Abstract
Advanced glycation end products (AGEs) are an abnormal modification of the collagenous matrix in bone, and their accumulation contributes to alteration of mechanical properties. Using a mouse model of focal external radiotherapy, we quantified the time-dependent changes in the glycation of bone collagen following unilateral hindlimb exposure to 4 daily fractions of 5 Gy. Fluorometric analysis of decalcified femurs demonstrated a significant and transient increase in the quantity of pentosidine, pyridinolines, and non-specific AGEs per unit of collagen at one week post-radiation. These differences did not persist at 4, 8, 12, or 26 weeks post-radiation. Radiation had no effect on bone collagen content. We hypothesize that following the transient increase in glycation products, these cross-links are then removed as a result of increased post-radiation osteoclast activity and continued mineralization of the bone.
INTRODUCTION
Although fragility fractures are a frequent complication associated with radiation therapy for soft tissue sarcoma, there are currently no viable prevention strategies. Mechanical testing and computational modeling indicate that decreased strength in irradiated bone cannot be explained by changes in mineral density or bone quantity alone but are also likely due to embrittlement of the bone tissue (1). There is substantial laboratory and epidemiological evidence for the role of material properties in regulation of bone mechanical properties, including increased fragility associated with diabetes and age (2–20).
The organic matrix, consisting primarily of type I collagen, contributes extensively to the mechanical integrity of bone (20). Modification of collagen can occur through glycosylation, cross-linking, and fibril fragmentation (3, 21). Enzymatic cross-linking of collagen is typically biologically regulated and indicative of tissue maturity, while non-enzymatic cross-links are influenced by the local microenvironment and closely associated with aging and disease processes (16, 20). One factor contributing to altered matrix mechanical properties may be the accumulation of advanced glycation end products (AGEs). AGEs are naturally occurring non-enzymatic modifications of proteins. Matrix cross-links resulting from formation of AGEs can alter matrix integrity, cell-matrix signaling, and rates of tissue turnover (16, 17, 22–24).
We hypothesize that radiotherapy induces increased accumulation of AGEs in bone. Modification of bone collagen by accumulation of AGEs would provide a potential explanation for the altered material properties of bone post-radiation. The aim of this study was to quantify accumulation of advanced glycation end products in bone following radiotherapy using a mouse model.
METHODS
Hindlimb Irradiation
Female BALB/F mice aged 12 weeks (Taconic, Germantown, NY) were anesthetized and their hindlimbs extended for unilateral localized irradiation (RTx) at a dose of 20 Gy delivered as four consecutive daily 5 Gy fractions (4×5 Gy) (n=6/group/time point). The body and contralateral hindlimb of each animal was covered with lead shielding, allowing the non-irradiated hindlimb to serve as an internal non-irradiated control (0 Gy). The BED (Biologically Effective Dose) was calculated to be 57.5 Gy2.8, with an EQD2 Gy (Equivalent Dose in 2 Gy Fractions) of 32.5 Gy2.8/2 (or ~16 fractions of 2 Gy each) where:
and
In this case, n is the number of fractions, d is the dose per fraction (here, d = 5 Gy), and α/β = 2.8 Gy for bone, which is considered a normal and therefore late-responding (non-tumor) tissue (25–27). All methods were approved by the SUNY Upstate Medical University Committee for the Humane Use of Animals.
Analysis of Cross-Links
Tibias were harvested at 1, 2, 4, 8, and 26 weeks post-RTx, cleaned of soft tissues, and wrapped in saline-soaked gauze for storage at −20°C until processing. The tibias were then decalcified with 30% formic acid in 20% aqueous sodium citrate for 24 hours (Cat #399388 and #W302600, Sigma-Aldrich, St. Louis, MO, USA) and lyophilized (17). This was followed by digestion in collagenase Type II (3 mg/ml at 37°C, Cat #LS004176, Worthington Biochemical, Lakewood, NJ, USA) with homogenization (Polytron Kinematica, Lucerne, Switzerland). Insoluble organic material was removed by centrifugation. The extent of collagen cross-linking was determined by autofluorescence of the soluble matrix digest (Tecan Infinite M200, Research Triangle Park, NC). All samples were run in triplicate and four fluorescence reads were done per well. Three glycation products were quantified, including pentosidine (excitation/emission λ 335/385), pyridinolines (297/395), and non-specific AGEs (370/440 and 335/400). Pentosidine was quantified by comparison to a quinine sulfate standard curve (17). Results are expressed as arbitrary units (AU, arbitrary fluorescence units as reported by the plate reader using a gain setting of 100), with the exception of pentosidine, which is expressed in relation to the quinine sulfate standard. Fluorescence values were normalized by the moles of collagen contained in the same sample volume. The resulting value reflects the degree of cross-linking per collagen molecule, not the total number of cross-links in each bone sample. The collagen content of each sample was calculated by assuming collagen to contain 13.5% hydroxyproline by mass, and a molecular weight of 399 kDa (28, 29). When quantifying cross-links in bone, results are typically normalized to the collagen or hydroxyproline content of the sample, as collagen is the primary organic component of bone (3, 10, 11, 15, 17–19, 23, 30–36).
Statistics
Data were analyzed with JMP software (SAS, Cary, NC) using a two-way ANOVA model including time, radiation dose, and their interaction as variables. Statistical significance was assumed at p ≤ 0.05. Post-hoc pairwise comparisons were conducted using Tukey’s post-hoc test, which accounts for multiple sampling errors.
RESULTS
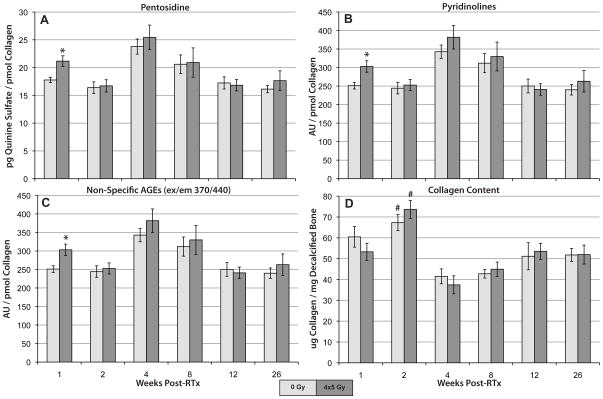
One week after the last dose fraction, specimens subjected to 4×5 Gy demonstrated a significant increase in the extent of collagen glycation (number of cross-links per unit of collagen). Specifically, the quantity of pyridinolines (p = 0.008), pentosidine (p = 0.013), and non-specific AGEs (p = 0.020 for ex/em = 370/440 and p = 0.0196 for ex/em = 335/400) accumulated by each molecule of collagen were elevated in irradiated samples compared to non-irradiated controls at one week (Figure 1A–C). There were no significant differences between treatment groups at any other time point. Pentosidine, pyridinolines, and non-specific AGEs were all significantly affected by time (p < 0.001), although there was no consistent positive or negative trend with increasing time. The interaction between radiation and time was not significant.
Figure 1.
Degree of collagen glycation following radiation (4×5 Gy) treatment (average ± SD, n = 4–6 per treatment per time point). Data are expressed as quantity of cross-links per picomole of collagen. At 1 week post-radiation, irradiated samples had significantly elevated content of (A) pentosidine, (B) pyridinolines, and (C) non-specific AGEs compared to controls. * denotes significantly different compared to non-irradiated controls at the same time point (p < 0.020). # denotes significantly different from all other time points (p < .013).
Radiation treatment did not significantly impact tibial collagen content (ug of collagen per mg of dry decalcified bone, p = 0.977, Figure 1D). Tibial collagen content was significantly affected by time (p < 0.001), with both control and irradiated groups demonstrating a significant increase in collagen content at two weeks compared to all other time points (p ≤ 0.013). Collagen content at one week was significantly higher than at four (p < 0.001) and eight weeks (p = 0.020).
DISCUSSION
Previously, we have documented radiation-induced alterations in bone quality and microarchitecture, including loss of trabecular bone, thickening of cortical bone, increased mineral density, and transient increase in osteoclast numbers followed by loss of viable osteoclasts (1, 37–41). The overall increase in bone volume and mineral density following radiotherapy is accompanied by a decrease in mechanical strength, indicating that radiation induces alterations to the material properties of the bone matrix (1). The cause of the post-radiation bone embrittlement is not yet fully understood, but there is evidence that alterations to the organic matrix may contribute significantly to altered mechanical properties.
There is extensive evidence that cross-linking and glycation-associated changes to collagen are related to the biomechanical properties of bone, particularly in the context of aging and diabetic pathologies. Garnero et al. induced collagen cross-linking in ex vivo bovine cortical femur specimens, and found that increased pyridinolines and pentosidine per unit of collagen correlated with reduced compressive yield stress and post-yield energy absorption; stiffness remained unaffected (3). Similarly, Tang et al. found that AGE accumulation in cadaveric trabecular bone specimens correlated with decreased stiffness of individual trabeculae and overall decreases in post-yield strain energy and energy dissipation (15). Stiffening of the collagenous matrix is strongly associated with accumulation of cross-links in bone, contributing to increased skeletal fragility and fracture risk (17, 34). Elevated pentosidine content in diabetic rats has been correlated with decreased modulus, energy absorption, and peak load in three-point bending (11). Viguet-Carrin et al. demonstrated no direct association between collagen cross-links and compressive mechanical properties (stiffness, failure load, work to fracture) for intact cadaveric vertebral bodies. Bivariate analysis of their data, however, indicated a significant association between increased failure load and high bone mineral density and low pentosidine content (35). There is also evidence that the type of cross-links accumulated in collagen may be important. Enzymatic and non-enzymatic cross-links may have differential effects in determining mechanical properties of bone (9, 20, 32, 42).
Reports of post-radiation changes to the organic matrix of bone suggest multiple factors may contribute to modification of collagen integrity. Specifically, it has been suggested that radiation induces increased collagen fragmentation, but not tissue collagen content. Açil et al. reported radiation-induced collagen fragmentation following irradiation of porcine mandibles ex vivo (21). Our data demonstrate no radiation-dependent changes in overall bone collagen content. This is consistent with the observations of Açil et al. in bone (murine model, 9.5 Gy focal hindlimb), Sassi et al. in skin (breast cancer patients, 30–56 Gy), and Lindburg et al. in cultured articular cartilage explants (porcine, 2 Gy) (43–45).
Documentation of radiation effects on collagen cross-linking and glycation in vivo is particularly sparse. Açil et al. treated rat hindlimbs with a single 9.5 Gy radiation exposure (BED = 42.8 Gy2.8, equivalent to 12 fractions of 2 Gy each, as calculated by us) and evaluated hindlimbs at 14 and 100 days post-RTx for hydroxyproline, lysylpyridinoline, and hydroxylysylpyridinoline content. The only radiation-associated effect was a decrease in lysylpyridinoline quantity per mg of bone at 14 days post-RTx, which returned to control levels by 100 days post-RTx (43).
In this study, we demonstrate an early, transient increase in both enzymatic (pentosidine, non-specific AGEs) and non-enzymatic (pyridinolines) cross-linking of bone collagen following focal radiation therapy. These data are consistent with our Raman spectroscopic results using this mouse model of focal radiotherapy (4×5 Gy), showed significantly increased collagen crosslink ratios, significantly decreased matrix depolarization ratios, and early decreased mineral depolarization ratios in irradiated tibias beginning at one week post-RTx (46). The current results also provide a potential at least partial explanation for the findings of Wernle et al., who subjected irradiated mouse femurs (single fraction doses of 0, 5, and 20 Gy) to compressive mechanical testing (1). Failure load was significantly increased in irradiated samples at 1 week, falling below that of controls at 12 weeks. Temporally this correlates with our findings of increased collagen glycation at 1 week post-RTx and fits with the wider body of literature documenting the roles of collagen glycation and cross-linking in regulating bone mechanical properties. Although Wernle et al. documented radiation-associated morphological changes, finite element modeling indicated that morphology alone insufficiently explained the altered mechanical properties. Incorporation of an embrittled failure model improved the predictive strength of the computation models, suggesting that material properties play a significant role in determining the mechanical integrity of bone post-radiation (1).
We hypothesize that accumulation of advanced glycation end products in irradiated bone may contribute to embrittlement of the collagenous matrix and thereby increase fracture risk. Equilibration of glycation product levels beyond the one-week time point in this animal model is likely the result of increased osteoclastic bone resorption that follows radiation exposure (beginning 2 to 4 weeks post-RTx) and continued deposition of new mineral (41, 47). Longer term, radiation induced bone fragility may be regulated by the interaction of altered bone morphology, vascularity, biochemical matrix alterations, and cellular activity. Future investigations will pursue identification of specific biological mechanisms contributing to post-radiation bone embrittlement, including the role of reactive oxygen species, cell-mediated matrix remodeling, and potential therapeutic interventions.
Acknowledgments
This study was funded by an award from the Carol M. Baldwin Breast Cancer Research Foundation (MEO), the Kate Allen Breast Cancer Research Fund (TAD), and the David G. Murray Endowment (TAD).
LIST OF ABBREVIATIONS
- AGEs
advanced glycation end products
- AU
arbitrary fluorescence units
- ANOVA
analysis of variance
- BED
biologically equivalent dose (Gy)
- ex/em
excitation/emission wavelength (nm)
- RTx
radiation treatment
References
- 1.Wernle JD, Damron TA, Allen MJ, Mann KA. Local irradiation alters bone morphology and increases bone fragility in a mouse model. J Biomech. 2010 Oct 19;43(14):2738–46. doi: 10.1016/j.jbiomech.2010.06.017. Epub 2010/07/27. eng. [DOI] [PubMed] [Google Scholar]
- 2.Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res. 2002 Sep;17(9):1621–8. doi: 10.1359/jbmr.2002.17.9.1621. Epub 2002/09/05. eng. [DOI] [PubMed] [Google Scholar]
- 3.Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, et al. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone. 2006 Mar;38(3):300–9. doi: 10.1016/j.bone.2005.09.014. Epub 2005/11/08. eng. [DOI] [PubMed] [Google Scholar]
- 4.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996 Oct;11(10):1531–8. doi: 10.1002/jbmr.5650111021. Epub 1996/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 5.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000 Aug;15(8):1526–36. doi: 10.1359/jbmr.2000.15.8.1526. Epub 2000/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005 Dec;37(6):825–32. doi: 10.1016/j.bone.2005.07.019. Epub 2005/09/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone. 2011 Jun 1;48(6):1260–7. doi: 10.1016/j.bone.2011.03.672. Epub 2011/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 8.Katayama Y, Akatsu T, Yamamoto M, Kugai N, Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996 Jul;11(7):931–7. doi: 10.1002/jbmr.5650110709. Epub 1996/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995 Oct;17(4 Suppl):365S–71S. doi: 10.1016/8756-3282(95)00328-b. Epub 1995/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 10.Oxlund H, Mosekilde L, Ortoft G. Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone. 1996 Nov;19(5):479–84. doi: 10.1016/s8756-3282(96)00283-9. Epub 1996/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006 Oct;17(10):1514–23. doi: 10.1007/s00198-006-0155-5. Epub 2006/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009 Jul;94(7):2380–6. doi: 10.1210/jc.2008-2498. Epub 2009/04/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26(1):93–100. doi: 10.1007/s00774-007-0784-6. Epub 2007/12/21. eng. [DOI] [PubMed] [Google Scholar]
- 14.Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010 Jan;46(1):148–54. doi: 10.1016/j.bone.2009.09.003. Epub 2009/09/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007 Apr;40(4):1144–51. doi: 10.1016/j.bone.2006.12.056. Epub 2007/01/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vashishth D. Advanced glycation end-products and bone fractures. IBMS BoneKEy 2009. 2009 Aug;6(8):268–78. doi: 10.1138/20090390. Epub 08/2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001 Feb;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. Epub 2001/02/22. eng. [DOI] [PubMed] [Google Scholar]
- 18.Viguet-Carrin S, Farlay D, Bala Y, Munoz F, Bouxsein ML, Delmas PD. An in vitro model to test the contribution of advanced glycation end products to bone biomechanical properties. Bone. 2008 Jan;42(1):139–49. doi: 10.1016/j.bone.2007.08.046. Epub 2007/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 19.Viguet-Carrin S, Follet H, Gineyts E, Roux JP, Munoz F, Chapurlat R, et al. Association between collagen cross-links and trabecular microarchitecture properties of human vertebral bone. Bone. 2010 Feb;46(2):342–7. doi: 10.1016/j.bone.2009.10.001. Epub 2009/10/20. eng. [DOI] [PubMed] [Google Scholar]
- 20.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319–36. doi: 10.1007/s00198-005-2035-9. Epub 2005/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 21.Acil Y, Springer IN, Niehoff P, Gassling V, Warnke PH, Acmaz S, et al. Proof of direct radiogenic destruction of collagen in vitro. Strahlenther Onkol. 2007 Jul;183(7):374–9. doi: 10.1007/s00066-007-1598-0. Epub 2007/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 22.Franke S, Ruster C, Pester J, Hofmann G, Oelzner P, Wolf G. Advanced glycation end products affect growth and function of osteoblasts. Clin Exp Rheumatol. 2011 Jul-Aug;29(4):650–60. Epub 2011/09/13. eng. [PubMed] [Google Scholar]
- 23.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007 Feb 23;282(8):5691–703. doi: 10.1074/jbc.M610536200. Epub 2006/12/05. eng. [DOI] [PubMed] [Google Scholar]
- 24.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008 Apr;1126:166–72. doi: 10.1196/annals.1433.044. Epub 2008/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 25.Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol. 1988;27(2):117–22. doi: 10.3109/02841868809090331. Epub 1988/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 26.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003 Sep;41(3):208–11. doi: 10.1002/mpo.10338. Epub 2003/07/18. eng. [DOI] [PubMed] [Google Scholar]
- 27.Fowler JF. Development of radiobiology for oncology-a personal view. Phys Med Biol. 2006 Jul 7;51(13):R263–86. doi: 10.1088/0031-9155/51/13/R16. Epub 2006/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 28.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. Epub 1950/05/01. eng. [PubMed] [Google Scholar]
- 29.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996 Jun;29(3):225–9. doi: 10.1016/0009-9120(96)00003-6. Epub 1996/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 30.Banse X, Devogelaer JP, Lafosse A, Sims TJ, Grynpas M, Bailey AJ. Cross-link profile of bone collagen correlates with structural organization of trabeculae. Bone. 2002 Jul;31(1):70–6. doi: 10.1016/s8756-3282(02)00800-1. Epub 2002/07/12. eng. [DOI] [PubMed] [Google Scholar]
- 31.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988 Jun 1;252(2):495–500. doi: 10.1042/bj2520495. Epub 1988/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010 Feb;21(2):195–214. doi: 10.1007/s00198-009-1066-z. Epub 2009/09/18. eng. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, et al. Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1–34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int. 2011 Aug;22(8):2373–83. doi: 10.1007/s00198-010-1454-4. Epub 2010/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 34.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009 Jun;20(6):887–94. doi: 10.1007/s00198-008-0754-4. Epub 2008/10/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, et al. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006 Nov;39(5):1073–9. doi: 10.1016/j.bone.2006.05.013. Epub 2006/07/11. eng. [DOI] [PubMed] [Google Scholar]
- 36.Sroga GE, Vashishth D. UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Feb 15;879(5–6):379–85. doi: 10.1016/j.jchromb.2010.12.024. Epub 2011/01/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oest ME, Rogers B, Spadaro JA, Strauss J, Damron TA. Osteoclast Activity Increases Early Following Localized Bone Irradiation. Annual Meeting of the Orthopaedic Research Society; Long Beach, CA. 2012. [Google Scholar]
- 38.Oest ME, Franken V, Wentz S, Spadaro JA, Strauss J, Damron TA. Early Increased Medullary Osteoclasts and Unopposed Cortical Mineral Apposition Explain Morphologic Changes After Limited Field Irradiation. Annual Meeting of the Orthopaedic Research Society; San Antonio, TX. 2013. [Google Scholar]
- 39.Keenawinna L, Oest ME, Mann KA, Spadaro J, Damron TA. Zoledronic acid prevents loss of trabecular bone after focal irradiation in mice. Radiat Res. 2013 Jul;180(1):89–99. doi: 10.1667/RR3200.1. Epub 2013/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 40.Willey JS, Livingston EW, Robbins ME, Bourland JD, Tirado-Lee L, Smith-Sielicki H, et al. Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone. 2010 Jan;46(1):101–11. doi: 10.1016/j.bone.2009.09.002. Epub 2009/09/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willey JS, Lloyd SA, Robbins ME, Bourland JD, Smith-Sielicki H, Bowman LC, et al. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat Res. 2008 Sep;170(3):388–92. doi: 10.1667/RR1388.1. Epub 2008/09/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito M, Marumo K, Ushiku C, Kato S, Sakai S, Hayakawa N, et al. Effects of alfacalcidol on mechanical properties and collagen cross-links of the femoral diaphysis in glucocorticoid-treated rats. Calcif Tissue Int. 2011 Apr;88(4):314–24. doi: 10.1007/s00223-011-9472-6. Epub 2011/02/18. eng. [DOI] [PubMed] [Google Scholar]
- 43.Acil Y, Gierloff M, Behrens C, Moller B, Gassling V, Niehoff P, et al. Effects of Zoledronate on Irradiated Bone In Vivo: Analysis of the Collagen Types I, V and Their Cross-links Lysylpyridinoline, Hydroxylysylpyridinoline and Hydroxyproline. Calcif Tissue Int. 2013 Mar;92(3):251–60. doi: 10.1007/s00223-012-9676-4. Epub 2012/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 44.Sassi M, Jukkola A, Riekki R, Hoyhtya M, Risteli L, Oikarinen A, et al. Type I collagen turnover and cross-linking are increased in irradiated skin of breast cancer patients. Radiother Oncol. 2001 Mar;58(3):317–23. doi: 10.1016/s0167-8140(00)00253-x. Epub 2001/03/07. eng. [DOI] [PubMed] [Google Scholar]
- 45.Lindburg CA, Willey JS, Dean D. Effects of low dose X-ray irradiation on porcine articular cartilage explants. J Orthop Res. 2013 Aug 1; doi: 10.1002/jor.22406. Epub 2013/08/06. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong B, Oest ME, Mann KA, Damron TA, Morris MD. Raman spectroscopy demonstrates prolonged alteration of bone chemical composition following extremity localized irradiation. Bone. 2013 Nov;57(1):252–8. doi: 10.1016/j.bone.2013.08.014. Epub 2013/08/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondo H, Searby ND, Mojarrab R, Phillips J, Alwood J, Yumoto K, et al. Total-body irradiation of postpubertal mice with (137)Cs acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. Radiat Res. 2009 Mar;171(3):283–9. doi: 10.1667/RR1463.1. Epub 2009/03/10. eng. [DOI] [PubMed] [Google Scholar]