Abstract
Adult neurogenesis is limited to specific brain regions in the mammalian brain, such as the hippocampal dentate gyrus and the subventricular zone/olfactory bulb system. Alterations in adult neurogenesis appear to be a common hallmark in different neurodegenerative diseases including Parkinson's disease (PD), Alzheimer's disease (AD), and Huntington's disease (HD). This is remarkable, because the distinct pathological proteins responsible for the different diseases induce the loss of different neural populations. Impaired adult neurogenesis was shown in numerous animal models of neurodegenerative diseases; however, only few postmortem studies have been performed. We will review concepts related to the interplay between cellular plasticity in regions of adult neurogenesis with a specific focus on cell-autonomous and non-cell-autonomous factors. Furthermore, various strategies aimed to stimulate neuronal plasticity will be discussed within the context of a potential translation into therapeutic approaches for neuropsychiatric symptoms associated with PD, HD, and AD.
Different neurodegenerative diseases (Parkinson's, Huntington's, and Alzheimer's) involve distinct pathological proteins. However, adult neurogenesis appears to be disrupted in all cases.
REGULATION OF NEUROGENESIS IN THE ADULT BRAIN
Altman and colleagues were the first scientists to report adult neurogenesis (Altman and Das 1965; Altman 1969), and this observation changed the dogma that the mammalian brain is incapable of generating new neurons. New neurons generated throughout life are one component of brain plasticity—a cellular process—however, limited to distinct brain regions harboring adult neural stem and precursor cells, primarily the subventricular zone (SVZ) adjacent to the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus. As described in detail elsewhere in the literature, adult neurogenesis involves several crucial steps of neural development. An important aspect regarding adult neurogenesis is its modulation as a result of a variety of genetic, epigenetic, and transcriptional factors as well as environmental factors, age, and acute and chronic diseases (Ma et al. 2010; Mu et al. 2010). At this point, one may ask: Why is the generation of new neurons in distinct regions of the brain important for neurodegenerative diseases?
Gradual loss of different neuronal populations occurs in monogenic and sporadic neurodegenerative diseases. Diseased neurons have deficits in synaptic transmission and this is associated with axonal and dendritic degeneration (reviewed in Luo and O’Leary 2005). Impaired adult neurogenesis in neurodegenerative diseases indicates that in addition to losing existing neurons, the adult brain's endogenous capacity for cell renewal and the putative function of these new neurons is compromised or lost.
Despite disease-specific patterns of neurite degeneration and loss of neurons within specific neurotransmitter populations, “pre-disease”-related symptoms observed in the early stages of Parkinson's disease (PD), Alzheimer's disease (AD), and Huntington's disease (HD) frequently include depression, anxiety, cognitive, or olfactory dysfunction, symptoms linked to olfactory or hippocampal function (Simuni and Sethi 2008; Stout et al. 2011; Hinnell et al. 2012), the main regions of adult neurogenesis. Therefore, there may be an increased vulnerability within regions of cellular plasticity caused by the underlying neurodegenerative processes (Braak et al. 2003; Pavese et al. 2010; Carlesimo et al. 2012). Specifically, there may be neurogenesis-related dysfunctions for distinct sensory, emotional, and cognitive processes in the context of different neurodegenerative diseases. In particular, some of the patients’ symptoms early in the course of these diseases may be connected to deficits in adult neurogenesis.
In addition to the primarily diseased neurons, many neurodegenerative diseases present with pathology in glial cells and changes in the brain environment. Therefore, an important aspect of neurodegeneration may affect surrounding cells and signals—in particular, glia. The fate of “newly generated neurons in a diseased brain” represents a unique model system to study early cell-autonomous and non-cell-autonomous changes in neurodegenerative diseases.
Therefore, the next question is, are new neurons a strategy to replace neurons lost during the progress of neurodegenerative diseases?
In PD, this question has been addressed by various approaches of cell-replacement therapies. Since the early 1980s, transplantation of human dopamine-producing fetal midbrain neurons into the striatum of PD patients was performed. These studies provided the proof of principle that transplanted fetal cells survive, produce dopamine, and functionally integrate, also shown by an increased fluorodopa uptake in the putamen (Hauser et al. 1999). Although open-label trials led to striking clinical improvements, the negative outcome of double-blind trials in the United States was a severe setback because of adverse side effects, namely, graft-induced dyskinesias and limited motor improvements, terminating transplantation programs for more than a decade (Freed et al. 2001). The limited occurrence of Lewy bodies (2%–5%) in grafted human fetal mesencephalic neurons and an increased microglial response within the graft in few patients with PD suggested an adverse host-to-graft interaction. More importantly, the presence of a fetal synucleinopathy triggered extensive studies on cell–cell interactions and disease-specific propagation of α-synuclein (α-syn) in PD (Kordower et al. 2008; Li et al. 2008). Currently, transplantation approaches are further explored with a specific focus on the precise selection of patients and the cell type chosen for transplantation. Specifically, human pluripotent stem cell–derived neuronal cells might provide a future cellular source for these techniques. Recently, a new trial has been instated that aims at reviving and refining transplantation techniques, funded by the EU as the multicenter project TRANSEURO (Petit et al. 2014).
All of these techniques will strongly rely on an in-depth understanding of how young neurons are able to integrate and survive in a rather hostile neurodegenerative microenvironment. To foster this understanding, the adult neural stem cell niche with its endogenous pool of neural stem cells for adult neurogenesis is an ideal “physiological” scenario. In this regard, a recently published differentiation paradigm for human pluripotent stem cells that enriches for hippocampal DG granule neurons will be of great value. This differentiation paradigm recapitulates the expression patterns of key developmental genes during hippocampal neurogenesis, shows characteristics of neuronal network maturation, and produces PROX1+ neurons that functionally integrate into the DG (Yu et al. 2014). Specifically, this advance will allow one to combine classical rodent model–based hippocampal neurogenesis studies with disease modeling using human neurons and will offer important insights into the neurodevelopmental aspects of neurodegenerative diseases. We will review current reports about adult neurogenesis in monogenic and sporadic neurodegenerative disorders and try to decipher common pathogenic mechanisms.
PD: DISEASE MECHANISMS
PD is the most common movement disorder with an increasing prevalence because of an aging population (de Lau and Breteler 2006). Motor symptoms include bradykinesia, rigidity, resting tremor, and postural instability, predominantly linked to the degeneration of dopaminergic neurons in the substantia nigra pars compacta.
The neuropathological hallmark of PD is the accumulation of α-syn as intracellular deposits in Lewy bodies and Lewy neurites (Spillantini et al. 1998). Mutations in the human α-syn gene (A30P, E46K, H50Q, and A53T) are observed in rare autosomal-dominant forms of PD (Polymeropoulos et al. 1997; Kruger et al. 1998; Zarranz et al. 2004; Appel-Cresswell et al. 2013).
Nonmotor symptoms in PD like depression, anxiety, cognitive and olfactory deficits, and autonomic dysfunction are likely associated with other neurotransmitter systems. These symptoms occur both early and throughout the entire disease course and have a strong impact on the quality of life. In particular, depression is an important nonmotor symptom, present in up to two-thirds of all PD patients (Tolosa et al. 2009; Gallagher et al. 2010; Hinnell et al. 2012). Moreover, large-scale epidemiological studies observed cognitive impairments in up to 20% of PD patients at disease onset (Aarsland et al. 2009); in advanced stages, more than 80% of patients may develop dementia (Hely et al. 2008). Recent studies in PD patients point to an early affection of the hippocampus and olfactory bulb (OB) in the disease process (Braak et al. 2003; Weintraub et al. 2011; Carlesimo et al. 2012), making the investigation of adult hippocampal and OB/SVZ neurogenesis the focus of research.
ADULT NEUROGENESIS IN PD
Human Disease
Postmortem analysis of adult neurogenesis in PD and related synucleinopathies are still very limited and technically challenging. In particular, the analysis of proliferation in the SVZ of human PD brains led to conflicting results. An interesting debate has emerged over the question of whether a decreased proliferation of SVZ progenitors is present in PD patients at a late stage of the disease (Höglinger et al. 2004; van den Berge et al. 2011).
A significant reduction of proliferating cell nuclear antigen (PCNA)-positive cells in the human SVZ (n = 4 per group) of patients with clinically and pathologically diagnosed PD (mean age 68.5 ± 3.2, postmortem time 26.5 ± 4.8) compared with controls (mean age 66.0 ± 11.1, postmortem time 27.8 ± 10.5) was reported (Höglinger et al. 2004). Fewer proliferating cells (epidermal growth factor receptor [EGFR]-positive cells) in the human SVZ were also reported in a different study (O’Keeffe et al. 2009). In general, the limited numbers of postmortem cases is still a common drawback of these studies.
The Hol group (van den Berge et al. 2011) investigated 10 controls, 10 PD patients, and five patients with incidental Lewy body disease and detected no difference in the number of proliferating cells for PCNA or the G2/M phase marker phosphohistone H3 for PD and age-and sex-matched controls. Similar amounts of PCNA and H3 positive cells were found in incidental Lewy body disease. This PD cohort (79.5 ± 5.5 for controls and 79.3 ± 5.0 for PD) was more than 10 years older than the population in the study from the Hirsch group with a very short postmortem time (means between 5 and 8 h within the different groups). The donor population was matched for a broad range of variables, most importantly Braak staging.
The published studies largely differ in the postmortem times, the analyzed area of the SVZ, the markers, and immunostaining protocols used. Although this controversy is not yet conclusively solved at present, sufficient study numbers and standardized fixation, immunostaining, and analysis methods will be necessary to draw a final conclusion regarding SVZ proliferation in PD.
Even less is known about neurogenic activity in the DG of the hippocampus in PD. The Hirsch group observed a decreased number of DG cells expressing nestin and β-III-tubulin in three PD patients and five patients suffering from PD with dementia (PDD) compared with three controls (Höglinger et al. 2004). Interestingly, PDD shows a more severely decreased number of nestin-expressing cells in the human DG. The number of Sox2-expressing cells in the DG is reduced in dementia with Lewy bodies (DLB), and this was paralleled by an increase in α-syn-positive cells (Winner et al. 2012). The current findings are of particular interest because a decreased hippocampal neurogenesis was also described in patients suffering from depression. Namely, the number of proliferating cells in the human DG stained with the marker minichromosome maintenance 2 was significantly reduced in depressed patients (n = 10) compared with 10 healthy controls (Lucassen et al. 2010), and increased in patients treated with selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (Boldrini et al. 2009, 2012).
At this point, we also will have to acknowledge that the human system may rely on different dynamics (Spalding et al. 2013). Specifically, neuronal precursor cells generated in the lateral ventricle wall, the site of origin of newly generated OB neurons in other mammals, do not migrate to the human OB. Recently, the Frisen group identified a unique pattern of adult neurogenesis in humans by retrospective birth dating and revealed continuous generation of striatal interneurons in humans (Ernst et al. 2014). Therefore, some hypotheses and data obtained in rodent models concerning stem cell niches and adult neurogenesis, but also more general mechanisms, may or may not be translated to the human condition.
Adult Neurogenesis in Transgenic Animal Models of PD
The majority of transgenic animal models for PD lack significant nigrostriatal degeneration. However, progressive neuropathology, and, specifically, nonmotor deficits frequently observed in PD have been obtained. Transgenic mice–carrying mutations or overexpressing PD-related genes are of particular interest for these purposes. Specifically, the functional role of genes encoding for α-syn, leucin-rich repeat kinase 2 (LRRK2), parkin, PINK1, and DJ-1 have been studied in transgenic animals (for review, see Rockenstein et al. 2007; Dawson et al. 2010).
The analysis of adult neurogenesis in the different transgenic animal models of PD revealed distinct impairments of proliferative activity and survival of newly generated neurons (reviewed in Winner et al. 2011; Marxreiter et al. 2013). Here, we will focus on α-syn and LRRK2 models.
α-syn has been identified in several species, indicating that an evolutionary conserved role and physiological modulation of α-syn was specifically attributed to neuronal remodeling, namely, songbird learning. Although not essential for cell survival or synapse formation, α-syn plays an important role in presynaptic dopamine recruitment and synaptic transmission (Iwai et al. 1995; Abeliovich et al. 2000). Recently, we used double α/β-syn knockout mice (Chandra et al. 2004), and observed that the knockdown of α/β-syn resulted in a proportional increase of new neurons in the hippocampal DG (Winner et al. 2012). To elucidate mechanisms of α-syn-mediated neurodegeneration in PD, transgenic α-syn models overexpressing of α-syn were used (Masliah et al. 2000; Hashimoto et al. 2003; Nuber et al. 2008).
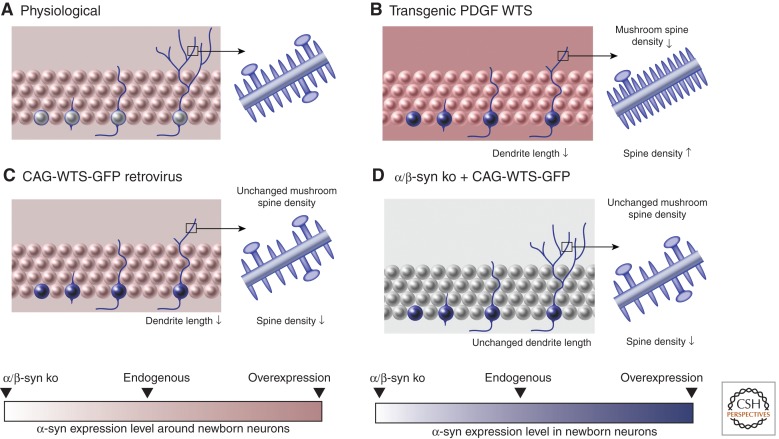
The first transgenic mouse model described for α-syn was a mouse-carrying human wild-type (wt) α-syn under the control of the human platelet–derived growth factor (PDGF)-β promoter (Masliah et al. 2000). Accumulation of human α-syn in various brain regions, including the cortex and the hippocampal formation and spatial memory deficits, are present (Masliah et al. 2011). In the hippocampal DG, coexpression of human wt α-syn and neural progenitor markers in the DG is found as early as Sox2 expression, but also later in developing doublecortin (DCX)-expressing neuroblasts and mature neurons (Winner et al. 2004, 2012). The overexpression of wt α-syn led to a decreased hippocampal neurogenesis paralleled by increased cell death. At the same time, the proliferation of neural stem cells was not affected (Winner et al. 2004). We labeled newly generated neurons in the DG of PDGF wt α-syn animals with an enhanced green fluorescent protein (eGFP) retrovirus, and we observed that dendrite outgrowth of newly generated neurons was significantly impaired in the wt α-syn mice. At the same time, an increased spine density in new neurons was observed in the wt α-syn mice, possibly indicating a compensatory effect because of reduced connectivity. Reductions in mushroom spines, reflecting a lack of maturation, most likely indicate that overexpression of α-syn hinders normal levels of presynaptic activity. To further distinguish cell-autonomous from non-cell-autonomous effects, we investigated selective overexpression of wt α-syn in newly generated neurons. Again, we observed a decrease in dendritic length and impaired dendritic branching (Winner et al. 2012). When endogenous mouse α/β-syn was absent, the effect of dendrite length reduction following selective overexpression of α-syn in newly generated neurons by injecting a retrovirus overexpressing human α-syn was no longer present in the α/β-syn knockout mice. These experiments showed that cell-autonomous overexpression of wt α-syn induced a reduction in dendrite length. This reduced dendrite length could be modulated by changes in the concentration of endogenous α-syn levels (Winner et al. 2012). These data strongly suggest differential effects of cell-intrinsic wt α-syn and overexpressed α-syn in surrounding DG cells on hippocampal neural stem cells and the maturation of newly generated DG neurons in this transgenic α-syn model, and provides evidence that gene dosage has an impact on neurite outgrowth and maintenance.
A conditional model expressing human wt α-syn under the control of the calcium/calmodulin-dependent protein kinase (CaMK)-IIα promoter showed widespread distribution of α-syn in the brain including the hippocampus, which was partly reversed by shutdown of the transgene (Nuber et al. 2008). These animals showed motor impairments and spatial learning deficits by 12 months of age. Interestingly, a decreased DG neurogenesis without affecting proliferation of adult neural stem cells was observed. More importantly, this detrimental effect on DG neurogenesis was reversible by conditional cessation of transgene expression, indicated by an increased number of DCX-expressing DG neuroblasts (Nuber et al. 2008).
Interestingly, the expression of human mutant A53T α-syn under the PDGF promoter led to a severe neuropathological phenotype (Hashimoto et al. 2003), paralleled by an even more severe decreased hippocampal neurogenesis. The number of newly generated neurons was significantly reduced; even the proliferation of neural stem cells in the DG of these transgenic mice was decreased (Crews et al. 2008; Kohl et al. 2012). Besides the contribution of α-syn to impaired proliferation, differentiation, and survival of newly generated DG neurons, compromised neurotransmitter systems (serotonergic or dopaminergic efferents) may play an additional role for the decreased DG neurogenesis.
In addition, animals transgenic for LRRK2 containing the most frequent G2019S mutation show increased anxiety-related behavior (Melrose et al. 2010). Moreover, LRRK2 is highly expressed in the hippocampus, in particular, in PSA-NCAM expression neuroblasts (Melrose et al. 2007). Importantly, transgenic G2019S LRRK2 mice showed reduced proliferation of neural stem cells, which finally led to a strong decrease (by 77%) of survival of newly generated neurons in the DG (Winner et al. 2011). In addition, transfection studies outlined that dendritic branching was severely impaired in primary neuronal cultures in vitro (MacLeod et al. 2006) as well as in newly generated neurons of the adult hippocampus in vivo (Winner et al. 2011), whereas knockout of LRRK2 resulted in an increase in dendrite length (Paus et al. 2013).
The impact of overexpressed α-syn on dendritic development and spine formation of new DG neurons (Winner et al. 2012) showed the significance of the α-syn protein itself on the integration of new neurons in the DG, a crucial step in hippocampal neurogenesis (van Praag et al. 2002). Moreover, the deleterious effect of mutant A53T α-syn on the proliferative capacity of neural stem cells in the DG in contrast to wt α-syn (Crews et al. 2008; Kohl et al. 2012) is suggestive of a differential effect of α-syn species as well as different structural states of α-syn (i.e., oligomers, fibrils, and aggregates) (Winner et al. 2011) on proliferating and maturing hippocampal neurons.
Further possible mechanisms are related to alterations in the expression of proneurogenic growth factors. Activation of the cAMP response element-binding (CREB) protein by the phosphodiesterase inhibitor rolipram was able to rescue the dendrite outgrowth defect following α-syn overexpression (Winner et al. 2012). Recently, we detected a reduction of brain-derived neurotrophic factor (BDNF) and glial cell–derived neurotrophic factor (GDNF) in A53T α-syn mice compared with nontransgenic littermates (Kohl et al. 2012). Although serum levels of BDNF are reduced in PD patients (Scalzo et al. 2010), impairments of the local trophic support of newly generated DG neurons might play a role in the reduction of neurogenesis in the A53T α-syn transgenic model (Fig. 1).
Figure 1.
Model of effects of human wild-type α-synuclein (WTS) and mouse endogenous α-synuclein in the dentate gyrus (DG). (A) A newly generated neuron integrating into the DG is shown on the left part of all images; the right part depicts a higher magnified insert of a dendrite showing spines and the larger mushroom spines (A–D). (B–D) Models showing the impact of WTS on dendrite and spine development. (B) Platelet-derived growth factor (PDGF) WTS transgenic mice. (C) Retroviral overexpression of WTS using a CAG-WTS-green fluorescent protein (GFP) construct. (D) Retroviral overexpression of WTS on an α/β-synuclein knockout background. Cell-specific overexpression of WTS results in decreased dendrite length and spine density (B,C), whereas nonspecific overexpression (C) leads to a decrease in mushroom spines, as indicated in the purple panels showing an example of magnification of the spines (according to Winner et al. 2012). CAG, Cytomegalovirus (CMV) enhancer fused to the chicken β-actin promoter.
AD: DISEASE MECHANISMS
AD, first described by the German psychiatrist and neuropathologist Alois Alzheimer in 1906, is the most common form of dementia in adulthood. Patient deficits include olfactory deficits, memory impairment, and cognitive and functional deterioration. Widespread neurodegeneration throughout the basal forebrain, cortex, and limbic system as a result of neuronal and synaptic loss is observed. Specific hallmarks of AD are neurofibrillary tangles caused by hyperphosphorylated tau proteins and amyloid plaque deposition (Hardy and Selkoe 2002). Amyloid-β (Aβ) is the product of proteolysis of amyloid precursor protein (APP) by β- and γ-secretase enzymes (reviewed in Crews et al. 2010b). No curative treatment exists for AD.
Genetic studies have identified mutations in genes like APP, presenilin (PSEN)1, and PSEN2 to cause familial AD. They result in toxic Aβ oligomers and deposition of the Aβ42 peptide and the accumulation of intracellular and/or extracellular Aβ species (i.e., K670N/M671 L, Swedish mutation; V717I, London mutation; V717F, Indiana mutation) or amyloid angiopathy (i.e., D23N, Iowa mutation) (Selkoe 1998; Walsh and Selkoe 2007). Presenilin is the catalytic component of γ-secretase cutting APP (De Strooper 2003) and, at the same time, plays a role in regulating the Notch and Wnt signaling mechanisms by sequentially cleaving the notch receptor to generate a notch intracellular domain (NICD) (Kojro and Fahrenholz 2005). For AD research, a model of great interest is the triple transgenic (tg) model (overexpression of Swedish mutant APP, mutant P301L tau in a homozygous mutant of PS1 [M146V]) knock-in mouse, because these mice develop hippocampal tangle-like pathology, neurological deficits, and amyloid deposition (Crews et al. 2010a).
ADULT NEUROGENESIS IN AD
Human Disease
Studies of human AD have not been able to clarify the role of AD pathology on adult neurogenesis yet. Although an increase in protein expression and cell numbers of neurogenesis-related proteins (e.g., DCX and PSA-NCAM, TUC-4 and NeuroD) from AD hippocampus (Jin et al. 2004) was reported, other studies found an increase in gliosis and vascular-associated changes in presenile AD human hippocampus (Boekhoorn et al. 2006) or a decrease in DCX- and Sox2-positive cells in human AD in combination with increased BMP6 levels (Crews et al. 2010a). Some of these contradictory findings might be explained by a gene expression study, showing that AD neuropathology in the prefrontal cortex is preceded by changes in gene expression that point to increased synaptic activity and plasticity in human AD brains at different Braak stages (Bossers et al. 2010). Clearly, future studies depend on defined clinical data providing treatment and precise disease stages. Approaches like measuring the concentration of the nuclear bomb test–derived 14C in genomic DNA might be a means to better analyze cell turnover dynamics in AD in the future (Spalding et al. 2013).
Adult Neurogenesis in Transgenic Animal Models of AD
When comparing studies of adult neurogenesis in animal models of AD, there seems to be a huge variability, depending on promoters, age of the animal and age of onset of the disease, transgene expression, neurotransmitter level, and amount of overexpression/loss of the disease-causing protein. In different AD transgenic models, adult neurogenesis was altered in both directions, decreased and increased (reviewed in Lazarov and Marr 2010; Marlatt and Lucassen 2010; Mu et al. 2010). Experimental conditions largely differ and have been extensively discussed (Lazarov and Marr 2010; Marlatt and Lucassen 2010). Another difficulty in characterizing adult neurogenesis in AD is that there are both extracellular (amyloid-mediated) and intracellular (tau-mediated) AD pathology and, thus, it is hard to delineate cell-autonomous and non-cell-autonomous effects of AD pathology in the stem cell niche.
Although a single APP transgene mutation (Indiana mutation) induced a decreased adult neurogenesis in animals with amyloid deposition (Donovan et al. 2006), double and triple mutations of APP (APP Swedish and Indiana) under many circumstances result in increased proliferation and, in some cases, survival of new neurons (Haughey et al. 2002; Mirochnic et al. 2009). A potential explanation of these divergent results might be a temporal distinct susceptibility in response to protein overexpression associated with glial response and aberrant cell-cycle changes, as described in other brain regions (Yang et al. 2001; Varvel et al. 2009).
An important study in adult neurogenesis was performed in the commonly used pan AD model, the triple transgenic mice (3xTg-AD) harboring three mutant genes (APP, PSEN1, and tau). Decreased proliferation was detected and correlated with the presence of β-amyloid plaques (Rodriguez et al. 2008).
Sisodia and colleagues studied the neural stem cell (NSC) autonomous versus nonautonomous effect by performing coculture experiments of NSCs and microglia derived from PSEN mutants or controls. A decreased proliferation and less neuronal lineage commitment could be achieved in wild-type NSCs when cocultured with PSEN1 mutated microglia (Choi et al. 2008). This indicates that the non-NSC-autonomous mechanisms might be an important key to understanding how AD-related proteins act on newly generated neurons. A detailed summary of the divergent reports on adult neurogenesis in PSEN models (reviewed in van Tijn et al. 2010) recently indicated the difficulty that most of these studies were performed in young mice and, rarely, old mice (e.g., PS1-P264 L/KI mice) (Zhang et al. 2007). More recently, it has become more evident that Aβ interferes with the homeostasis of intrahippocampal neurotransmitters, such as the γ-aminobutyric acid (GABA) and glutamate (Schinder et al. 2009). In particular, Aβ-mediated alterations of the GABAergic neurotransmission or an imbalance between hippocampal GABAergic and glutamatergic neurotransmission result in an impaired hippocampal neurogenesis in AD. Specifically, Aβ modifies the balance between excitatory and inhibitory inputs on adult-born neurons (Sun et al. 2009). Furthermore, risk genes for AD, such as the apolipopotein E, may have a detrimental effect on adult hippocampal neurogenesis as a result of altered signaling that promotes glial differentiation at the expense of neurogenesis (Li et al. 2009).
Investigating adult neurogenesis in AD is involved in a larger picture of alterations in synaptic plasticity, spine morphology, and axonal pathology. A systematic comparison of different AD transgenic models under the same promoter with a similar labeling paradigm, sex, age, and background including analysis of neuronal circuits, activity-dependent modifications, and functional testing is a crucial requirement to draw meaningful conclusions for the relevant AD preclinical models.
HD: DISEASE MECHANISMS AND CURRENT TREATMENT
Cytomegalovirus (CMV) enhancer fused to the chicken β-actin promoter (CAG) trinucleotide-repeat expansion (>39) within the disease-causing huntingtin gene causes HD, a progressive autosomal-dominant neurodegenerative disease and the most common hereditary hyperkinetic movement disorder (for review, see Pringsheim et al. 2012). An extended polyglutamine tract in the huntingtin (htt) protein was found (MacDonald et al. 1993). The clinical symptoms of HD are progressive involuntary choreatic movements, cognitive decline, and affective symptoms (reviewed in Walker 2007). Impaired olfactory and cognitive functions as well as depressive symptoms are frequently present in patients and presymptomatic gene carriers and precede the onset of motor symptoms for many years (Mochel et al. 2007; Stout et al. 2011). Despite the fact that, since the discovery of the HD gene in 1993, the disease is diagnosed years before the onset of first symptoms; so far, no disease-modifying therapy exists. Oligomerization and aggregation of the mutant htt result in neuronal damage in medium spiny neurons of the neostriatum and other neurons, such as in the cortex (reviewed in Li and Li 2004; Sathasivam et al. 2010).
ADULT NEUROGENESIS IN HD
Human Disease
Curtis et al. (2003) described an increased cell proliferation in the SVZ of human HD patients. Increased PCNA numbers correlated with severity of disease and the number of CAG repeats. In the human ventricle wall, the PCNA/glial fibrillary acidic protein (GFAP)-colabeled cells were found in the most superficial regions close to the ventricular edge (Curtis et al. 2003, 2005). The same group could not detect significant differences in cell proliferation in the DG of HD patients compared with healthy controls (Low et al. 2011), using PCNA as a proliferation marker. The patient samples used in this study were at a much later stage of progression of the disease compared with studies in HD mice, and medication, such as antidepressant treatment (SSRIs), might have had an additional effect on DG neurogenesis (Boldrini et al. 2009, 2012; Lucassen et al. 2010).
PCNA/β-III tubulin-positive cells were reported close to the human HD caudate nucleus (Curtis et al. 2005), which might suggest potential migration of neural precursors to the degenerating striatum. This phenomenon of neuroblasts migrating toward the striatum was also reported in transgenic HD mice (R6/2) (Phillips et al. 2005; Kohl et al. 2010). However, these striatal neuroblasts were not able to mature into functional neurons, indicating that the striatal microenvironment did not allow functional integration. New insights might arise from further investigation of the quiescent stem cell population in these brains, specifically NSCs that express Sox2 and GFAP. Using a novel carbon-14 dating approach, a recent study indicates that newly generated neuronal precursors exist in the striatum that may have migrated from the SVZ (Ernst et al. 2014). The newly generated neurons in the striatum appear to be interneurons. The medium spiny neurons depleted in HD are most likely not renewed postnatally at a significant level; however, in HD brains, a reduction of postnatally generated interneurons and cells of the oligodendrocyte lineage was detected (Ernst et al. 2014). These new labeling techniques might be crucial to unravel the distinct turnover of specific neuronal populations within the HD striatum.
Adult Neurogenesis in Transgenic Animal Models of HD
The R6/1 and R6/2 lines are the most widely used animal models for HD. These transgenic mice carry highly expanded CAG repeats by introduction of exon 1 of the human HD gene into the mouse germline. They differ in their number of CAG repeats and develop a progressive neurological phenotype that shows some of the features of HD, including involuntary stereotypic movements, tremor, and epileptic seizures, as well as nonmotor symptoms (Mangiarini et al. 1996).
Several studies have reported reduced progenitor proliferation rates in the DG in both mouse models (the slower progressing R6/1 line) (Lazic et al. 2004, 2006) and R6/2 mice with a fast disease progression (Gil et al. 2004, 2005; Phillips et al. 2005; Kohl et al. 2007). This resulted in a reduction of newly generated neurons, although, in most reports, neuronal differentiation was not compromised. Several studies also observed a reduced number of neuroblasts and immature neurons (Kohl et al. 2007; Peng et al. 2008; Fedele et al. 2011).
Different stimuli known to increase adult neurogenesis were tested. Physical activity and environmental enrichment (van Dellen et al. 2000; Spires et al. 2004) had positive effects on survival, cognitive performance, and striatal BDNF levels (Pang et al. 2006), as well as reduction of neuronal intranuclear inclusion load (Benn et al. 2010). Neither asialoerythropoietin (Gil et al. 2004), running (Kohl et al. 2007), nor seizures (Phillips et al. 2005) were able to reverse the reduction of adult neurogenesis in these HD models; only environmental enrichment could increase levels of hippocampal neurogenesis to some extent (Lazic et al. 2006).
In contrast to decreased proliferation in the DG, SVZ proliferation was reported to be unchanged in R6/2 mice (Phillips et al. 2005; Kohl et al. 2010). A reduction in newly generated neurons was present in the OB, which more severely affected GABAergic than dopaminergic newly generated neurons (Kohl et al. 2010). In the OB, huntingtin aggregates were described in mature neurons in the granule cell layer (GCL) and glomerular layer, but not in GFAP-positive B cells or epidermal growth factor (EGF) receptor–positive C cells in the SVZ or DCX-positive newly generated neuroblasts in the SVZ or OB. This finding suggests a non-cell-autonomous mechanism of aggregated huntingtin on newly generated neurons (Kohl et al. 2010).
An important observation in regard to striatal plasticity was that adenoviral overexpression of BDNF and Noggin in the ventricular wall increased the number of new neurons in the striatum of R6/2 htt mutant mice. Under these conditions, newly recruited striatal neurons expressed striatal neuronal markers, such as DARPP-32 and GAD67. In addition, extended neuronal fibers to the ipsilateral globus pallidus were noted, indicating that these new striopallidal projection neurons survived and integrated as GABAergic striatal neurons. This integration was accompanied by substantial motor improvement and longer survival (Cho et al. 2007), and indicates that changing the striatal microenvironment will be crucial for survival of new neurons in HD.
Other promising models for HD are a full-length human mutant huntingtin mouse with 97 glutamine repeats on a bacterial artificial chromosome (Gray et al. 2008) and yeast artificial chromosome mice expressing normal (YAC18) and mutant (YAC46 and YAC72) huntingtin (Hodgson et al. 1999). These mice show progressive motor deficits, neuronal synaptic dysfunction, and late-onset cortical and striatal neuropathology. Nine- and 12-mo-old YAC128 mice showed significant reductions in hippocampal neurogenesis (Simpson et al. 2011). Moreover, younger YAC128 animals showed a slight, but significant reduction in DC cell proliferation and in the number of neuroblasts at 3 mo of age (Simpson et al. 2011). The analysis of 15-wk-old mice of the knock-in Hdh Q111 model revealed no differences in cell proliferation and numbers of DCX-positive neuroblasts, but, in male mice, a reduced number of mature DCX neuroblasts was found (Orvoen et al. 2012).
In addition, a rat model of huntingtin has been established (truncated huntingtin cDNA fragment encoding for 51 CAG repeats under the control of the rat huntingtin promoter [von Horsten et al. 2003]). These rats show adult onset of reduced anxiety, cognitive impairment, and slowly progressive motor dysfunction.
The 51 CAG repeats in this model more closely reflect the human disease, and the longer survival of these animals allows age-related studies. Adult neurogenesis was analyzed in 8- and 12-mo-old HD rats. The decrease in hippocampal progenitor cells was accompanied by an expansion of the quiescent stem cell pool (characterized by BrdU and Sox-2 coexpression) and diminished CREB signaling (Kandasamy et al. 2010). Phospho-Smad 2, which is involved in transforming growth factor (TGF)-β1 signaling that is physiologically not present in subgranular stem cells, is increased in neural quiescent stem cells in these HD transgenic rats, indicating that TGF-β signaling is involved in modulating adult neurogenesis in HD (Kandasamy et al. 2010).
A next crucial step for this area of research will be a detailed analysis of stem-cell-autonomous versus non-cell-autonomous actions in HD, as well as exploring the impact of different size aggregates for adult neurogenesis and testing compounds that have been shown to be protective to HD in rodent models to determine whether they have an impact in modulating adult neurogenesis (Miller et al. 2010).
CONCLUDING REMARKS
For PD, AD, and HD, the transfer of promising therapeutic approaches into clinical applications will depend on early-stage treatment. In this regard, compounds that are effectively able to restore distinct aspects of adult neurogenesis might be of specific interest for future clinical studies. Specifically, improving neuropsychiatric and severely disabling symptoms is an urgent need and will have a major impact on the quality of life for all patients suffering from these devastating disorders.
ACKNOWLEDGMENTS
This work is supported by the Interdisciplinary Centre for Clinical Research (IZKF, University Hospital of Erlangen). Additional support comes from the German Federal Ministry of Education and Research (BMBF, 01GQ113), the Bavarian Ministry of Education and Culture, Science and the Arts in the framework of the Bavarian Molecular Biosystems Research Network, and ForIPS.
Footnotes
Editors: Fred Gage, Gerd Kempermann, and Hongjun Song
Additional Perspectives on Neurogenesis available at www.cshperspectives.org
REFERENCES
- Aarsland D, Marsh L, Schrag A 2009. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord 24: 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. 2000. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252. [DOI] [PubMed] [Google Scholar]
- Altman J 1969. Autoradiographic and histological studies of postnatal neurogenesis: IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433–457. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, et al. 2013. α-Synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord 28: 811–813. [DOI] [PubMed] [Google Scholar]
- Benn CL, Luthi-Carter R, Kuhn A, Sadri-Vakili G, Blankson KL, Dalai SC, Goldstein DR, Spires TL, Pritchard J, Olson JM, et al. 2010. Environmental enrichment reduces neuronal intranuclear inclusion load but has no effect on messenger RNA expression in a mouse model of Huntington disease. J Neuropathol Exp Neurol 69: 817–827. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ 2006. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis 24: 1–14. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V 2009. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34: 2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V 2012. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossers K, Wirz KT, Meerhoff GF, Essing AH, van Dongen JW, Houba P, Kruse CG, Verhaagen J, Swaab DF 2010. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer's disease. Brain 133: 3699–3723. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E 2003. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G 2012. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology 78: 1939–1945. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, et al. 2004. Double-knockout mice for α- and β-synucleins: Effect on synaptic functions. Proc Natl Acad Sci 101: 14966–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA 2007. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest 117: 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS 2008. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron 59: 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E 2008. α-Synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci 28: 4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E 2010a. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci 30: 12252–12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Rockenstein E, Masliah E 2010b. APP transgenic modeling of Alzheimer's disease: Mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct Funct 214: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL 2003. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci 100: 9023–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL 2005. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience 132: 777–788. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL 2010. Genetic animal models of Parkinson's disease. Neuron 66: 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM 2006. Epidemiology of Parkinson's disease. Lancet Neurol 5: 525–535. [DOI] [PubMed] [Google Scholar]
- De Strooper B 2003. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron 38: 9–12. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ 2006. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol 495: 70–83. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J 2014. Neurogenesis in the striatum of the adult human brain. Cell 156: 1072–1083. [DOI] [PubMed] [Google Scholar]
- Fedele V, Roybon L, Nordstrom U, Li JY, Brundin P 2011. Neurogenesis in the R6/2 mouse model of Huntington's disease is impaired at the level of NeuroD1. Neuroscience 173: 76–81. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. 2001. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 344: 710–719. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, Lees AJ, Schrag A 2010. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 25: 2493–2500. [DOI] [PubMed] [Google Scholar]
- Gil JM, Leist M, Popovic N, Brundin P, Petersen A 2004. Asialoerythropoietin is not effective in the R6/2 line of Huntington's disease mice. BMC Neurosci 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil JM, Mohapel P, Araujo IM, Popovic N, Li JY, Brundin P, Petersen A 2005. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington's disease mice. Neurobiol Dis 20: 744–751. [DOI] [PubMed] [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, et al. 2008. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci 28: 6182–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ 2002. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Masliah E 2003. Transgenic models of α-synuclein pathology: Past, present, and future. Ann NY Acad Sci 991: 171–188. [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP 2002. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem 83: 1509–1524. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW 1999. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 56: 179–187. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG 2008. The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Mov Disord 23: 837–844. [DOI] [PubMed] [Google Scholar]
- Hinnell C, Hurt CS, Landau S, Brown RG, Samuel M, PROMS-PD Study Group. 2012. Nonmotor versus motor symptoms: How much do they matter to health status in Parkinson's disease? Mov Disord 27: 236–241. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, et al. 1999. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23: 181–192. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC 2004. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7: 726–735. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T 1995. The precursor protein of non-A β component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron 14: 467–475. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA 2004. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci 101: 13363–13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M, Couillard-Despres S, Raber KA, Stephan M, Lehner B, Winner B, Kohl Z, Rivera FJ, Nguyen HP, Riess O, et al. 2010. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-β signaling in an animal model of Huntington disease. J Neuropathol Exp Neurol 69: 717–728. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S, Bogdahn U, Aigner L, Winkler J 2007. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease. Brain Res 1155: 24–33. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Regensburger M, Aigner R, Kandasamy M, Winner B, Aigner L, Winkler J 2010. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease. BMC Neurosci 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Munch M, Barlow C, Carter T, Masliah E, Winkler J 2012. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci 35: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Fahrenholz F 2005. The non-amyloidogenic pathway: Structure and function of α-secretases. Subcell Biochem 38: 105–127. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 14: 504–506. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O 1998. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet 18: 106–108. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Marr RA 2010. Neurogenesis and Alzheimer's disease: At the crossroads. Exp Neurol 223: 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Grote H, Armstrong RJ, Blakemore C, Hannan AJ, van Dellen A, Barker RA 2004. Decreased hippocampal cell proliferation in R6/1 Huntington's mice. Neuroreport 15: 811–813. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Grote HE, Blakemore C, Hannan AJ, van Dellen A, Phillips W, Barker RA 2006. Neurogenesis in the R6/1 transgenic mouse model of Huntington's disease: Effects of environmental enrichment. Eur J Neurosci 23: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Li SH, Li XJ 2004. Huntingtin and its role in neuronal degeneration. Neuroscientist 10: 467–475. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 14: 501–503. [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y 2009. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoportein E4 knockin mice. Cell Stem Cells 5: 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low VF, Dragunow M, Tippett LJ, Faull RL, Curtis MA 2011. No change in progenitor cell proliferation in the hippocampus in Huntington's disease. Neuroscience 199: 577–588. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E 2010. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58: 940–949. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DD 2005. Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28: 127–156. [DOI] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H 2010. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci 13: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor S, James M, Groot Ni 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A 2006. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron 52: 587–593. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, et al. 1996. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ 2010. Neurogenesis and Alzheimer's disease: Biology and pathophysiology in mice and men. Curr Alzheimer Res 7: 113–125. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, Regensburger M, Winkler J 2013. Adult neurogenesis in Parkinson's disease. Cell Mol Life Sci 70: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L 2000. Dopaminergic loss and inclusion body formation in α-synuclein mice: Implications for neurodegenerative disorders. Science 287: 1265–1269. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, et al. 2011. Passive immunization reduces behavioral and neuropathological deficits in an α-synuclein transgenic model of Lewy body disease. PLoS ONE 6: e19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose HL, Kent CB, Taylor JP, Dachsel JC, Hinkle KM, Lincoln SJ, Mok SS, Culvenor JG, Masters CL, Tyndall GM, et al. 2007. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience 147: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Melrose HL, Daechsel JC, Behrouz B, Lincoln SJ, Yue M, Hinkle KM, Kent CB, Korvatska E, Taylor JP, Witten L, et al. 2010. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis 40: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP, Holcomb J, Al-Ramahi I, de Haro M, Gafni J, Zhang N, Kim E, Sanhueza M, Torcassi C, Kwak S, et al. 2010. Matrix metalloproteinases are modifiers of huntingtin proteolysis and toxicity in Huntington's disease. Neuron 67: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirochnic S, Wolf S, Staufenbiel M, Kempermann G 2009. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus 19: 1008–1018. [DOI] [PubMed] [Google Scholar]
- Mochel F, Charles P, Seguin F, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, et al. 2007. Early energy deficit in Huntington disease: Identification of a plasma biomarker traceable during disease progression. PLoS ONE 2: e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH 2010. Signaling in adult neurogenesis. Curr Opin Neurobiol 20: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, Boy J, Kuhn M, Nguyen HP, Teismann P, et al. 2008. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci 28: 2471–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA 2009. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci 106: 8754–8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvoen S, Pla P, Gardier AM, Saudou F, David DJ 2012. Huntington's disease knock-in male mice show specific anxiety-like behaviour and altered neuronal maturation. Neurosci Lett 507: 127–132. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ 2006. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience 141: 569–584. [DOI] [PubMed] [Google Scholar]
- Paus M, Kohl Z, Ben Abdallah NM, Galter D, Gillardon F, Winkler J 2013. Enhanced dendritogenesis and axogenesis in hippocampal neuroblasts of LRRK2 knockout mice. Brain Res 1497: 85–100. [DOI] [PubMed] [Google Scholar]
- Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ 2010. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain 133: 3434–3443. [DOI] [PubMed] [Google Scholar]
- Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, Duan W 2008. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol 210: 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit GH, Olsson TT, Brundin P 2014. The future of cell therapies and brain repair: Parkinson's disease leads the way. Neuropathol Appl Neurobiol 40: 60–70. [DOI] [PubMed] [Google Scholar]
- Phillips W, Morton AJ, Barker RA 2005. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington's disease are attributable to the in vivo microenvironment. J Neurosci 25: 11564–11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N 2012. The incidence and prevalence of Huntington's disease: A systematic review and meta-analysis. Mov Disord 27: 1083–1091. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Crews L, Masliah E 2007. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev 59: 1093–1102. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A 2008. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS ONE 3: e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathasivam K, Lane A, Legleiter J, Warley A, Woodman B, Finkbeiner S, Paganetti P, Muchowski PJ, Wilson S, Bates GP 2010. Identical oligomeric and fibrillar structures captured from the brains of R6/2 and knock-in mouse models of Huntington's disease. Hum Mol Genet 19: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL 2010. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. J Neurol 257: 540–545. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Morgenstern NA 2009. Adult neurogenesis is altered by GABAergic imablance in models of Alzheimer's disease. Cell Stem Cell 5: 573–574. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ 1998. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol 8: 447–453. [DOI] [PubMed] [Google Scholar]
- Simpson JM, Gil-Mohapel J, Pouladi MA, Ghilan M, Xie Y, Hayden MR, Christie BR 2011. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol Dis 41: 249–260. [DOI] [PubMed] [Google Scholar]
- Simuni T, Sethi K 2008. Nonmotor manifestations of Parkinson's disease. Ann Neurol 64: S65–S80. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M 1998. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 251: 205–208. [DOI] [PubMed] [Google Scholar]
- Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ 2004. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J Neurosci 24: 2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, et al. 2011. Neurocognitive signs in prodromal Huntington disease. Neuropsychology 25: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Halabisky B, Zhou Y, Jorge JP, Yu G, Mucke L, Gan L 2009. Imbalance between GABAergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer's disease. Cell Stem Cell 5: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E, Gaig C, Santamaria J, Compta Y 2009. Diagnosis and the premotor phase of Parkinson disease. Neurology 72: S12–S20. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ 2000. Delaying the onset of Huntington's in mice. Nature 404: 721–722. [DOI] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, et al. 2011. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain 134: 3249–3263. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH 2002. Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tijn P, Kamphuis W, Marlatt MW, Hol EM, Lucassen PJ 2010. Presenilin mouse and zebrafish models for dementia: Focus on neurogenesis. Prog Neurobiol 93: 149–164. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Bhaskar K, Kounnas MZ, Wagner SL, Yang Y, Lamb BT, Herrup K 2009. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J Clin Invest 119: 3692–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Horsten S, Schmitt I, Nguyen HP, Holzmann C, Schmidt T, Walther T, Bader M, Pabst R, Kobbe P, Krotova J, et al. 2003. Transgenic rat model of Huntington's disease. Hum Mol Genet 12: 617–624. [DOI] [PubMed] [Google Scholar]
- Walker FO 2007. Huntington's disease. Semin Neurol 27: 143–150. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ 2007. Aβ oligomers—A decade of discovery. J Neurochem 101: 1172–1184. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE, Wolk DA, Moberg PJ, Xie SX, Clark CM 2011. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 68: 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J 2004. Human wild-type α-synuclein impairs neurogenesis. J Neuropathol Exp Neurol 63: 1155–1166. [DOI] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH 2011. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci 33: 1139–1151. [DOI] [PubMed] [Google Scholar]
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, et al. 2012. Role of α-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci 32: 16906–16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K 2001. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci 21: 2661–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al. 2004. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R 2007. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol 204: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]