Abstract
Context:
Currently, there is limited data on the prevention of chemotherapy-induced nausea and vomiting (CINV) in Indian population with aprepitant containing regimens.
Aims:
The aim was to assess the Efficacy and Safety of Aprepitant for the prevention of nausea and vomiting associated with highly emetogenic chemotherapy/moderately emetogenic chemotherapy (HEC/MEC) regimens.
Settings and Design:
Investigator initiated, multicentric, open-label, prospective, noncomparative, observational trial.
Subjects and Methods:
Triple drug regimen with aprepitant, palonosetron, and dexamethasaone administration was assessed for the prevention of CINV during acute, delayed, and the overall phase (OP) for HEC/MEC Regimens. The primary endpoint was complete response (CR; no emesis and no use of rescue medication) and the key secondary endpoint was the complete control (CC; no emesis, no rescue medication and no more than mild nausea) during the OP.
Statistical Analysis Used:
Perprotocol efficacy was analyzed for the first cycle with results represented in terms of CR/CC rates using descriptive statistics.
Results:
Seventy-five patients were included in the study with median age of 49.7 years and 89.7% being females. The CR rate (OP) for patients administered HEC or MEC regimens during the first cycle were 92% and 90.9%, respectively. Similarly, the CC rates (OP) were 75% and 90% for these regimens, respectively. 7 (9.2%) patients reported adverse drug reactions that were mild and transient with no reports of any serious adverse events.
Conclusions:
Use of aprepitant containing regimen for patients receiving HEC/MEC regimen resulted in significantly high CR and CC response rates, which further consolidate its potential role to improve patient quality of life and compliance to disease management.
Keywords: Aprepitant, chemotherapy-induced nausea and vomiting, dexamethasone, highly emetogenic chemotherapy regimens, moderately emetogenic chemotherapy regimens, palonosetron
Introduction
Chemotherapy remains a common treatment modality of cancer. Irrespective of the fact that chemotherapy expands survival, this often leads to the most debilitating and distressing side effects in the form of nausea and vomiting. Distinct obstacle to the furtherance of chemotherapy is the failure to prevent chemotherapy-induced nausea and vomiting (CINV) completely which may result in down gradation of the physical and mental status of the patient while leading to poor compliance rate for the next cycle of chemotherapy. Therefore, avoidance or alleviation of CINV is of utmost importance for better patient outcomes to chemotherapy.[1,2,3]
The incidence of CINV varies from 30% to >90% within the first 24 h of introducing moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC) regimen without antiemetic prophylaxis. Preventing CINV from the initiation of chemotherapy is important because effective control in the acute phase (AP) (0–24 h after chemotherapy) is linked with decreased incidence of CINV in the delayed phase (DP) (day 2 onward).[4] Similarly, control in the first cycle is linked with decreased incidence in subsequent cycles with few patients developing anticipatory nausea and vomiting in later cycles.[5,6]
Various clinical studies suggest the incidence of 28–50% for Delayed emesis in patients receiving HEC/MEC regimens.[7,8] In AP 5-HT3 receptor thought to be is the main causative agent, which is well controlled with 5-HT3 RA and dexamethasone. In the contrary to this DP is under the control of substance P and neurokinin-1 (NK-1) receptor system. Two drug therapies without the aprepitant has not demonstrated good control in DP with improbable maintenance of antiemetic efficacy, especially over repeated administration of HEC regimens. Triple therapy with aprepitant improves complete response (CR) rate in DP. Clinicians are likely to underestimate DP CINV which is more frequent than the AP and patient who did not have suffered AP is likely to suffer DP CINV.[5,9] Moreover, negative effects on quality of life (QoL) are more with DP CINV rather than AP CINV indicating addition of aprepitant to prevent DP CINV would benefit patients receiving chemotherapy.[6,10,11]
There are limited data available for use of aprepitant in Indian patients and therefore the current study was conducted to evaluate the efficacy and safety of this triple regimen containing the same on CINV related outcomes when administered to patients receiving HEC/MEC drug regimens for various cancers in the first cycle.
Subjects and Methods
An investigator-initiated, multicentric, noncomparative, prospective observational study was conducted at different oncology centers of Maharashtra in India. The study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice with study approval from an Independent Ethics Committee and written informed consent from the patients.
Inclusion criteria
Seventy-five male or female patients from Oncology department with age ≥ 18 years, with various malignancies including breast (n = 29), colorectal (n = 5), gastrointestinal (n = 7), gynecological (n = 13), head and neck (n = 9), hematological (n = 6), lung (n = 4), bone (n = 2), who had an Eastern Cooperative Oncology Group Performance Status of 0–2 and likely to undergo 4–6 cycles of HEC or MEC drugs or regimens were enrolled in the study. The emetogenicity of the chemotherapy was defined as HEC or MEC as per National Comprehensive Cancer Network (NCCN) guidelines, 2013.
Exclusion criteria
Main exclusion criteria included: Emesis within prior 24 h of starting chemotherapy; symptomatic primary brain tumor or metastasis in the brain; previous radiation to the brain, abdomen or pelvis, any active infection or uncontrolled disease; and any systemic steroid therapy.
Medication and dosage
All eligible patients received aprepitant p.o. 125 mg (day 1), 80 mg (day 2–3) along with intravenous (IV) palonosetron injection 0.25 mg (day 1) and dexamethasone IV injection and p.o. on day 1 and subsequent days.
Patients recorded any episodes of nausea and/or vomiting episodes and their use of rescue medication a (dopamine antagonist) in a diary during the first 120 h after initiation of chemotherapy. Based on patient observations, the investigator assessed the severity of CINV according to a four-point Likert scale (any, mild, moderate or severe nausea). A vomiting episode was defined as one or more distinct episodes of emesis (expulsion of stomach contents through the mouth) or retches (an attempt to vomit that is not productive of stomach contents).
Endpoints
The primary efficacy endpoint was CR defined as no emetic episodes and no use of any rescue therapy during the 5 days (120 h) following the initiation of chemotherapy. The key secondary efficacy endpoint was complete control (CC) defined as no emesis, no rescue medication and no more than mild nausea during the 5 days (120 h) following initiation of chemotherapy. The AP response was recorded on day 1 (0–24 h) of chemotherapy and DP from day 2 to 5 (25–120 h), while overall phase (OP) response being calculated from day 1 to 5.
Evaluation of safety
Adverse events and laboratory data were compiled according to the Common Terminology Criteria for Adverse Events (version 4).
Statistics
According to the protocol-defined exclusion criteria, the perprotocol efficacy was analyzed for the first cycle with results represented in terms of CR/CC rates. Similarly, the concomitant risk factors, including baseline patient demographics such as age and sex, past history a primary cancer site and HEC/MEC drug administered were also assessed. Data were analyzed by descriptive statistics.
Results
Patient characteristics and treatment
Seventy-five outpatients who completed at least four cycles of chemotherapy for various malignancies including breast cancer (n = 29), colorectal (n = 5), gastrointestinal tract (n = 7), gynecological (n = 13), head and neck (n = 9), hematological (n = 6), lung (n = 4) and bone (n = 2) were included for analysis with results represented for the first cycle. Overall, 89.7% of patients were females, and the mean age was 49.7 years. Nineteen patients (26.3%) had concomitant comorbid condition including hypertension, type 2 diabetes or COPD. The demographic data is mentioned in Table 1.
Table 1.
Baseline demographics for the patients enrolled in the study
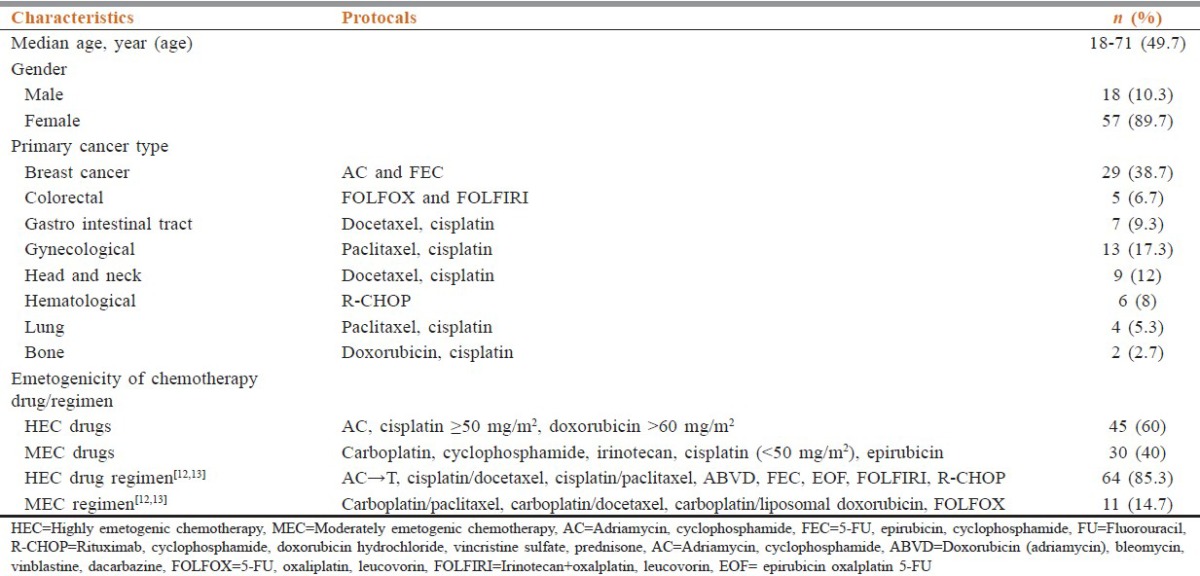
Chemotherapy drugs were grouped as per their emetogenicity into HEC and MEC. 60% of patients received HEC drugs, and rest received MEC drugs. The most common HEC drugs were an anthracycline plus cyclophosphamide and cisplatin ≥ 50 mg/m2. The most commonly used HEC and MEC drugs included anthracycline plus cyclophosphamide and carboplatin respectively. Polovinch et al. (2009) reclassified the emetogenicity of MEC drugs as HEC regimen when used in combination. The HEC regimens, therefore, included AC, Cisplatin-Docetaxel, Cisplatin-Paclitaxel, ABVD, FEC, EOF, FOLFIRI. These consisted of 85.3% of all the regimens being administered.
Antiemetic efficacy
The occurrence of nausea and vomiting was evaluated using patient questionnaires. The CR and CC rates were compared among all, MEC and HEC regimens used during the study [Figures 1 and 2]. The CR rates for overall, acute, DP were 96.8%, 93.7% and 92% respectively for patients receiving HEC regimen.
Figure 1.
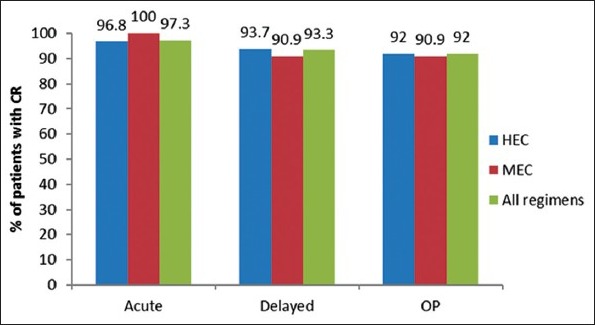
Comparison of complete response for highly emetogenic chemotherapy, moderately emetogenic chemotherapy and all regimens
Figure 2.
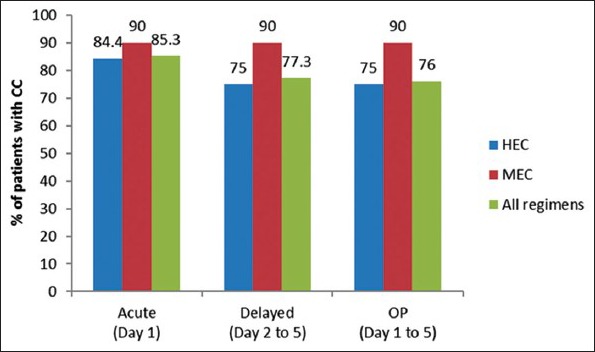
Comparison of complete control for highly emetogenic chemotherapy, moderately emetogenic chemotherapy and all regimens
Similarly the CC rates for overall, acute, DP were 75%, 85.4% and 75% respectively for HEC regimen. Five cases of persistent CIN or CINV were noted. Two of these cases were labeled as Grade 3 and 4 severities that were managed with rescue medication involving injection metoclopramide.
Safety
Overall 7 (9.2%) patients experienced adverse reactions which were mild and well tolerated. The most common side-effect observed was hiccough in 2 (2.7%). Other adverse drug reactions observed included mucositis (1.3%), dysphagia (1.3%), generalized weakness (1.3%), constipation (1.3%) and body pain (1.3%). No Grade 3 or 4 adverse events were reported during the study.
Risk analysis
For the five cases with persistent CIN or CINV, categorical or continuous variable risk factor analyses were performed. Most of these cases may be influenced by gender status (females) with Age ≤ 40 years (50%) for not achieving CR with the administered prophylactic regime. Rescue antiemetic treatment was administered in alone case.
Discussion
In this prospective observational multicenter CINV prophylaxis study in Indian patients with various malignancies, triple therapy with aprepitant (an NK-1 receptor antagonist), palonosetron (a second generation 5 HT3 receptor antagonist), and dexamethasone was associated with high CR and CC rates over 5 days of cycle 1 of HEC or MEC administered.[3,4,14,15]
There is scarce data available about the risk of CINV and the efficacy of antiemetic Regimens in Indian or Asian patients involving HEC and/or MEC regimens.
Most CINV prophylaxis studies with effect on the QoL are characterized by narrow patient selection criteria and are limited to well-defined chemotherapy regimens of different levels of emetogenicity with less due representation to local geographic population.[10,16]
Several researchers have demonstrated that Aprepitant-containing antiemetic regimen for patients with a variety of cancer types treated with MEC has shown greater efficacy than the control regimen. As a result, a three-drug regimen including 5HT3-receptor antagonist, dexamethasone, and aprepitant has been incorporated into the Multinational Association of Supportive Care in Cancer, European Society for Medical Oncology, American Society of Clinical Oncology and the NCCN guidelines for prevention of CINV in patients receiving selected MEC regimens. This strategy is especially relevant for select patients receiving certain moderate emetic risk chemotherapy (i.e. carboplatin, cisplatin, doxorubicin, epirubicin, ifosfamide, irinotecan or methotrexate).[17]
The results of our study are consistent with those from previous similar studies of CINV prophylaxis for patients receiving HEC [Table 2] or MEC regimens.[5]
Table 2.
Comparison of CR rates from various studies receiving triple regimen with aprepitant
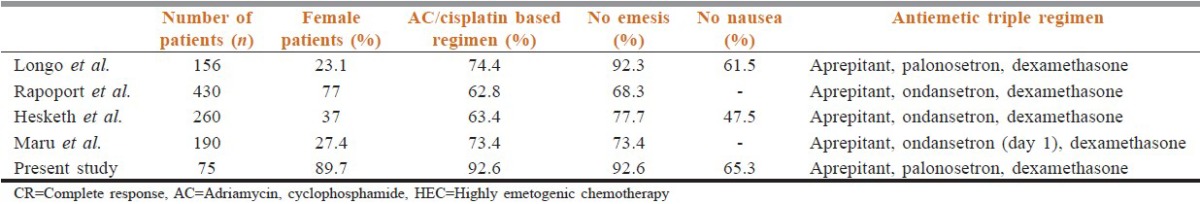
In this study, the prophylaxis regimen resulted in CR of 92.6% for OP in patients receiving HEC based regimens with similar rates of “No emesis.” Maru et al. showed CR of 73.4% that was again similar in the “No emesis” group and probably related to the single day administration of ondansetron.
Five of the enrolled patients showed in CR to the prophylaxis therapy with one showing grade 3 severity as per NCI-Toxicity criteria and was well controlled with rescue medication.
The CR rates in the current study (90%) for patients treated with MEC Regimens was comparable to study results from Ito et al.[5] (80.3%) and Ryu et al. (96.3%).[17]
Chemotherapy cycles administered repeatedly may lead to CINV that becomes progressively more difficult to control. The efficacy of antiemetic prophylaxis is, therefore, evaluated during the first chemotherapy cycle as it is known that pretreated patients are at a higher risk of emesis and anticipatory vomiting in the following cycles.[18]
In the current study, supplementation of antiemesis regimen with aprepitant was well tolerated with mild AEs including hiccough (2.7%) being reported. The reported incidence of hiccough was again comparable to other studies including Jordan et al.[9] Other AEs noted were mucositis, dysphagia, generalized weakness, constipation and body pain where the causality could not be confirmed with the use of aprepitant.
Overall the safety experience in the Indian population was similar to the reported incidence for aprepitant in published literature.
In the current study, the incidence of Nausea reported by patients (34.7%) was comparable to results from Longo et al., (38.5%). The relative high incidence of Nausea in both the studies could be partly related to the lack of conformity for NCCN guidelines (2013) where steroids are recommended for 4 days, especially for HEC regimens.[19] This may be attributable to the relative persistence of Nausea (moderate intensity) in 5 (7.8%) patients on day 4 and 5 for the CINV prophylaxis regimen administered group.
The study was limited by small sample size which narrates further need of a large randomized study trial with the standardized dosage schedule of NK-1-RA (3 days), 5-HT3-RA (day 1) and dexamethasone (4 days) by NCCN recommendations (2013) to confirm the benefits of such treatment strategies especially for HEC drug combination Regimens.
Conclusion
The study confirms the clinical benefits of aprepitant supplementation in patients receiving HEC/MEC Regimens for various malignancies along with 5-HT3 antagonist and steroids, thereby offering improved prevention of CINV. The combination is well-tolerated with no augmentation of any adverse drug-drug interaction with most of the commonly co-prescribed medications in patients receiving chemotherapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 2.Dewan P, Singhal S, Harit D. Management of chemotherapy-induced nausea and vomiting. Indian Pediatr. 2010;47:149–55. doi: 10.1007/s13312-010-0023-4. [DOI] [PubMed] [Google Scholar]
- 3.Takeshima N, Matoda M, Abe M, Hirashima Y, Kai K, Nasu K, et al. Efficacy and safety of triple therapy with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy for gynecological cancer: KCOG-G1003 phase II trial. Support Care Cancer. 2014;22:2891–8. doi: 10.1007/s00520-014-2280-6. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract. 2014;10:68–74. doi: 10.1200/JOP.2012.000816. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Karayama M, Inui N, Kuroishi S, Nakano H, Nakamura Y, et al. Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer. 2014;84:259–64. doi: 10.1016/j.lungcan.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 7.Longo F, Mansueto G, Lapadula V, Stumbo L, Del Bene G, Adua D, et al. Combination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapy. Int J Clin Pract. 2012;66:753–57. doi: 10.1111/j.1742-1241.2012.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–8. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 9.Jordan K, Jahn F, Jahn P, Behlendorf T, Stein A, Ruessel J, et al. The NK-1 receptor-antagonist aprepitant in high-dose chemotherapy (high-dose melphalan and high-dose T-ICE: Paclitaxel, ifosfamide, carboplatin, etoposide): Efficacy and safety of a triple antiemetic combination. Bone Marrow Transplant. 2011;46:784–9. doi: 10.1038/bmt.2010.205. [DOI] [PubMed] [Google Scholar]
- 10.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–8. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 11.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–8. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 12.Polovich M, Whitford JM, MiKaela MO. 3rd ed. Pittsburgh, PA: Oncology Nursing Society; C 2009. Chemotherapy and Biotherapy for Practice. [Google Scholar]
- 13.Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double-blind study. Support Care Cancer. 2010;18:423–31. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 14.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–98. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouganim N, Dranitsaris G, Hopkins S, Vandermeer L, Godbout L, Dent S, et al. Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol. 2012;19:e414–21. doi: 10.3747/co.19.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maru A, Gangadharan VP, Desai CJ, Mohapatra RK, Carides AD. A phase 3, randomized, double-blind study of single-dose fosaprepitant for prevention of cisplatin-induced nausea and vomiting: Results of an Indian population subanalysis. Indian J Cancer. 2013;50:285–91. doi: 10.4103/0019-509X.123580. [DOI] [PubMed] [Google Scholar]
- 17.Ryu JW, Park SJ, Park M, Kim JS, Lee KY. The use of aprepitant in the first cycle of moderately emetogenic chemotherapy in patients with colorectal cancer: Its preventive effect on chemotherapy-induced nausea and vomiting. Korean J Clin Oncol. 2013;9:47–52. [Google Scholar]
- 18.Grunberg S, Clark-Snow RA, Koeller J. Chemotherapy-induced nausea and vomiting: Contemporary approaches to optimal management: Proceedings from a symposium at the 2008 Multinational Association of Supportive Care in Cancer (MASCC) Annual Meeting. Support Care Cancer. 2010;18(Suppl 1):1–10. doi: 10.1007/s00520-009-0807-z. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN): Antiemesis Guidelines; 2013. Available from: http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf .


