Abstract
Background:
Flunarizine, a Ca2+ channel blocker, crosses blood brain barrier (BBB), antagonizes calcium influx and interferes with neurotransmitter system. Flunarizine 20 mg/kg exhibited significant antidepressant activity in our previous study using forced swim test (FST) in mice, which was contradictory to the findings of other authors. Hence, the present study was designed to strengthen the results of our previous study, using the modified tail suspension test (TST) in rats.
Aim:
Aim of this study was to evaluate the antidepressant activity of flunarizine versus standard antidepressant drug fluoxetine in modified TST in rats.
Materials and Methods:
The study approved by Institutional Animal Ethics Committee was conducted using 24 adult albino rats (n = 6 in each group). Antidepressant effect of normal saline (0.1 ml/100 g), fluoxetine (10 mg/kg, intraperitoneally (ip)), and flunarizine (2 and 10 mg/kg, ip) was evaluated by using modified TST in rats. Thirty minutes after administration of all test drugs the duration of immobility was recorded for a period of 5 min in all rats by using modified TST. The data was analyzed by Student's t-test and one-way analysis of variance (ANOVA) and P < 0.05 was considered significant.
Results:
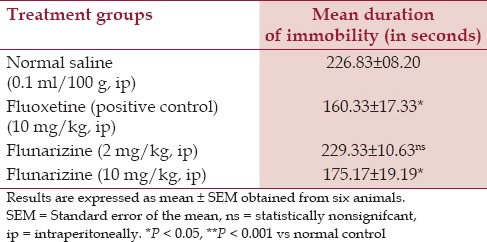
Mean duration of immobility was significantly reduced in fluoxetine and flunarizine (10 mg/kg, ip) group as compared to the normal saline, that is, 160.33, 175.17, and 226.83 s, respectively (P < 0.05). Decrease in immobility with flunarizine (10 mg/kg, ip) was statistically significant compared with normal saline, but was not found to be significant when compared to fluoxetine (P > 0.05). Also, currently used human dose of flunarizine when extrapolated to rats (i. e., 2 mg/kg, ip) failed to show significant antidepressant effect in modified TST in rats.
Conclusion:
The results of the present study indicate antidepressant-like activity of flunarizine.
Keywords: Antidepressant, Flunarizine, Fluoxetine, Tail suspension test
Introduction
The calcium channel blocker flunarizine is a lipophilic diphenyl piperazine derivative; it is a relatively weak calcium channel blocker that also inhibits Na+ channels.[1] Flunarizine has the ability to cross the blood brain barrier (BBB), antagonize calcium influx, and to interfere with the neurotransmitter system.[2] The recognition of calcium channel antagonists (CCAs) binding sites in the limbic regions of the brain has raised the hope that these drugs might also be useful in some behavioral disorders.[3] Dihydropyridine CCAs (DHP-CCAs) like nifedipine, nitrendipine, and nimodipine have been reported to have significant antidepressant and antianxiety activity at 0.1, 1, and 10 mg/kg intraperitoneally (ip) dose in mouse forced swim test (FST).[4,5] It is suggested that antidepressant activity of these DHP-CCAs might be due to their interaction with central dopaminergic system.[4,6] Also higher lipophilicity of nimodipine with better BBB penetration contributed for maximal antidepressant activity of nimodipine in comparison with other CCAs.[7] Calcium channel blocker flunarizine also penetrates BBB effectively due to its higher liphophilicity.[2,8] So flunarizine might have antidepressant action similar to DHP-CCAs, which could be useful in treating various depressive disorders. Despite these facts, there is no clear scientific work regarding the antidepressant activity of flunarizine. Previous two studies in rodents reject its antidepressant activity,[5,9] while another study supports its antidepressant effect.[10] In our previous study, we investigated antidepressant and anxiolytic activity of flunarizine in mice. Our results indicate that in FST, flunarizine in dose of 20 mg/kg, ip showed significant antidepressant activity in mice.[11] Therefore, the present study was designed to strengthen the results of our previous study, using the different antidepressant model, that is, modified tail suspension test (TST) in rats.
Modified TST, which is an animal model for depression first proposed by Steru et al.,[12] and adapted by Chermat et al.,[13] for rats. It is based on the principle that suspending rat upside down leads to characteristic behavior of immobility after initial momentary struggle. The immobility displayed by these rodents reflects behavioral despair; which, in turn, reflects depressive disorders in humans. TST is simple, inexpensive, and reliable for screening of several different classes of antidepressant drugs. It has been reported that TST is less stressful and has higher pharmacological sensitivity than FST, the other commonly employed model to study antidepressant activity.[14]
Materials and Methods
Animals
All the pharmacological experiments were conducted using albino rats (n = 6), weighing between 150 and 200 g. The animals were maintained under controlled environmental conditions such as temperature (21 ± 2°C), relative humidity (30-70%), and photoperiod of 12/12 h period. They were provided with standard commercial pelleted diet and Aquaguard drinking water ad libitum. They were acclimatized for at least 7 days before the start of experiments. The care and use of laboratory animals were strictly in accordance with the guidelines prescribed by the Institutional Animal Ethics Committee constituted under the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India).
Drugs
The following drugs were used: flunarizine, fluoxetine, and normal saline. Fluoxetine was used as the positive control to compare the results of flunarizine. Flunarizine and fluoxetine were dissolved in sterile normal saline. Freshly prepared drug solutions were injected ip, 30 min prior to the testing, in the volume of 0.1 ml/100 g body weight. Concurrent control group animals received appropriate volume of normal saline ip.
Experimental design
Twenty-four naïve albino rats were used for this study, and they were divided into four groups (n = 6). First group treated as control and received normal saline (0.1 ml/100 g, ip). Second group received fluoxetine (10 mg/kg, ip), and served as positive control.[15,16] Third and fourth group were treated with two different doses of flunarizine (2 and 10 mg/kg, ip). From the two doses of flunarizine, first dose 2 mg/kg obtained by extrapolating human dose for rats,[17] second dose, that is, 10 mg/kg in rats obtained by extrapolating mice dose used in previous studies.[9,10] The study was approved by Institutional Review Board and Institutional Animal Ethics Committee (Protocol number: 16:01/2014).
TST
TST is behavior despair model of depression, employed in rodents to predict antidepressant potential by decreasing immobility period produced by several different classes of antidepressant drugs.[18] In this test, adapted from Chermat et al.,[13] rat both acoustically and visually isolated were suspended 58 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. A square platform made of plywood was positioned horizontally 15-20 cm (depending on the animal's size) below the bench, just under the rat's forepaws, in such a way that the rat could lightly touch the platform, and thus minimize the weight sustained by its tail.[13] Rats were considered immobile only when they hung passively and completely motionless. The total duration of immobility induced by tail suspension was recorded during a 5-min period. The observer was in the room where experiments were performed and was blind to the different drug treatments in the animals.
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM) from six animals. Behavioral data were analyzed using one-way ANOVA and Student's t-test. P - values < 0.05 were considered as statistically significant.
Results
Mean duration of immobility was significantly reduced in fluoxetine and flunarizine (10 mg/kg) group as compared to the normal saline. The decrease in immobility with flunarizine (10 mg/kg) was statistically significant compared with normal saline (P < 0.05), but was not found to be significant when compared to fluoxetine (P > 0.05). Also, mean duration of immobility was nonsignificant in rats who received 2 mg/kg dose of flunarizine (P > 0.05) [Table 1].
Table 1.
Effects of flunarizine on duration of immobility in modified tail suspension test in rats
Discussion
The present work investigated the antidepressant effect of flunarizine, a calcium channel blocker, in modified TST in rats. The result of the present work provided evidences that mean duration of immobility was significantly reduced in fluoxetine and flunarizine group as compared to the normal saline. The decrease in immobility with flunarizine was statistically significant compared with normal saline, but was not found to be significant when compared to fluoxetine. The present study showed an antidepressant-like activity of flunarizine at a dose of 10 mg/kg in rats, which supports its possible use as an antidepressant.
In the present study, 2 mg/kg dose of flunarizine in rats was extrapolated from currently used doses of flunarizine, for prophylactic treatment of migraine in humans. At this dose we did not observe any significant antidepressant effect in rats. This suggests that, flunarizine may not have an antidepressant effect at presently used human dose and might need higher or more frequent doses to be effective.
In our previous study, we investigated antidepressant and anxiolytic activity of flunarizine in mice. Our results indicate that in FST, flunarizine in dose of 20 mg/kg, ip showed significant antidepressant activity in mice.[11] Results of our previous study are in agreement with the study of Kataki et al.,[10] who observed significant antidepressant activity of flunarizine (20 mg/kg, ip) in FST in mice. Czyrak et al.,[9] and Cohen et al.,[5] also studied antidepressant activity of flunarizine and found that flunarizine was inactive in FST in mice. In most of these studies only one antidepressant model, that is, FST was used in mice. As false positive and false negative results are common with FST in rodents, so few authors recommend using three different test to evaluate new test substance for its antidepressant effect, instead of relying on single test procedure.[19] Also, Czyrak et al.,[9] studied single oral doses of flunarizine in FST in mice, which might not be more conclusive to study antidepressant effect in rodents.
The precise mechanisms by which flunarizine produced antidepressant-like effects in rodents is not completely understood. DHP-CCAs like nifedipine, nitrendipine, and nimodipine have been reported to have significant antidepressant activity in mouse FST.[4,5,6] Also higher lipophilicity of nimodipine with better BBB penetration contributed for maximal antidepressant activity of nimodipine in comparison with other CCAs.[7] The efficiency of flunarizine may be related to the fact that flunarizine readily crosses the BBB and interferes with various neurotransmitter systems, for example, it exerts antihistaminergic and antiserotonergic activities; modulating effects on dopaminergic; and adenosine and opioid transmission.[20]
Jensen et al.,[21] demonstrated that flunarizine inhibited serotonin uptake in a concentration dependent manner with half maximal inhibitory concentration (IC50 ) value of 1 μmol/L in blood platelets and 5 μmol/L in rat synaptosomes. Findings of this in vitro study suggest that antidepressant activity of flunarizine might be due to inhibition of serotonin uptake at nerve endings through serotonin transporter (SERT). Also it is well-known fact that selective serotonin reuptake inhibitors (SSRIs) like drugs sometimes produces false negative results in FST in rodents.[19] This possibly explains the false negative results obtained with flunarizine on antidepressant activity in some of the previous behavioral studies in rodents.
Experimental data suggests that flunarizine exerts multiple effects on different neurotransmitter system in the brain. Notable advantage of flunarizine is its cost, which is 10-30 times lower in comparison with other antidepressants and cognition enhancers. But, detailed animal and radioligand studies are required to establish the efficacy of flunarizine in molecular level, so that the data will be strong enough to consider this drug for some clinical trials and to facilitate the therapeutic repositioning of this drug in near future for variety of neuropharmacological disorders.
Conclusion
The results of present study indicate antidepressant-like activity of flunarizine in modified TST in albino rats.
Acknowledgment
The authors are thankful to The Dean, SKNMC and GH for providing necessary facility and granting financial support to carry out the research project. Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tripathi KD. 5-Hydroxytryptamine, its Antagonists and Drug Therapy of Migraine. In: Tripathi KD, editor. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers; 2013. p. 180. [Google Scholar]
- 2.Agarwal VK, Jain S, Vaswani M, Padma MV, Maheshwari MC. Flunarizine as add-on therapy in refractory epilepsy: A open trial. J Epilepsy. 1996;9:20–2. [Google Scholar]
- 3.Cortes R, Supavilai P, Karobath M, Palacois JM. Calcium antagonists binding sites in the rat brain: Quantitative autoradiographic mapping using the 1, 4-dihydro-pyridine [3H] PN-200-110 and [3H] PY 108-068. J Neural Transm. 1984;60:169–97. doi: 10.1007/BF01249092. [DOI] [PubMed] [Google Scholar]
- 4.Mogilnicka E, Czyrak A, Maj J. Dihydropyridine calcium channel antagonists reduce immobility in the mouse behavioral despair test; antidepressants facilitate nifedipine action. Eur J Pharmacol. 1987;138:413–6. doi: 10.1016/0014-2999(87)90480-8. [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Perrault G, Sanger DJ. Assessment of the antidepressant-like effects of L-type voltage-dependent channel modulators. Behav Pharmacol. 1997;8:629–38. doi: 10.1097/00008877-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Rataboli P, Garg A, Muchandi K. Antidepressant effect of low dose nimodipine in the mouse behavior despair model. Internet J Pharmacol. 2009:8. [Google Scholar]
- 7.Sushma M, Sudha S, Guido S. Effect of calcium channel blockers on antidepressant activity following electroconvulsive shock in mice. Indian J Pharmacol. 2004;36:317–9. [Google Scholar]
- 8.Piccini P, Nuti A, Paoletti AM, Napolitano A, Mells GB, Bonuccelli U. Possible involvement of dopaminergic mechanisms in the antimigraine action of flunarizine. Cephalalgia. 1990;10:3–8. doi: 10.1046/j.1468-2982.1990.1001003.x. [DOI] [PubMed] [Google Scholar]
- 9.Czyrak A, Mogilnicka E, Maj J. Some central pharmacological effects of the calcium channel antagonist flunarizine. J Nueral Transm Gen Sect. 1991;83:179–88. doi: 10.1007/BF01253388. [DOI] [PubMed] [Google Scholar]
- 10.Kataki MS, Senthil Kumar KT, Rajkumari A. Neuropsychopharmacological profiling of flunarizine: A calcium channel blocker. Int J Pharm Tech Res. 2010;2:1703–13. [Google Scholar]
- 11.Kotal A, Yegnanarayan R, Ingole S, Khobragade R, Zalte N, Quraishi N, et al. Preclinical study of antidepressant, nootropic and antianxiety activity of flunarizine: A calcium channel blocker in mice. Indian J Pharmacol. 2013;45:S30. [Google Scholar]
- 12.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacolgy (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 13.Chermat R, Thierry B, Mico JA, Stéru L, Simon P. Adaptation of the tail suspension test to the rat. J Pharmacol. 1986;17:348–50. [PubMed] [Google Scholar]
- 14.Thierry B, Steru L, Simon P, Porsolt RD. The tail suspension test: Ethical considerations. Psychopharmacology (Berl) 1986;90:284–5. doi: 10.1007/BF00181261. [DOI] [PubMed] [Google Scholar]
- 15.Hesham ER, Hasan SA. Effects of antidepressants on behavioral assessment in adolescent rats. Bahrain Med Bull. 2011;33:1–12. [Google Scholar]
- 16.Raid M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the pet radioligand [18F] MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–6. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. U. S. Dept of Health and Human Services, Food and Drug Administration and Center for Drug Evaluation and Research. 2005. [Accessed May 3, 2013]. at http://www.fda.gov/cder/guidence/index.htm .
- 18.Huwiler A. Tail suspension test. In: Vogel HG, editor. Drug Discovery and Evaluation: Pharmacological Assays. 3rd ed. Vol. 1. Springer-Verlag: Berlin, New York; 2008. pp. 791–3. [Google Scholar]
- 19.Castagné V, Moser P, Porsolt RD. Behavioral assessment of antidepressant activity in rodents. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd ed. Boca Raton: CRC Press; 2009. Ch. 6. [PubMed] [Google Scholar]
- 20.Fischer W, Kittner H, Regenthal R, de Sarro GD. Anticonvulsant profile of flunarizine and relation to Na + channel blocking effects. Basic Clin Pharmacol Toxicol. 2004;94:79–88. doi: 10.1111/j.1742-7843.2004.pto940205.x. [DOI] [PubMed] [Google Scholar]
- 21.Jensen PN, Smith DF, Poulsen JH, Moller HJ, Rosenberg R. Effect of flunarizine and calcium on serotonin uptake in human and rat blood platelets and rat synaptosomes. Biol Psychiatry. 1994;36:118–23. doi: 10.1016/0006-3223(94)91192-4. [DOI] [PubMed] [Google Scholar]


