Abstract
The periodontal pathogen Tannerella forsythia possesses a glycosylated S-layer as an outermost cell decoration. While the S-layer provides a selection advantage to the bacterium in the natural habitat, its virulence potential remains to be investigated. In the present study, the immune responses of human macrophages and gingival fibroblasts upon stimulation with wild-type T. forsythia and an S-layer-deficient mutant were investigated. The mRNA expression levels of the pro-inflammatory mediators IL-1β, TNF-α, and IL-8 were analyzed by qPCR, and the production of the corresponding cytokines was investigated by ELISA. The S-layer-deficient T. forsythia mutant induced significantly higher levels of pro-inflammatory mediators compared with wild-type T. forsythia, especially at the early phase of response. Analysis of these data suggests that the S-layer of T. forsythia is an important virulence factor that attenuates the host immune response to this pathogen by evading the bacterium’s recognition by the innate immune system.
Keywords: Tannerella forsythia, S-layer, immunology, cytokine production, macrophages
INTRODUCTION
The “red complex bacteria”, including Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, are strongly associated with clinical measurements of periodontitis (Socransky et al., 1998; Kinane, 2001; Holt and Ebersole, 2005) and, additionally, pose a potential risk for the development of systemic disorders (Beck et al., 2000).
Among the identified virulence factors of T. forsythia are cell-surface proteolytic enzymes (Moncla et al., 1990; Saito et al., 1997), glycosidases (Hughes et al., 2003), envelope lipoproteins (Hasebe et al., 2004), lipopolysaccharides (Bodet et al., 2006a; Bodet and Grenier, 2010), surface antigens (Sharma et al., 1998), and the S(surface)-layer (Kerosuo, 1988; Lee et al., 2006). While S-layers, as outermost bacterial cell-surface layers (Sleytr et al., 2010), generally provide a selection advantage to the bacteria, the S-layer of T. forsythia has been shown to mediate adhesion/invasion to human gingival epithelial cells and to epidermal carcinoma cells of the mouth (Sabet et al., 2003; Sakakibara et al., 2007); its involvement in hemagglutination is reported to be contradictory (Sakakibara et al., 2007). In addition, a significantly elevated serum IgG level to the S-layer was found in early-onset periodontitis patients, indicating an increased interaction between the host adaptive immune mechanisms and T. forsythia during disease progression (Yoneda et al., 2003). Despite these findings, the role of the S-layer in the host response to T. forsythia remains largely unknown.
The aim of the present study was to investigate how deletion of the S-layer from T. forsythia influences the cellular immune response. The experimental set-up included T. forsythia wild-type cells and an S-layer-deficient mutant acting as stimuli for macrophages as integral parts of the innate immune system, and for human gingival fibroblasts (HGFs), as the most abundant cells in the periodontium, playing a crucial role in the host response (Ara et al., 2009). To characterize the elicited immune response, we determined the release of the pro-inflammatory mediators IL-1β, TNF-α, and IL-8 by ELISA and analyzed the expression of the corresponding genes by qPCR. A possible scenario of how the T. forsythia S-layer may contribute to evading the host immune defense is discussed.
MATERIALS & METHODS
Bacterial Strain and Growth Conditions
T. forsythia ATCC 43037 (Tfwt) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). An S-layer-deficient T. forsythia mutant (Tf ΔtfsAB) was obtained upon insertional inactivation of the S-layer genes (Sakakibara et al., 2007). T. forsythia cells were grown anaerobically at 37°C for 4 days in tryptic soy broth (30 g/L; Gerbu, Gaiberg, Germany), supplemented with yeast extract (5 g/L; Becton Dickinson, Heidelberg, Germany), phytone peptone (5 g/L; Becton Dickinson), cysteine (0.2 g/L; Sigma, Vienna, Austria), horse serum (20 mL/L; PAA, Linz, Austria), hemin (2.5 μg/mL, Sigma), menadione (2 μg/mL, Sigma), and N-acetylmuramic acid (10 μg/mL, Sigma). Cells were harvested by centrifugation (15 min, 4500 × g), and the OD600 was set to 1.0 with MEM medium containing 5% FCS (PAA) before stimulation, corresponding to ~109 cells/mL of MEM medium.
Ultrathin-sectioning of T. forsythia Cells
Ultrathin-sectioning of bacterial cells and electron microscopy were performed as described previously (Messner et al., 1986).
Cell Lines
The U937 monocytic cell line was purchased from ATCC. HGFs were isolated from the gingival tissue of periodontally healthy patients undergoing routine third molar tooth extraction (Qu et al., 2010). Patients were informed before the surgical procedures and gave written agreement. The study protocol was approved by the Ethics Committee of the Medical University of Vienna. U937 cells and HGFs were cultured in RPMI1640 medium (Invitrogen, Vienna, Austria) and Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen), respectively, supplemented with 10% of FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. For differentiation into adherent macrophages, U937 cells were seeded (106 cells/mL) and treated with phorbol 12-myristate 13-acetate (Sigma, St. Louis, MO, USA) at a concentration of 0.2 μg/mL for 72 hrs.
Stimulation of Macrophages and Human Gingival Fibroblasts
Adherent U937 macrophages and HGFs were seeded in a 24-well plate at a density of 105 cells per well containing 0.5 mL of RPMI1640 and DMEM medium without FBS, respectively. Subsequently, either viable Tfwt (107 cells/mL) or viable Tf ΔtfsAB cells (107 cells/mL) were added to the medium to mimic the native scenario of infection. This corresponds to a multiplicity of infection of 50 bacteria per mammalian cell, which is recommended for minimizing proteolytic degradation of cytokines (Bodet et al., 2005, 2006b). Cells stimulated with 1 μg/mL of P. gingivalis LPS (Invitrogen) as well as non-stimulated cells were taken as controls. Each experimental group included 4 wells. After stimulation for either 3 or 24 hrs, the cellular mRNA expression levels of IL-1β, TNF-α, and IL-8 in macrophages and GFBs as well as the content of corresponding proteins in the conditioned medium were determined. Experiments were repeated at least 3 times.
Viability Test of Mammalian Cells
Cells were seeded at a density of 104 cells per well containing 0.1 mL of corresponding medium without FBS and stimulated with Tf wt and Tf ΔtfsAB cells for 24 hrs. Each experimental group included 8 wells. After stimulation, a 10-μL quantity of MTT dye (5 mg/mL in PBS) was added, and culture plates were incubated at 37°C for 4 hrs. Subsequently, the medium was discarded, a 100-μL quantity of DMSO was added, and the OD550 was measured on a Spectramax Plus microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Quantitative PCR
The mRNA expression levels of IL-1β, TNF-α, and IL-8 were determined by qPCR (Bertl et al., 2009), with the GAPDH encoding gene as internal reference. Isolation of mRNA from 105 cells, each, and transcription into cDNA was performed with the TaqMan® Gene Expression Cells-to-CT™ kit (Ambion/Applied Biosystems, Foster City, CA, USA). qPCR was performed on an ABI Prism SDS 7000 device (Applied Biosystems) in paired reactions with Taqman® gene expression assays with the following ID numbers (all from Applied Biosystems): IL-1β, Hs01555413_m1; TNF-α, Hs99999043_m1; IL-8, Hs00174103_m1; and GAPDH, Hs99999905_m1. qPCR reactions were performed in triplicate with the following thermocycling conditions: 95°C for 10 min, 40 cycles, each for 15 sec at 95°C and at 60°C for 2 min. The point at which the PCR product was first detected above a fixed threshold (cycle threshold, Ct) was determined for each sample. Changes in the expression of target genes were calculated with the 2−ΔΔCt method, where ΔΔCt = (Cttarget – CtGAPDH)sample – (Cttarget – CtGAPDH)control, with an untreated sample as a control.
Determination of Cytokines
The levels of IL-1β, TNF-α, and IL-8 in the conditioned medium were determined by ELISA with Ready-SET-Go kits (Ebioscience, San Diego, CA, USA). For measurement, IL-1β and TNF-α after three-hour stimulation were undiluted, IL-1β and TNF-α after 24-hour stimulation and IL-8 after three-hour stimulation were diluted 1:5, and IL-8 after 24-hour stimulation was diluted 1:20. The detection limit for all cytokines was 2 pg/mL.
Statistical Analysis
Statistical differences were analyzed by ANOVA’s statistic, and paired comparisons were performed by Tukey’s post hoc test. Statistical analyses were performed with the SPSS 17.0 program. Data are expressed as mean ± SD. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Electron Microscopy of T. forsythia Wild-type and S-layer-deficient Cells
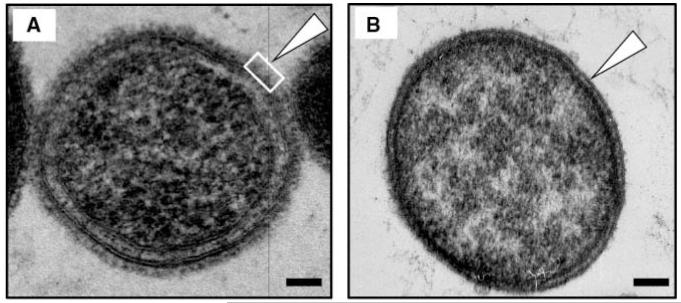
Transmission electron microscopy of ultrathin-sectioned Tf wt cells indicated the presence of a ~10-nm-thick S-layer completely surrounding the cells, while the S-layer was completely missing on Tf ΔtfsAB cells (Figs. 1A, 1B).
Figure 1.
TEM micrographs of an ultrathin-sectioned (A) T. forsythia ATCC 43037 wild-type cell and (B) an S-layer-deficient T. forsythia ΔtfsAB mutant cell. Open triangles point to the outermost surface of either cell. The S-layer-covered cell surface is indicated by an open square. Bars, 100 nm.
Viability of Macrophages and Human Gingival Fibroblasts
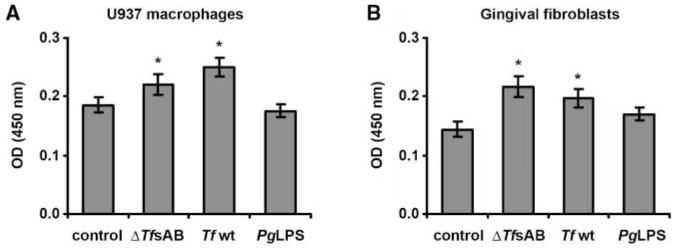
The viability of U937 macrophages and HGFs was significantly increased upon stimulation with both Tf wt and Tf ΔtfsAB mutant cells (Fig. 2), while P. gingivalis LPS did not increase cell viability. At the same time, no significant difference in the effects of the 2 types of T. forsythia on viability of macrophages and HGFs was observed.
Figure 2.
Viability of U937 macrophages (A) and human gingival fibroblasts (B) upon stimulation with T. forsythia ATCC 43037. Cells were stimulated with wild-type T. forsythia (107 cells/mL), T. forsythia ΔtfsAB mutant (107 cells/mL), or P. gingivalis LPS (1 μg/mL) for 24 hrs, and the cell viability was measured in an MTT assay. Each value represents the mean value ± SD of 8 values measured in one representative assay. A similar tendency was also observed in the other viability experiments. *Significantly different from control, with P < 0.01.
Cytokine Expression in U937 Macrophages
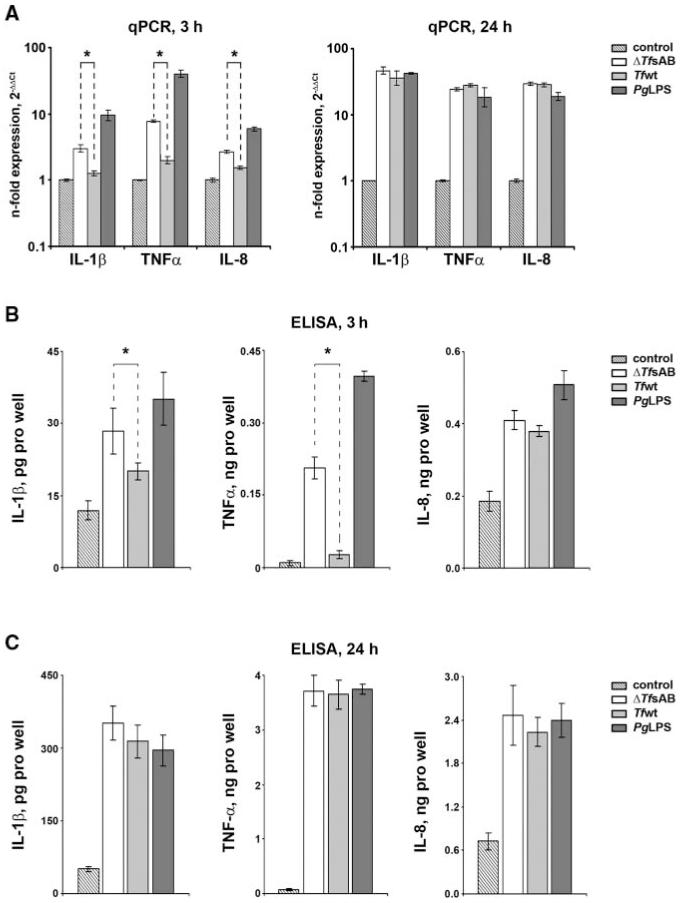
Changes in the levels of IL-1β, TNF-α, and IL-8 in U937 macrophages upon stimulation with Tf wt, Tf ΔtfsAB, and P. gingivalis LPS were determined by qPCR and ELISA (Fig. 3). After both three- and 24-hour stimulation, all stimuli induced significantly higher expression levels of pro-inflammatory mediators in U937 macrophages compared with non-stimulated cells. After 3 hrs of stimulation, macrophages treated with Tf ΔtfsAB exhibited significantly higher mRNA expression levels of all 3 cytokines in comparison with macrophages stimulated with Tfwt (Fig. 3A). This observation was supported by the measurements of cytokine levels in conditioned medium, showing significantly increased IL-1β and TNF-α production by U937 macrophages stimulated with Tf ΔtfsAB compared with macrophages stimulated with Tfwt (Fig. 3B). A similar tendency was observed also for IL-8, although the difference was not statistically significant. Both types of T. forsythia cells induced significantly lower cytokine expression levels in comparison with P. gingivalis LPS after 3 hrs of stimulation. After 24 hrs of stimulation, however, the difference in cytokine expression between differently stimulated cells was no longer observed (Figs. 3A, 3C).
Figure 3.
Cytokine expression in U937 macrophages upon stimulation with T. forsythia ATCC 43037. U937 macrophages were stimulated with wild-type T. forsythia (107 cells/mL), T. forsythia ΔtfsAB mutant cells (107 cells/mL), and P. gingivalis LPS (1 μg/mL) for 3 and 24 hrs, and the mRNA expression levels of IL-1β, TNF-α, and IL-8 (A), as well as the content of corresponding proteins in conditioned medium (B,C), were measured by qPCR and ELISA, respectively. Changes in the gene expression are presented by the relative amount of mRNA with the formula 2(−ΔΔCt), with non-stimulated macrophages as a control. Cytokine levels measured in the conditioned medium of non-stimulated macrophages were subtracted from those measured in conditioned medium of stimulated cells. The data are given as mean value ± SD of 4 different wells originating from one representative experiment. A similar tendency was also observed in the other qPCR experiments. *Significantly different, with P < 0.01.
IL-8 Expression in Human Gingival Fibroblasts
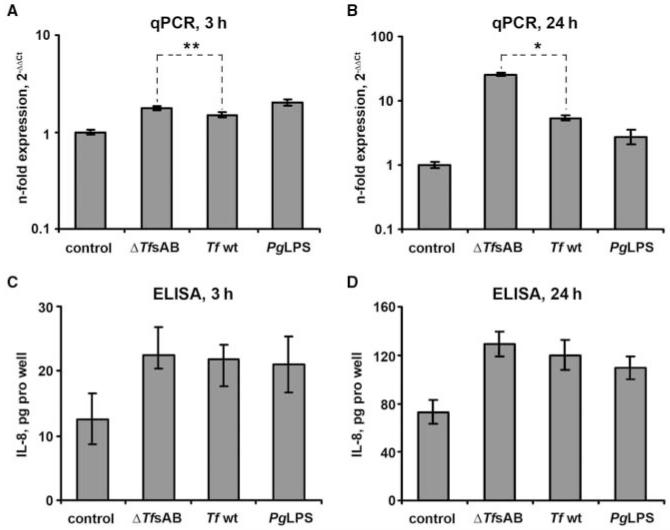
The IL-8 expression level in HGFs was significantly increased upon stimulation with Tfwt, Tf ΔtfsAB, and P. gingivalis LPS in comparison with non-stimulated cells after both 3 and 24 hrs of stimulation (Fig. 4). HGFs stimulated with Tf ΔtfsAB exhibited a significantly higher IL-8 mRNA expression level than those stimulated with the wild-type species (Figs. 4A, 4B). However, no significant difference in IL-8 production between HGFs stimulated with ΔtfsAB and Tf wt was observed by cytokine measurement (Figs. 4C, 4D). The mRNA levels of IL-1β and TNF-α as well as the levels of the corresponding cytokines in conditioned medium were below the methodological detection limit (data not shown).
Figure 4.
IL-8 expression in human gingival fibroblasts upon stimulation with T. forsythia ATCC 43037. Gingival fibroblasts were stimulated with wild-type T. forsythia (107 cells/mL), ΔtfsAB T. forsythia mutant cells (107 cells/mL), or P. gingivalis LPS (1 μg/mL) for either 3 or 24 hrs, and the change in the IL-8 expression level was measured by qPCR (A,B) and ELISA (C,D). Data are presented as in Fig. 3. *,** Significantly different, with P < 0.01 and P < 0.05, respectively.
DISCUSSION
We investigated the role of the S-layer of the periodontal pathogen T. forsythia in the host immune responses of macrophages and HGFs by determining the release of pro-inflammatory mediators. The study focused on the determination of changes in the expression of IL-1β, TNF-α, and IL-8, which are released by macrophages during the early phase of host cell stimulation and are associated with the acute phase of host response (Le and Vilcek, 1987). Both IL-1β and TNF-α may directly stimulate bone resorption in vitro and in vivo (Mundy, 1993) or stimulate production of prostaglandin E2 (Nakao et al., 2002; Rausch-Fan et al., 2005), which, in turn, is a potent stimulator of bone resorption (Offenbacher et al., 1993). IL-8 attracts neutrophils into the inflamed tissue, promoting the development of acute inflammation (Baggiolini et al., 1994).
T. forsythia at the concentration used in the present study had no cytotoxic effect on either macrophages or HGFs. An MTT assay (Mosmann, 1983) indicated that viability of both mammalian cell types was significantly increased by T. forsythia. In contrast, P. gingivalis LPS, which was used as a control, did not have such an effect. Thus, it can be speculated that while LPS activates Toll-like receptors, T. forsythia could be internalized by macrophages and stimulate intracellular receptors. Although the exact mechanism underlying increased viability is currently unclear, it might be important for providing long-lasting viability of host cells, which is critical for an effective immune response and appropriate bacterial clearance (Marriott et al., 2005).
The main observation of this study is that after 3 hrs of stimulation, macrophages treated with S-layer-deficient T. forsythia produced significantly higher levels of pro-inflammatory mediators than macrophages treated with wild-type T. forsythia, whereas after 24 hrs of stimulation, these differences were no longer observed. Thus, it is conceivable that the S-layer of T. forsythia delays the host immune response to this pathogen. The responses of macrophages upon stimulation with T. forsythia are complex, also involving autocrine responses to produced cytokines. In parallel, degradation of cytokines takes place. Obviously, the contributions of these processes are substantially higher after 24 hrs of stimulation than after 3 hrs of stimulation, which may be manifested in time-dependent differences of results.
The increased virulence activity of S-layer-deficient T. forsythia compared with that of the wild-type species was also confirmed in experiments with HGFs. Particularly, a Tf ΔtfsAB mutant induced a significantly higher mRNA expression level of IL-8 compared with Tfwt. A similar trend was observed by the measurement of the IL-8 level in HGFs’ conditioned medium, although in this case, the difference was not statistically significant. The differences observed for HGFs upon stimulation with different kinds of T. forsythia are especially important, because these cells are primary cells and, thus, partially mimic the situation in periodontal pockets. Expression of IL-1β and TNF-α in HGFs could not be detected, which is in agreement with a previous study with HGFs (Kent et al., 1996).
The functional importance of the S-layer for the modulation of the host immune response to T. forsythia is currently unclear. Since the primary aim of a host immune response is the removal of invading pathogens, one can assume that the S-layer of T. forsythia is a strategy to evade recognition by the innate immune system. Indeed, the S-layer of T. forsythia attenuates the host response at the initial phase and, thus, delays the clearance of this pathogen by the immune system. Several components of T. forsythia could be involved in the immune response to this pathogen. Particularly, LPS and bacterial DNA of T. forsythia were shown to induce production of pro-inflammatory cytokines by human macrophages, with the IL-8 secretion level of LPS from T. forsythia being about 1.5 times the effect of LPS from P. gingivalis (Bodet et al., 2006a; Sahingur et al., 2010). Considering the cell envelope architecture of T. forsythia, in which a rough LPS (G. Posch, O. Holst, C. Schäffer, manuscript in preparation) is proposed to serve as anchor for the S-layer to the outer membrane (Noonan and Trust, 1997), it is conceivable that the S-layer shields the LPS from recognition by the immune system, at least at the early stage of infection. The later response could be induced by some intracellular structures, for instance, bacterial DNA, and could be similar for both T. forsythia species. The exact mechanisms responsible for the differences in virulence activity of S-layer-deficient and wild-type T. forsythia remain to be further investigated.
ACKNOWLEDGMENTS
The authors thank Dr. Yukitaka Murakami (Aichi-Gakuin University, Nagoya) for providing the T. forsythia S-layer mutant, and Nguyen Phuong Quynh and Hedwig Rutschek for technical assistance. Financial support came from the Austrian Science Fund FWF, project P20605-B12 (to C.S.).
Abbreviations
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- GAPDH
glycerinaldehyde-3-phosphate-dehydrogenase
- HGFs
human gingival fibroblasts
- LPS
lipopolysaccharide
- MEM
minimal essential medium
- MTT
3,4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide
- OD
optical density
- PBS
phosphate-buffered saline
- qPCR
quantitative polymerase chain-reaction
- SD
standard deviation
- Tf wt
Tannerella forsythia ATCC 43037
- Tf ΔtfsAB
Tannerella forsythia ATCC 43037 S-layer mutant
REFERENCES
- Ara T, Kurata K, Hirai K, Uchihashi T, Uematsu T, Imamura Y, et al. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J Periodontal Res. 2009;44:21–27. doi: 10.1111/j.1600-0765.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Beck JD, Slade G, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontol 2000. 2000;23:110–120. doi: 10.1034/j.1600-0757.2000.2230111.x. [DOI] [PubMed] [Google Scholar]
- Bertl K, An N, Bruckmann C, Dard M, Andrukhov O, Matejka M, et al. Effect of enamel matrix derivative on proliferation/viability, migration and expression of angiogenic factor and adhesion molecules in endothelial cells in vitro. J Periodontol. 2009;80:1622–1630. doi: 10.1902/jop.2009.090157. [DOI] [PubMed] [Google Scholar]
- Bodet C, Grenier D. Synergistic effects of lipopolysaccharides from periodontopathic bacteria on pro-inflammatory cytokine production in an ex vivo whole blood model. Mol Oral Microbiol. 2010;25:102–111. doi: 10.1111/j.2041-1014.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Modulation of cytokine production by Porphyromonas gingivalis in a macrophage and epithelial cell co-culture model. Microbes Infect. 2005;7:448–456. doi: 10.1016/j.micinf.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J Dent Res. 2006a;85:235–239. doi: 10.1177/154405910608500306. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Inflammatory responses of a macrophage/epithelial cell co-culture model to mono and mixed infections with Porphyromonas gingivalis, Trepomena denticola, and Tannerella forsythia. Microbes Infect. 2006b;8:27–35. doi: 10.1016/j.micinf.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Hasebe A, Yoshimura A, Into T, Kataoka H, Tanaka S, Arakawa S, et al. Biological activities of Bacteroides forsythus lipoproteins and their possible pathological roles in periodontal disease. Infect Immun. 2004;72:1318–1325. doi: 10.1128/IAI.72.3.1318-1325.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Hughes CV, Malki G, Loo CY, Tanner AC, Ganeshkumar N. Cloning and expression of α-D-glucosidase and N-acetyl-β-glucosaminidase from the periodontal pathogen, Tannerella forsythensis (Bacteroides forsythus) Oral Microbiol Immunol. 2003;18:309–312. doi: 10.1034/j.1399-302x.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Kent LW, Dyken RA, Rahemtulla F, Allison AC, Michalek SM. Effect of in vitro passage of healthy human gingival fibroblasts on cellular morphology and cytokine expression. Arch Oral Biol. 1996;41:263–270. doi: 10.1016/0003-9969(95)00127-1. [DOI] [PubMed] [Google Scholar]
- Kerosuo E. Ultrastructure of the cell envelope of Bacteroides forsythus strain ATCC 43037T. Oral Microbiol Immunol. 1988;3:134–137. doi: 10.1111/j.1399-302x.1988.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000. 2001;25:8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. [DOI] [PubMed] [Google Scholar]
- Le J, Vilcek J. Tumor necrosis factor and interleukin-1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56:234–248. [PubMed] [Google Scholar]
- Lee SW, Sabet M, Um HS, Yang J, Kim HC, Zhu W. Identification and characterization of the genes encoding a unique surface (S-)layer of Tannerella forsythia. Gene. 2006;371:102–111. doi: 10.1016/j.gene.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Marriott HM, Bingle CD, Read RC, Braley KE, Kroemer G, Hellewell PG, et al. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P, Pum D, Sleytr UB. Characterization of the ultrastructure and the self-assembly of the surface layer of Bacillus stearothermophilus strain NRS 2004/3a. J Ultrastruct Mol Struct Res. 1986;97:73–88. doi: 10.1016/s0889-1605(86)80008-8. [DOI] [PubMed] [Google Scholar]
- Moncla BJ, Braham P, Hillier SL. Sialidase (neuraminidase) activity among Gram-negative anaerobic and capnophilic bacteria. J Clin Microbiol. 1990;28:422–425. doi: 10.1128/jcm.28.3.422-425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Role of cytokines in bone resorption. J Cell Biochem. 1993;53:296–300. doi: 10.1002/jcb.240530405. [DOI] [PubMed] [Google Scholar]
- Nakao S, Ogata Y, Shimizu E, Yamazaki M, Furuyama S, Sugiya H. Tumor necrosis factor α (TNF-α)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFκB in human gingival fibroblasts. Mol Cell Biochem. 2002;238:11–18. doi: 10.1023/a:1019927616000. [DOI] [PubMed] [Google Scholar]
- Noonan B, Trust TJ. The synthesis, secretion and role of virulence of the paracrystalline surface layer protein layers of Aeromonas salmonicida and A. hydrophila. FEMS Microbiol Lett. 1997;154:1–7. doi: 10.1111/j.1574-6968.1997.tb12616.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432S–444S. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- Qu Z, Laky M, Ulm C, Matejka M, Dard M, Andrukhov O, et al. Effect of Emdogain on proliferation and migration of different periodontal tissue associated cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:924–931. doi: 10.1016/j.tripleo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Rausch-Fan X, Ulm C, Jensen-Jarolim E, Schedle A, Boltz-Nitulescu S, Rausch WD, et al. Interleukin-1β-induced prostaglandin E2 production by human gingival fibroblasts is upregulated by glycine. J Periodontol. 2005;76:1182–1188. doi: 10.1902/jop.2005.76.7.1182. [DOI] [PubMed] [Google Scholar]
- Sabet M, Lee SW, Nauman RK, Sims T, Um HS. The surface (S-) layer is a virulence factor of Bacteroides forsythus. Microbiology. 2003;149:3617–3627. doi: 10.1099/mic.0.26535-0. [DOI] [PubMed] [Google Scholar]
- Sahingur SE, Xia X-J, Alamgir S, Honma K, Sharma A, Schenkein HA. DNA from Porphyromonas gingivalis and Tannerella forsythia induce cytokine production in human monocytic cell line. Mol Oral Microbiol. 2010;25:123–135. doi: 10.1111/j.2041-1014.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ishihara K, Kato T, Okuda K. Cloning, expression, and sequencing of a protease gene from Bacteroides forsythus ATCC 43037 in Escherichia coli. Infect Immun. 1997;65:4888–4891. doi: 10.1128/iai.65.11.4888-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara J, Nagano K, Murakami Y, Higuchi N, Nakamura H, Shimozato K, et al. Loss of adherence ability to human gingival epithelial cells in S-layer protein-deficient mutants of Tannerella forsythensis. Microbiology. 2007;153:866–876. doi: 10.1099/mic.0.29275-0. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sojar HT, Glurich I, Honma K, Kuramitsu HK, Genco RJ. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect Immun. 1998;66:5703–1570. doi: 10.1128/iai.66.12.5703-5710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr UB, Egelseer EM, Ilk N, Messner P, Schäffer C, Pum D, et al. Nanobiotechnological applications of S-layers. In: König H, Claus H, Varma A, editors. Prokaryotic cell wall compounds – structure and biochemistry. Springer-Verlag; Berlin: 2010. pp. 459–481. [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Hirofuji T, Motooka N, Nozoe K, Shigenaga K, Anan H, et al. Humoral immune responses to S-layer-like proteins of Bacteroides forsythus. Clin Diagn Lab Immunol. 2003;10:383–387. doi: 10.1128/CDLI.10.3.383-387.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]