Abstract
Background:
Grading of breast carcinoma on fine needle aspiration cytology (FNAC) is beneficial for selecting patients for neoadjuvant chemotherapy.
Aims:
To grade the breast carcinoma on FNAC using Robinson grading system and to assess the concordance of cytological grading (CG) with histological grading (HG) using Elston-Ellis modification of Scarff-Bloom-Richardson grading system.
Materials and Methods:
The study was conducted for 1-year, comprising of 50 female patients attending outpatient departments (OPD) as well as admitted in various surgical wards of a teaching hospital, diagnosed as breast carcinoma. FNAC smears were stained with May–Grunwald–Giemsa and Papanicolaou (Pap) stains and CG was done using Robinson system on Pap stained smears. The results were compared with HG system after resection of tumors.
Results:
Of 50 cases, 14 (28%) cases were graded as grade I, 24 (48%) grade II, and 12 (24%) grade III by CG, whereas 9 (18%), 28 (56%) and 13 (26%) cases were graded as grade I, II and III by HG. The result showed overall 72% concordance of CG with HG, with grade II and grade III showing highest degree of concordance (83.33%), which is comparable to previous studies. Kappa measurement showed a higher degree of agreement in high-grade tumors compared with low-grade tumors (0.73 in grade III, 0.53 in grade II and 0.39 in grade I).
Conclusion:
Cytological grading is comparable to HG in majority of cases. Because neoadjuvant chemotherapy is becoming increasingly popular as primary treatment modality of breast cancer, CG could be a useful parameter in selecting the mode of therapy and predicting tumor behavior.
Keywords: Breast carcinoma, fine needle aspiration cytology, Robinson grading, Scarff-Bloom-Richardson's grading
Introduction
Among the malignant neoplasms, breast is one of the most common organs to be inflicted, and it is the most common non skin malignancy in women.[1] Carcinoma of the breast is a leading cause of malignant death in women, with more than 1,000,000 cases occurring annually. Prognosis of breast carcinoma depends on various parameters, such as tumor type, histological grading (HG), hormone receptor status, DNA ploidy, cell proliferation markers and expression of different oncogenes.[2] HG of breast carcinoma using the Elston-Ellis modification of Scarff-Bloom-Richardson (SBR) grading system is a widely accepted tumor grading system and has been found to have good prognostic correlation.[3] In recent years fine-needle aspiration cytology (FNAC) is routinely being used for preoperative diagnosis of breast carcinoma. Based on the cytological features, various grading systems have evolved.[4,5,6] Of the different cytological grading (CG) methods corresponding to Elston-Ellis modified SBR HG, the method described by Robinson et al.[4] was found to be useful in grading breast carcinoma in fine needle aspiration (FNA).[7,8,9] Hence in the era of neo-adjuvant chemotherapy, grading of breast carcinoma should be incorporated in FNAC reports for prognostication. Grading of breast carcinoma on FNAC is also very useful in patients with locally advanced disease, older patients with accompanying chronic diseases and patients who rejects surgery.[10] Keeping this in mind, in the present study was undertaken with the objective to find the concordance of CG of breast carcinoma using Robinson method with HG using Elston-Ellis modified SBR method.
Materials and Methods
The study comprised of 50 female patients, diagnosed as breast carcinoma on FNAC, later confirmed by histopathology following mastectomy for a period of 1-year in a teaching hospital of North-Eastern India from August 2006 to July 2007. The inclusion criteria were: (i) All female patients, irrespective of age diagnosed as carcinoma of the breast cytologically and histologically confirmed were included in the study. The exclusion criteria were: (i) uncooperative patient, (ii) patients having history of recurrence of breast carcinoma after mastectomy (iii) patients having history of chemo or radiotherapy prior to mastectomy and (iv) male patients. Informed consent was obtained from every patient to participate in the study. Ethical Committee clearance of the institution was obtained prior to this study.
Fine needle aspiration cytology was done using 23G needle, fixed to a 10 ml syringe. The aspirated materials were deposited on the slides and minimum 4-5 slides were made using a flat pressure by another slide with a smooth edge. Some of the slides were air-dried, and some of them are immediately placed in 95% ethyl alcohol for fixation. Air-dried smears were stained with May–Grunwald–Giemsa stain. Alcohol fixed smears were stained using routine Papanicolaou (Pap) method. Pap stained smears were used to grade breast carcinoma using the criteria described by Robinson et al.[4] It took into account of six different cytological parameters; namely cell dissociation, nuclear size, cell uniformity, nucleolus, nuclear margin and chromatin pattern. Each of these parameters was given a score of 1-3 and scores were added to get the final score. Cancers that scored in the range of 6-11 were graded I, scores of 12-14 were graded II and grade III was given for a score ranging from 15 to 18. HG of breast carcinoma was performed on formalin-fixed paraffin-embedded sections stained with H and E using Elston-Ellis modification of SBR grading system. It uses 3 parameters namely; tubule formation, nuclear pleomorphism and mitotic count. Mitotic count was done using Labomed vision 2000 microscope using 40X objective with a field diameter of 0.65 mm. Each of these parameters was assigned a score ranging from 1 to 3. The scores of each are then added together for a final sum that ranges between 3 and 9. Scores 3-5 were graded I, 6-7 were graded II and score 8-9 was graded as III. The results of CG were compared with a gold standard modified SBR grading. Kappa (ĸ) measurement of agreement was also calculated for each grade to compare the agreement.
Results
In the present study, a total number of 50 cases of breast carcinomas were included. Ages of the patient's ranges from 25 to 83 years with a maximum number of patients were in 40–49 years age group. Using Robinson's CG system, 14 cases were graded as grade I, 24 cases as grade II and 12 cases as grade III. These tumors were again graded postoperatively using Elston-Ellis modification of SBR HG system. On HG, 7 cases were graded as grade I, 20 cases as grade II and 10 cases as grade III [Table 1].
Table 1.
Concordance of Robinson's cytological grading with histological grading (concordance rate=Approximate sensitivity)
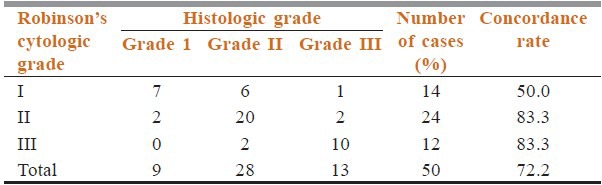
From the Table 1, it is seen that highest degree of concordance rate observed in grade II and grade III tumors which are 83.3%; while grade I tumors showed only 50% concordance rate. The overall sensitivity of CG was 72.2%, and specificity was 100%. Most of the disconcordance was observed in grade I tumors (50%). 13 cases showed discordant cytological grading; out of which 12 cases showed one grade and only 1 case showed two grade difference. The higher grade tumors (grade II and III) showed good concordance (83.3%) than lower grade (grade I) tumors (50%).
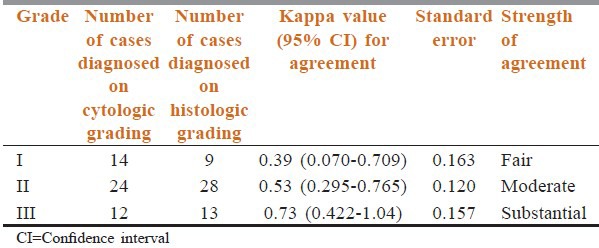
The kappa (ĸ) value of the agreement was calculated for each grade to compare the agreement between CG and HG [Table 2]. We found that grade I and grade II tumors showed κ value of 0.39 and 0.53 indicating fair and moderate agreement between CG and HG respectively. However, κ value for grade III tumors is 0.73 signifying substantial agreement between cytologic and histologic grading. Thus, higher grade tumors showed stronger agreement in our study.
Table 2.
Comparison of cytological and histological grades using kappa coefficient
Discussion
Breast cancer is one of the most common causes of death in many developed countries in middle-aged women and is becoming frequent in developing countries. In India, breast cancer is the second most prevalent cancer in women after cervical cancer.[11]
The idea of CG is to assess the tumor in situ, so that the most suitable treatment could be selected immediately, and the morbidity associated with overtreatment of low grade tumors could be avoided. According to uniform approach to breast FNAC as recommended by the National Cancer Institute, tumor grading on FNA material should be in reports of FNAC for prognostication.[12] Again simultaneous performance of CG and HG helps in measuring accuracy of CG in breast carcinoma. Histological concordance gives the cytopathologist a feedback and helps in increasing the efficiency of work.
Various CG systems of breast carcinoma are presently in use. Robinson's grading system is found to be better in various studies because of its simplicity, specificity and reproducibility.[9,13,14,15] It uses six different parameters namely; cell dissociation, cell size, cell uniformity, nucleolus, nuclear margin and nuclear chromatin. Robinson's CG had a concordance rate ranging from 56.9% to 89.1% with HG in different previous studies.[16]
In the present study, out of total 50 cases, 14 (28.0%), 24 (48%) and 12 (24%) cases were graded as grade I, II and grade III respectively. Hence majority of cases were in CG grade II which is comparable with previous studies. Robinson et al. in their study of 608 cases had the distribution of cases as 38.3%, 38.5% and 23.2% in cytological grades I, II and III respectively.[4] Pandit and Parekh et al. graded 75 breast carcinomas by same method and found 34.7% each in grades I and II, and 30.6% in grade III.[8] A similar study was carried out using Robinson's criteria by Das et al. showed that 28.8% cases were grade I, 46.2% as grade II and 25.0% as grade III.[9] The result of the present study showed similar concordance with these studies.
Regarding concordance of CG with HG, the present study showed 50% concordance in grade I, and 83.3% concordance in grade II and III [Table 1]. The overall concordance of CG with HG is 72.2% which is comparable with other published data. The original study by Robinson et al. found only 57% concordance, while Das et al., Sinha and Sinha and Lingegowda et al. found 71.2%, 73.0% and 64.0% concordance between CG and HG respectively.[4,9,17,18] Sood et al. found highest concordance (75%) in grade I tumors and lowest (60%) in grade III tumors with overall concordance of 68.67%.[19] This finding is contrasted to our study where we found highest concordance in grade III tumors. A study carried out by Saha et al. found absolute concordance of 77.19% between CG and HG using Robinson's grading system involving 57 cases of breast carcinoma.[15]
Majority of discordance between CG and HG was observed in grade I tumors (7/14). Of the 14 cases grades as grade I by CG, only 7 cases were graded as grade I by HG and out of other 7 cases, 6 were grade II and 1 was grade III. From the Table 1, it is clear that a total of 13 cases (26.0%) showed discordant grading and in the majority of cases there is one grade difference (12 cases, i.e. 92.3%). Similar results were obtained by Pandit and Parekh et al.[8] and Das et al.[9] Only 1 case showed two grade difference (2%), which is comparable to Das et al.[9] In our study, only 1 case was under graded by two grades while no case was two grades over graded.
Histological grading was based on the degree of tubule formation, mitosis and nuclear pleomorphism. As tubule formation and mitotic index were difficult to assess on cytology, it might be the cause of discordance between cytological and HG systems.[20,21,22] In CG, much importance have been given to nuclear features like nuclear size, nucleoli, nuclear membrane and chromatin pattern in contrast to HG; in which nuclear feature in only one component. This can also lead to cytohistological disparity in grading of breast carcinomas.
Conclusion
In the present study, a high degree of concordance was seen between cytological and HG system. Preoperative grading using FNAC helps in determining neo adjuvant chemotherapy as well as prognostication. This grading system is relatively a new approach in diagnostic pathology, and its arena is ever increasing. The method is in its infancy. It could be said in confidence that this grading system will be fruitful in prognostication of malignant breast lesions in the near future.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Lester SC. The breast. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier; 2004. p. 1129. [Google Scholar]
- 2.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 3.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson IA, McKee G, Nicholson A, D’Arcy J, Jackson PA, Cook MG, et al. Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. 1994;343:947–9. doi: 10.1016/s0140-6736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 5.Hunt CM, Ellis IO, Elston CW, Locker A, Pearson D, Blamey RW. Cytological grading of breast carcinoma – a feasible proposition? Cytopathology. 1990;1:287–95. doi: 10.1111/j.1365-2303.1990.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 6.Mouriquand J, Gozlan-Fior M, Villemain D, Bouchet Y, Sage JC, Mermet MA, et al. Value of cytoprognostic classification in breast carcinomas. J Clin Pathol. 1986;39:489–96. doi: 10.1136/jcp.39.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson IA, McKee G, Kissin MW. Typing and grading breast carcinoma on fine-needle aspiration: Is this clinically useful information? Diagn Cytopathol. 1995;13:260–5. doi: 10.1002/dc.2840130315. [DOI] [PubMed] [Google Scholar]
- 8.Pandit AA, Parekh HJ. Cytologic grading of breast carcinoma; comparison of four grading systems. J Cytol. 2000;17:39–44. [Google Scholar]
- 9.Das AK, Kapila K, Dinda AK, Verma K. Comparative evaluation of grading of breast carcinomas in fine needle aspirates by two methods. Indian J Med Res. 2003;118:247–50. [PubMed] [Google Scholar]
- 10.Skrbínc B, Babic A, Cúfer T, Us-Krásovec M. Cytological grading of breast cancer in Giemsa-stained fine needle aspiration smears. Cytopathology. 2001;12:15–25. doi: 10.1046/j.1365-2303.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- 11.Rao DN, Ganesh B. Estimate of cancer incidence in India in 1991. Indian J Cancer. 1998;35:10–8. [PubMed] [Google Scholar]
- 12.The uniform approach to breast fine needle aspiration biopsy. A synopsis. Acta Cytol. 1996;40:1120–6. doi: 10.1159/000333969. [DOI] [PubMed] [Google Scholar]
- 13.Wani FA, Bhardwaj S, Kumar D, Katoch P. Cytological grading of breast cancers and comparative evaluation of two grading systems. J Cytol. 2010;27:55–8. doi: 10.4103/0970-9371.70738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rekha TS, Nandini NM, Dhar M. Validity of different cytological grading systems of breast carcinoma – a hospital-based study in South India. Asian Pac J Cancer Prev. 2011;12:3013–6. [PubMed] [Google Scholar]
- 15.Saha K, Raychaudhuri G, Chattopadhyay BK, Das I. Comparative evaluation of six cytological grading systems in breast carcinoma. J Cytol. 2013;30:87–93. doi: 10.4103/0970-9371.112647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandya AN, Shah NP. Comparative evaluation of Robinson's cytological grading with Elston and Ellis’ Nottingham Modification of Bloom Richardson histopathology grading for breast carcinoma. Natl J Community Med. 2012;3:491–5. [Google Scholar]
- 17.Sinha S, Sinha N, Bandyopadhyay R, Mondal SK. Robinson's cytological grading on aspirates of breast carcinoma: Correlation with Bloom Richardson's histological grading. J Cytol. 2009;26:140–3. doi: 10.4103/0970-9371.62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingegowda JB, MuddeGowda PH, Ramakantha CK, Chandrasekar HR. Cytohistological correlation of grading in breast carcinoma. Diagn Cytopathol. 2011;39:251–7. doi: 10.1002/dc.21374. [DOI] [PubMed] [Google Scholar]
- 19.Sood N, Nigam JS, Yadav P, Rewri S, Sharma A, Omhare A, et al. Comparative Study of Cytomorphological Robinson's Grading for Breast Carcinoma with Modified Bloom-Richardson Histopathological Grading. Patholog Res Int 2013. 2013:146542. doi: 10.1155/2013/146542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabbs DJ, Silverman JF. Prognostic factors from the fine-needle aspirate: Breast carcinoma nuclear grade. Diagn Cytopathol. 1994;10:203–8. doi: 10.1002/dc.2840100302. [DOI] [PubMed] [Google Scholar]
- 21.Howell LP, Gandour-Edwards R, O’sullivan D. Application of the Scarff-Bloom-Richardson tumor grading system to fine-needle aspirates of the breast. Am J Clin Pathol. 1994;101:262–5. doi: 10.1093/ajcp/101.3.262. [DOI] [PubMed] [Google Scholar]
- 22.Masood S. Prognostic factors in breast cancer: Use of cytologic preparations. Diagn Cytopathol. 1995;13:388–95. doi: 10.1002/dc.2840130507. [DOI] [PubMed] [Google Scholar]


