Abstract
Purpose:
Akt is a serine/threonine protein kinase and has emerged as a crucial regulator of widely divergent cellular processes, including apoptosis, proliferation, differentiation, and metabolism. Activation of Akt/protein kinase B has been positively associated with human epidermal growth-factor receptor 2 (HER2)/neu overexpression in breast carcinoma and a worse outcome among endocrine treated patients. The Akt signaling pathway currently attracts considerable attention as a new target for effective therapeutic strategies. We therefore investigated the relationship between activation of Akt and clinicopathologic variables including hormone receptor and HER2/neu status.
Methods:
Archival tumor tissues from 100 patients with invasive breast carcinoma were analyzed by immunocytochemistry. This study describes the results of immunocytochemical pAkt expression in breast carcinoma imprints, prepared from cut surfaces of freshly removed tumors. Both nuclear and cytoplasmic expressions were evaluated for pAkt.
Results:
Nuclear and cytoplasmic positive scores of 72% (72/100) and 42% (42/100), respectively, were found. Coexistence of nuclear and cytoplasmic staining was observed in 32 cases (32/100). Nuclear positive staining correlated with HER2/neu overexpression (P = 0.043) and was significantly associated with positive involvement of axillary lymph nodes (P = 0.013). No correlation was found between cytoplasmic pAkt rate and clinicopathological parameters, estrogen receptor, progesterone receptor or HER2/neu expression.
Conclusions:
pAkt expression can be evaluated in cytological material and may add valuable information to current prognostic models for breast cancer. pAkt overexpression appears to be linked with potentially aggressive tumor phenotype in invasive breast carcinoma.
Keywords: Hormone receptors, human epidermal growth-factor receptor 2/neu, imprint cytology, pAkt
INTRODUCTION
Breast cancer is a heterogeneous disease, and this term encompasses a variety of entities with distinct morphological features and clinical behavior. In recent years, it has become apparent that this diversity is the result of distinct genetic, epigenetic, and transcriptomic alterations.[1] The current clinical management of breast cancer still relies on traditional prognostic and predictive factors like histology, clinical parameters and well-defined biologic factors like estrogen receptor (ER), progesterone receptor (PR), as well as human epidermal growth factor receptor 2 (HER2), all of which present an association with prognosis and treatment outcome. It is known that two morphologically similar tumors presenting in any assigned stage may behave in different fashions, a fact that seriously impedes the potential to accurately predict the clinical outcome in a given case.
Akt (also named protein kinase B [PKB]) is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.[2] It has recently garnered significant interest due to its putative role as an inhibitor of apoptosis. Akt family comprises three highly homologous members known as Akt1, Akt2, and Akt3 in mammalian cells. The activation mechanism remains to be fully characterized but occurs downstream of phosphoinositide 3-kinase (PI-3K). PI-3K generates a lipid second messenger essential for the translocation of PKB/Akt to the plasma membrane where it is phosphorylated and activated. Constitutive PKB/Akt signaling protects cells from apoptosis and mediates growth factor-induced cell proliferation.[3] The Akt signaling pathway plays a critical role in controlling the balance between cell survival and apoptosis, and the disruption of normal Akt signaling frequently occurs in several human cancers.[4] Akt phosphorylation is a major molecular event occurring after activation of growth-factor receptors and has been proposed as a surrogate marker for HER2.[5]
This study was performed to evaluate the immunocytochemical (ICC) expression of the pAkt protein on direct imprint smears in correlation to the conventional clinicopathological parameters (age, tumor size, grade, axillary node involvement), as well as HER2/neu and hormonal receptors expression (ER and PR).
METHODS
The study group was a sequential hundred specimens (n = 100) of primary breast carcinomas obtained by wide local excision (lumpectomy) or mastectomy. The study population consisted of 100 female patients with a mean age of 54 years (range: 31–85). All patients were treated and followed at the same institution (Mitera General Hospital and Maternity Clinic in Athens) between 2008 and 2012. The study has been approved by Ethical Review Board. The patients of study group gave informed consent orally, after clearly explaining to these persons how the biological material will be extracted and used. A total of 82 patients (82%) showed pathologic involvement of axillary lymph nodes. The 63 patients had 1–3 positive nodes (pN1), 19 had 4–9 positive nodes (pN2) while in 18 patients the sentinel lymph nodes were negative (pN0). Tumor samples were collected and freshly fixed in buffered formalin according to a standardized protocol. Imprint smears were prepared from cut surfaces of freshly removed tumors before fixation in buffered formalin. Each tumor was imprinted on three glass slides and fixed in 96% ethanol. One imprint smear was stained with Papanicolaou stain for morphologic evaluation of the adequacy, and the other two were used for ICC analysis. The demographic information, clinical presentation, pathologic stage, and follow-up were extracted from the medical charts. Tumor grading and staging were determined according to the principles outlined by World Health Organization (WHO 2004) and TNM classification of International Union Against Cancer. Nuclear grading was based on nuclear polymorphism and mitotic activity.
Immunohistochemistry
All slides were analyzed by two independent pathologists. The interobserver variability was low. In cases of disagreement, a final score was determined by consensus after re-examination. Assessment of all staining results was blinded to knowledge of the clinical outcome of patients. Immunohistochemical (IHC) and ICC reactions were performed using a Bond-X automated staining system (Vision biosystems bond, Newcastle, UK). This specific assay is based on a soluble, dextran-polymer system which yields a high signal to noise ratio and minimizes any background that may be caused by endogenous biotin. Polymer-based detection technology is an advancement in IHC visualization chemistry. pAkt immunoreactivity, specifically phosphorylation of serine 473, was evaluated using the rabbit polyclonal antibody (Cell Signaling Technology, New England Biolabs, Beverly, MA, USA). HER2/neu IHC expression was detected using a monoclonal anti-HER2/neu antibody (CB11; Novocastra, Newcastle, UK) at a dilution of 1:800. Two slides were also stained with anti-ER (clone 6F11; Novocastra, Burlingame, CA) and anti-PR (clone 1A6; Ventana Medical Instruments, tuscon, AR, USA) antibodies according to the manufacturers’ recommendations. For each case, the proportion of positive cells was determined on at least 400 cells in adjacent selected areas.
Staining was considered positive for pAkt when >10% of the tumor cells presented nuclear or cytoplasmic staining. ER and PR were recorded as positive if 10% or more of the nuclei in the invasive component of the tumor were stained.[6] HER2 immunostaining was scored according to described methods. The HER2 expression level was classified into four groups according to published guidelines.[7] These scores are defined by a lack of staining or membranous staining in <10% of the cells (score 0), faint or barely perceptible membranous staining in >10% of the cells (score 1), complete membranous staining in >10% of the cells of weak to moderate intensity (score 2) and complete membranous staining in >10% of the cells of strong intensity (score 3). In this scoring system, scores of 2 or higher are considered to indicate HER2 overexpression. In statistical analysis, HER2 expression was taken as a dichotomous variable: Negative (score 0, 1+) versus positive (2+, 3+).
Statistical analysis
All statistical analyses were conducted using the SPSS 12.0 statistical software program (SPSS, Chicago, IL, USA). Correlation between markers and clinicopathological parameters was determined using Chi-square analysis. The results were considered to be statistically significant at P < 0.05.
RESULTS
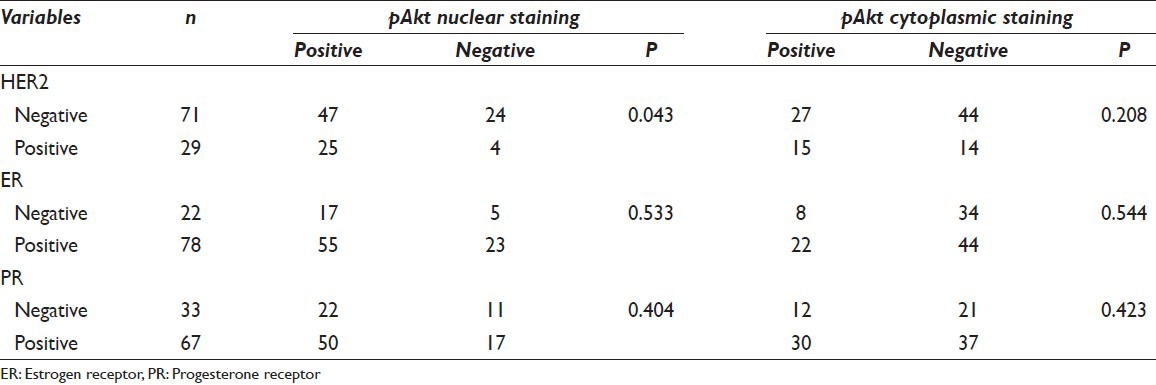
Associations between pAkt expression and clinicopathologic factors are shown in Table 1. A summary of the results of the Chi-square analysis of the studied variables is also displayed in the same table. According to the chosen cut-off point at 10%, we found a nuclear and cytoplasmic positive score 72% (72/100) and 42% (42/100), respectively. Expression patterns of pAkt staining are depicted in Figure 1. There was an obvious predominant nuclear staining pattern in 72 cases and a diffuse faint cytoplasmic staining in 42 samples. Coexistence of nuclear and cytoplasmic staining was observed in 32 cases. We did not achieve to demonstrate any relation between cytoplasmic pAkt rate and clinicopathological parameters [Table 1]. On the contrary, nuclear positive staining correlated strongly with HER2 overexpression (P = 0.043) and was significantly associated with positive involvement of lymph nodes (P = 0.013) as determined by the Chi-square test. The pAkt scores did not correlate with age (P = 0.153), tumor size (P = 0.928), and grade (P = 0.219). There was no significant correlation with other IHC variables such as hormone receptors. High levels of nuclear pAkt were not associated with high levels of ER and PR expression (P = 0.533, P = 0.404, respectively) [Table 2].
Table 1.
Correlation between clinicopathological parameters and pAkt nuclear and cytoplasmic staining pattern
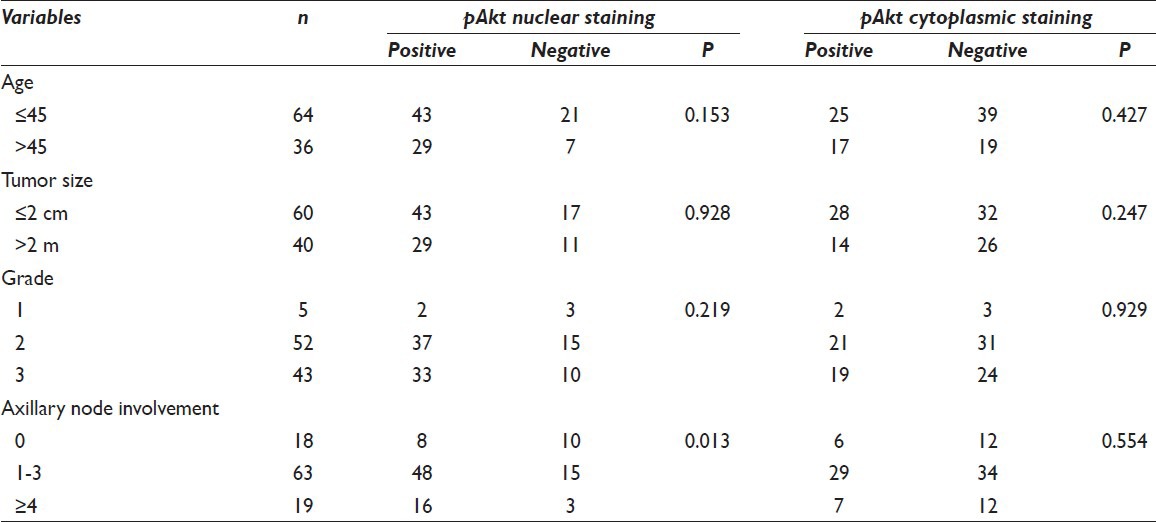
Figure 1.
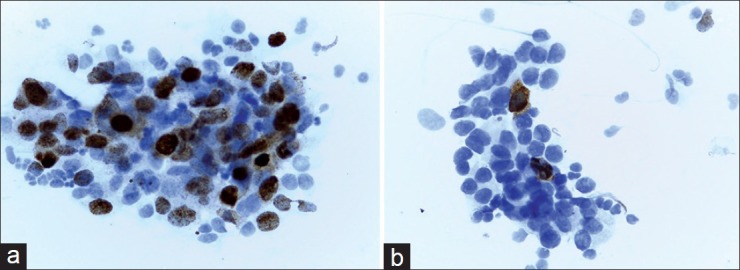
(a) Positive staining of pAkt in a high-grade breast tumor. About 40% of tumor cells demonstrate an obvious positive nuclear staining. In this case, cytoplasmic staining was noted as negative (×400). (b) Negative expression of pAkt in a breast tumor. Positive staining is limited to sporadic cells. Note coexistence of nuclear and cytoplasmic expression (×400)
Table 2.
Correlation between immunohistochemical variables and pAkt nuclear and cytoplasmic staining pattern
DISCUSSION
Akt is a serine/threonine kinase which is fully activated when phosphorylated (pAkt). After stimulation with growth factors and cytokines, PI-3K is activated and recruits Akt to the plasma membrane where it is phosphorylated. Phosphorylation of both threonine and serine residues fully activates Akt, which provides a survival signal that protects cells from apoptosis and mediates growth factor-induced cell proliferation.[3,8] In the present study, we observed both cytoplasmic and nuclear expressions of pAkt, which links this study to earlier in vitro studies suggesting that a fraction of activated Akt is translocated to the nucleus.[9,10]
Although the activation of the Akt pathway appears to be a potentially major event in the survival of breast cancer cells, few studies have investigated its prognostic and predictive value in tissue from breast cancer patients. As far as we know, this is the first report that investigated the status of pAkt expression on cytological imprint smears of breast cancer. Previous IHC studies using the pAkt antibody focused mostly on the cytoplasmic staining[11,12,13] while nuclear phosphorylation of Akt may have more value in determining its effects.[14,15] In our study, both nuclear and cytoplasmic expression were noted for pAkt. Few retrospective studies investigated the correlation between pAkt staining and clinical characteristics, outcome, or both in breast cancer. Particularly, recent studies have been focused on the association of pAkt activation and HER2/neu overexpression.[11,16,17]
Human epidermal growth-factor receptor 2/neu is a transmembrane glycoprotein involved in cell growth control. It represents a significant target for immunologically based antitumor therapy due to its limited expression in nonmalignant tissues and its contribution to the malignant phenotype of a transformed cell. HER2 encodes an 185kD orphan receptor tyrosine kinase that is constitutively active as a dimer and displays potent oncogenic activity when overexpressed.[18] There is mounting evidence of the role of HER2 overexpression in patients with breast carcinoma. It has been amply demonstrated that HER2 overexpression is associated with intensive proliferation and aggressive development of breast cancer.[19] HER2 is overexpressed in 25–30% of all breast cancers.[20] Only membranous staining was considered as positive being consistent with c-erbB-2 protein's role as a transmembrane molecule. Tokunaga et al. reported a close correlation between HER2 and pAkt expression in a population of 252 primary breast carcinomas. This finding was confirmed by Kirkegaard et al.[21] who reported a higher frequency of pAkt expression in HER2-positive tumors than in HER2-negative tumors in 402 patients with ER-positive tumors. Park and Kim[14] reported that pAkt activation was significantly elevated in primary ductal carcinoma with HER2 overexpression. Our study's findings are concordant with these. These data confirm that the Akt pathway is activated in a significant proportion of HER2-positive breast cancers, indicating that this latter transmembrane receptor is activated in a subset of cases. Furthermore, we demonstrated in our study that pAkt expression was significantly elevated in ductal carcinomas with positive axillary lymph node involvement indicating that pAkt overexpression appears to be linked with potentially aggressive tumor phenotype in invasive breast carcinoma.
Estrogen receptor and progesterone receptor (PR) assays are crucial for the management of patients with breast cancer, in both adjuvant and palliative settings. It is generally established that human breast cancer, which is rich in ERs, responds well to endocrine therapy and that patients with ER-positive breast cancer have a more favorable clinical course and prognosis than those with ER-negative cancer.[22,23] Our study showed no correlation between pAkt nuclear or cytoplasmic staining with hormone receptors. These results confirm previous reports.[13,21]
We propose that pAkt is a potential molecular marker for prediction of breast cancer's behavior. We also propose the possible use of ICC analysis on imprint smears or in fine-needle aspiration material to evaluate pAkt as prognostic factor in breast carcinoma. Our findings have to receive further validation in larger prospective series.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE.
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with the approval from Institutional Review Board (IRB) of all the institutions associated with this study as applicable.
Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Contributor Information
Olympia Vasou, Email: elempia77@yahoo.gr.
Lazaros Skagias, Email: skacyt@yahoo.gr.
Margariti Anastasia, Email: skalaz@gmail.com.
Athanasiadou Paulina, Email: linaathens@aol.com.
Efstratios Patsouris, Email: epatsour@med.uoa.gr.
Ekaterini Politi, Email: ekpoliti@med.uoa.gr.
REFERENCES
- 1.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–29. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 3.Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 5.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 6.Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808–16. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999;17:1983–7. doi: 10.1200/JCO.1999.17.7.1983. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–26. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 9.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–7. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 10.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–24. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 11.Vestey SB, Sen C, Calder CJ, Perks CM, Pignatelli M, Winters ZE. Activated Akt expression in breast cancer: Correlation with p53, Hdm2 and patient outcome. Eur J Cancer. 2005;41:1017–25. doi: 10.1016/j.ejca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Andre F, Nahta R, Conforti R, Boulet T, Aziz M, Yuan LX, et al. Expression patterns and predictive value of phosphorylated AKT in early-stage breast cancer. Ann Oncol. 2008;19:315–20. doi: 10.1093/annonc/mdm429. [DOI] [PubMed] [Google Scholar]
- 13.Stål O, Pérez-Tenorio G, Akerberg L, Olsson B, Nordenskjöld B, Skoog L, et al. Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res. 2003;5:R37–44. doi: 10.1186/bcr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SS, Kim SW. Activated Akt signaling pathway in invasive ductal carcinoma of the breast: Correlation with HER2 overexpression. Oncol Rep. 2007;18:139–43. [PubMed] [Google Scholar]
- 15.Schmitz KJ, Otterbach F, Callies R, Levkau B, Hölscher M, Hoffmann O, et al. Prognostic relevance of activated Akt kinase in node-negative breast cancer: A clinicopathological study of 99 cases. Mod Pathol. 2004;17:15–21. doi: 10.1038/modpathol.3800002. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 17.Tokunaga E, Kimura Y, Oki E, Ueda N, Futatsugi M, Mashino K, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–9. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- 18.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–6. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 19.Albanell J, Codony J, Rovira A, Mellado B, Gascón P. Mechanism of action of anti-HER2 monoclonal antibodies: Scientific update on trastuzumab and 2C4. Adv Exp Med Biol. 2003;532:253–68. doi: 10.1007/978-1-4615-0081-0_21. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson J, Nordgren H, Sjöström J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer. 2004;90:2344–8. doi: 10.1038/sj.bjc.6601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–46. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 22.Mouridsen HT, Rose C, Brodie AH, Smith IE. Challenges in the endocrine management of breast cancer. Breast. 2003;12(Suppl 2):S2–19. doi: 10.1016/s0960-9776(03)80158-3. [DOI] [PubMed] [Google Scholar]
- 23.Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]


