Nichols RL, Condon RE, Gorbach SL, et al. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg 1972;176:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucher P, Gervaz P, Soravia C, et al. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 2005;92:409–414 [DOI] [PubMed] [Google Scholar]
- 28.Washington JA, 2nd, Dearing WH, Judd ES, et al. Effect of preoperative antibiotic regimen on development of infection after intestinal surgery: Prospective, randomized, double-blind study. Ann Surg 1974;180:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg 2002;45:173–180 [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeppner J, Crnogorac V, Hopt UT, et al. The pig as an experimental model for colonic healing study of leakage and ischemia in colonic anastomosis. J Invest Surg 2009;22:281–285 [DOI] [PubMed] [Google Scholar]
- 31.Milsom JW, Pavoor RS, Shukla PJ. Evaluating the vascularity of intestinal anastomoses–can narrow band imaging play a role? Med Hypotheses 2011;77:290–293 [DOI] [PubMed] [Google Scholar]
- 32.Buchner AM, Shahid MW, Heckman MG, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology 2010;138:834–842 [DOI] [PubMed] [Google Scholar]
- 33.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61:1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backman V, Roy HK. Light-scattering technologies for field carcinogenesis detection: A modality for endoscopic prescreening. Gastroenterology 2011;140:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kast RE, Serhatkulu GK, Cao A, et al. Raman spectroscopy can differentiate malignant tumors from normal breast tissue and detect early neoplastic changes in a mouse model. Biopolymers 2008;89:235–241 [DOI] [PubMed] [Google Scholar]