Abstract
Rhythms govern many endocrine functions. Examples of such rhythmic systems include the insulin-secreting pancreatic beta-cell, which regulates blood glucose, and the gonadotropin-releasing hormone (GnRH) neuron, which governs reproductive function. Although serving very different functions within the body, these cell types share many important features. Both GnRH neurons and beta-cells, for instance, are hypothesized to generate at least two rhythms endogenously: (1) a burst firing electrical rhythm and (2) a slower rhythm involving metabolic or other intracellular processes. This review discusses the importance of hormone rhythms to both physiology and disease and compares and contrasts the rhythms generated by each system.
Keywords: LHRH, Beta-cell, Islet, Oscillations, Rhythms, Metabolism, Endocrine, Hormone
Introduction
Rhythmic activity at various frequencies forms the basis of many neural and endocrine systems. The thalamocortical system relating to sleep [1], vasopressin neurons signaling thirst and osmoregulation, [2, 3], and PreBoetzinger neurons governing breathing [4], for example, demonstrate rhythmic firing with periods on the order of seconds. Neurons of the suprachiasmatic nucleus, in contrast, generate much slower circadian (~24 h) rhythms [5, 6], while ultradian rhythms (<24 h, but typically ~ 30–120 min) occur in systems governing, for example, appetite and satiety [7], sleep and dreaming [8, 9], cardiac and respiratory function [10], as well as the transcription and translation of several genes [11, 12]. Rhythms thus abound in biology as a fundamental means of communication and organization.
This review contrasts two such rhythmic systems: the insulin-secreting pancreatic beta-cell, which regulates plasma glucose, and the gonadotropin-releasing hormone (GnRH) neuron, which governs reproductive function. Each cell type has its own endogenous rhythms, which can be observed in their electrical activity patterns and rhythmical secretory patterns. There are also additional rhythmic complexities to each system [13–18]. The purpose of this introductory review is to demonstrate the importance of rhythmic function to normal physiology and to compare and contrast the distinct mechanisms and functions of rhythmic activity in each of these systems.
Pulsatility in health and disease
For both the GnRH neuron and the insulin-secreting beta-cell, rhythmicity is a raison d’être. Properly timed, intermittent bursts of activity are crucial to normal organ function, and the loss of this capacity is associated with dysfunction and disease [19–23]. At a cellular and organismal level, there are several potential advantages to pulsatile signaling: (1) Information can be coded within the frequency and/or amplitude of pulses that permit complex downstream signaling. (2) Pulsatility may help to coordinate the activity of cells within a network that are otherwise isolated from one another, as is the case for GnRH neurons and pancreatic islets. (3) Intermittent cycles of activity and rest may be more energetically efficient and may reduce potential cellular stress. In this section, we introduce these two pulsatile endocrine systems and then highlight pathological conditions that are known to be associated with disrupted pulsatility.
GnRH neurons
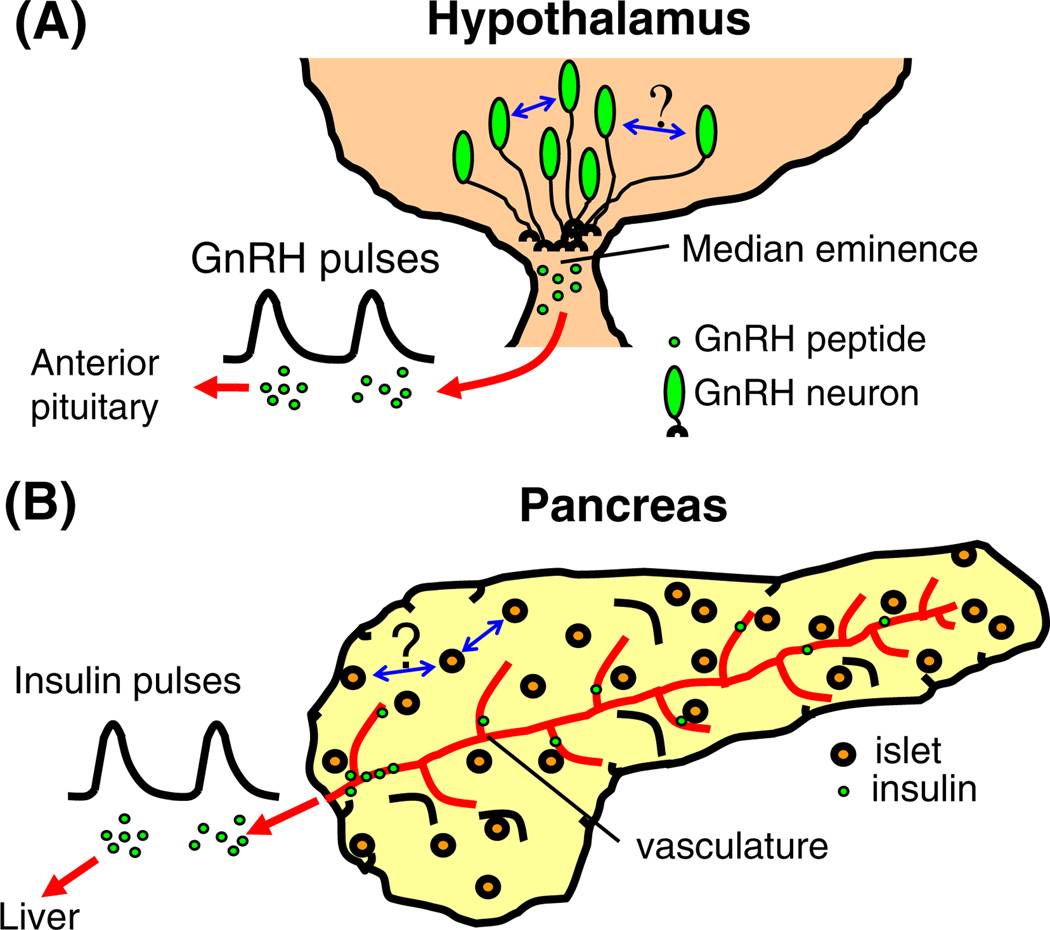
A network of some 800–2,000 GnRH neurons is widely distributed throughout the basomedial hypothalamus and preoptic area of the brain [24, 25]. Most GnRH neurons target their axons to the median eminence, where GnRH peptide is secreted into the bloodstream and carried by the portal vasculature to the anterior pituitary (Fig. 1a). Pituitary gonadotropes respond to GnRH signals by secreting both luteinizing hormone (LH) and follicle stimulating hormone (FSH). These pituitary hormones act on the gonads to promote the production of eggs or sperm and the synthesis of different steroids (estrogen, testosterone, etc.). The steroids, in turn, provide both positive and negative feedback at the level of the brain and the pituitary to complete a reproductive regulatory loop called the hypothalamic–pituitary–gonadal (HPG) axis (Fig. 1b). The role of GnRH neurons in the HPG-axis is to integrate the activity of neural afferents, environmental cues, and steroidal cues related to fertility and, in turn, to form the final common pathway for the central control of reproduction.
Fig. 1.
GnRH neurons and the HPG-axis. a GnRH neurons at the base of the hypothalamus target axons to the median eminence where GnRH is released and travels to the pituitary gland. b The HPG-axis.
The output of this final common pathway is the pulsatile release of GnRH peptide [26, 27], a crucial element in maintaining reproductive function. Pulsatile GnRH maintains appropriate levels of LH and FSH, while continuous GnRH suppresses the synthesis and secretion of LH and FSH [28], leading ultimately to infertility [20, 29, 30]. Modulation of the frequency of GnRH pulses is equally critical [31, 32], as GnRH pulse frequency is a primary determinant of differential pituitary responses [33, 34]. Specifically, higher-frequency pulses favor LH release, whereas lower-frequency pulses favor FSH release (Fig. 2) [31, 32]. To ensure the proper development of the ovarian follicle, GnRH neurons must therefore respond to changes in the steroid milieu, the stage of the reproductive cycle, and other cues by modulating GnRH pulse intervals for a few minutes to hours.
Fig. 2.
The frequency of GnRH pulses codes for different rates of LH and FSH secretion from pituitary gonadotropes. Adapted from Wildt et al. [216] with permission of the Endocrine Society, © 1981
Pulsatility in the GnRH system is required for proper reproductive function, and an inability to modulate GnRH pulse frequency can result in a loss of reproductive function. An imbalance in steroids or stress levels, or a significant change in energy balance toward obesity or anorexia, can result in disruptions or even the outright arrest of the pulsatile GnRH signal. Hyperprolactinemia and hypothalamic amenorrhea, for example, are associated with chronically low-frequency GnRH pulses [35, 36], and polycystic ovary syndrome (PCOS) is associated with persistent high-frequency GnRH pulses [22, 37]. These reproductive disorders thus demonstrate that pulsatility and pulse frequency coding in the GnRH system are crucial to fertility. A summary of reproductive disorders related to dysfunctional GnRH pulsatility can be found in [38].
Beta-cells
Islets of Langerhans are micro-organs that constitute ~ 1–2 % of the total mammalian pancreas by volume and are responsible for regulating plasma glucose and body energy metabolism [39]. Islets vary in size and shape, and possess their own microvasculature to rapidly carry insulin and other secreted factors from the islet cells to the wider circulation, as well as to receive circulating nutrients and regulatory factors involved in glucose homeostasis, providing an additional level of control [40–42]. In addition, islets are innervated by neurons of the autonomic nervous system [43, 44]. The islet is composed of several endocrine cell types, including glucagon-secreting alpha-cells, insulin-secreting beta-cells, somatostatin-secreting delta-cells, and others [45, 46] (see Fig. 3a). The proportion and organization of these cells differ somewhat from species to species [46, 47], but beta-cells compose the majority of the total islet mass (50–80 %).
Fig. 3.
a Illustration of the cellular composition of a pancreatic islet. b The actions of insulin and glucagon to regulate blood sugar
The primary function of beta-cells is to synthesize and secrete insulin, a critical regulator of blood glucose. In response to a rise in blood glucose concentration following the ingestion of a meal, beta-cells secrete insulin to stimulate the conversion of glucose to glycogen in the liver, suppress hepatic glucose output and facilitate the uptake of glucose into insulin target tissues, particularly skeletal muscle and fat (see Fig. 3b). When blood glucose levels drop as a result of insulin acting at its receptor, insulin secretion is in turn reduced, thus completing the regulatory loop. Blood glucose is also regulated by additional factors including the hormone glucagon, which is secreted by islet alpha-cells and counterbalances the effects of insulin by mobilizing glucose from the liver.
Pulsatility is important to the overall regulation of blood glucose. Delivering intermittent insulin to healthy individuals results in a more effective insulin response than continuous insulin in most [48–51] but not all, studies [52, 53]. In addition, patients with diabetes respond more effectively to pulsatile insulin compared to continuous delivery [54–56]. More recently, a series of elegant studies have shown in both dog and rat that pulsatile insulin is more efficacious in suppressing hepatic glucose production than is continuous insulin as pulsatile presentation potentiated insulin receptor signal transduction in liver [57–59].
Loss of pulsatile insulin secretion also occurs during the early stages of Type 2 diabetes, with a reduction in the amplitude and possibly the frequency of the insulin pulses being linked to subsequent diabetes [60–64]. The close relatives of diabetic patients, even those with normal glucose homeostasis, also demonstrate considerable degradation of pulsatile insulin secretion despite reporting clinically normal responses to glucose challenges and normal levels of insulin resistance [65, 66]. Even among islets observed under control conditions in vitro, oscillatory islets are associated with greater glucose sensitivity than non-oscillatory islets [67]. Aberrant insulin pulsatility has also been reported in specific forms of diabetes and in other metabolic disorders, including maturity onset diabetes of the young type 2 (MODY2) [68], Tarui’s disease or glycogen-storage disease type-VII [62], and maternally inherited diabetes and deafness (MIDD) [69], as well as hypertension [70] and obesity [71, 72]. Thus, considerable evidence suggests that pulsatile insulin secretion is important to the proper regulation of blood glucose and is a potential early warning sign of emerging disease [73]. A review of metabolic disorders related to dysfunctional pulsatile insulin secretion can be found in [74, 75].
Anatomical organization and communication
One similarity between GnRH neurons and pancreatic islets is their overall anatomical organization. GnRH neurons, as mentioned previously, form a loose network of cells spread throughout the medial basal hypothalamus that act in unison to release GnRH in pulses at the median eminence (Fig. 4a). Islets are similarly distributed throughout the pancreas, and although islets are composed of hundreds of endocrine cells, each islet acts as a discrete unit that secretes insulin pulses into the vasculature (Fig. 4b).
Fig. 4.
Anatomy and organization of two rhythmic endocrine systems, a GnRH neurons are located in the base of the hypothalamus and target axons to the median eminence, where GnRH is released in pulses. It is not known whether GnRH neurons communicate either at the level of the cell body or at median eminence. Blue arrows illustrate the unknown mechanism(s) for communication and synchronization in each system. b Islets are spread throughout the pancreas and typically are not in direct contact with one another. Islets are highly vascularized, so that pulses of insulin and other secretory products can be carried to the liver
The diffuse distribution of intrinsic oscillators in the body or within specific tissues represents a communications dilemma. How these systems communicate to produce synchronized hormone pulses is not well understood for either GnRH neurons or for islets. It has been proposed that islets can be entrained to a single-pancreatic secretory pattern by a number of putative synchronizing mechanisms, such as an intra-pancreatic ganglion pacemaker [76–78], circulating inter-islet factors [79], or feedback interactions with the liver [80, 81]. No matter what mechanism is involved in synchronizing islets, the ability to maintain similar pulse frequencies may aid in the process. Of interest, isolated islets lacking any in vivo inputs still maintain calcium oscillations with remarkably similar frequencies to one another; this “imprinted” frequency is tightly maintained among islets isolated from an individual mouse and reflects insulin pulsatility in vivo, but may differ markedly from one mouse to the next [82, 83].
GnRH neurons, likewise, are separated from one another within the hypothalamus and appear to have some contact, although it is limited [84–86]. The neurons may not need to be in physical contact in order to secrete GnRH in a pulsatile fashion, however, as GnRH pulses can still be observed from acutely dissociated hypothalamic neurons [87]. Because most GnRH neurons project their axons to the median eminence, this is one potential point of contact or near contact. Of interest in this regard, isolated median eminence tissue containing only GnRH axons and synaptic terminals has also been shown to maintain pulsatile GnRH release [88]. Further, GnRH neuron projections to the median eminence appear to have a spike initiation site for action potentials as well as spines and synaptic appositions along its entire length [89], which could aid both in generating and synchronizing pulses. This suggests that the synchronization of GnRH secretion could occur by any number of methods, including the release of diffusible factors such as nitric oxide [90, 91] and GnRH [92, 93].
Although the advantage of having a dispersed distribution of intrinsic oscillators is not known, one possibility is that the distributed network could be a nexus for gathering and integrating different inputs from different brain regions. Neuronal afferents to GnRH neurons transmit numerous cues related to metabolism, stress, steroids, and circadian cues to the reproductive axis, among other effects [94]. It is reasonable to suspect that an individual GnRH neuron, for example, might receive certain inputs related to reproduction such as photoperiod [95] or nutrition [96], but not others, based on the anatomical location of the specific GnRH neuron in the hypothalamus. Under various physiological and pathological conditions, hormonal and metabolic signals either regulate GnRH neurons directly or act on upstream neuronal circuits to influence the pattern of pulsatile GnRH secretion into the hypophysial portal circulation. Particularly, powerful inputs to GnRH neurons include arcuate projections from cells co-expressing kisspeptin, neurokinin, and dynorphin, collectively known as KNDy neurons [97, 98].
A network of many widely distributed GnRH neurons might thus collect a wider array of inputs in order to integrate their activity into the overall reproductive output signal. Islets, likewise, might receive different signals based on their anatomical location within the pancreas, although this remains to be established. It is known though that individual islets from different parts of the pancreas exhibit certain defining characteristics with regard to their size and intra-islet architecture [99]. Also, certain circulating factors are closely linked to both reproduction and energy metabolism. Adipose-derived adiponectin and leptin, in particular, can modulate the activity of both GnRH neurons [100–102] and beta-cells [103], suggesting a potential common thread between these two endocrine systems. Dysfunction in such inputs could help in explaining the reproductive features of metabolic disorders and the metabolic features of reproductive disorders [101, 104].
Unlike GnRH neurons, beta-cells are contained within micro-organs, the islets of Langerhans, containing their own microvasculature and neuronal inputs together with multiple types of endocrine cells. This scenario provides an additional layer of anatomical organization and possibilities for integration. Within the islet, beta-cells form an electrical syncytium as they are coupled to one another via gap junctions that are mediated by connexin 36 [105–107]. This coupling allows the network of beta-cells to function as a unit, with near synchronous activity of beta-cells preserved across the islet [108]. This strong coordination is important, as its loss in CX36 knockout mice leads to asynchronous beta-cell activity within the islet, a loss of the initial, transient phase of insulin secretion, and a reduced amplitude of pulsatile plasma insulin [109].
The generation of pulsatile secretion (period in minutes)
Secretory rhythms on the order of minutes to hours have long been associated with reproductive function. Endogenous secretory rhythms related to reproductive function were first observed in LH at circhoral (~ hourly) intervals [110]. These hormone pulses were subsequently shown to correlate with episodic volleys of hypothalamic electrical activity (Fig. 5a) [18, 111]. The collection of hypothalamic neurons producing these rhythms has been termed the “GnRH pulse generator.”
Fig. 5.
a Pulses of LH, the downstream target of GnRH signaling, directly follow episodes of neural activity from the hypothalamus, detected as multiunit activity (MUA) from an implanted electrode. Adapted from Wilson et al. [111] with permission of S. Karger AG, Basel © 1984. b Pulsatile GnRH secretion from cultures of GT1-1 cells. Adapted from Pitts et al. [127] with permission of the Endocrine Society, ©2001. c Episodes of electrical activity from a single-GnRH neuron recorded within a coronal brain slice of an estradiol-treated ovariectomized mouse. Modified from data originally presented in Nunemaker et al. [217]
The molecular basis of the low-frequency rhythm comprising the GnRH pulse generator is largely unknown, in part, because GnRH neurons are few in number and widely distributed in a configuration that is neither nuclear nor laminar in organization, making the identification of GnRH neurons difficult. Thus, the only electrophysiological report to appear in the first 25 years following the identification of GnRH described results obtained in four immunocytochemically identified GnRH neurons out of 102 attempts total using guinea pig hypothalamic slices [112]. Subsequent techniques developed to identify or isolate GnRH neurons have demonstrated pulsatile GnRH release from cultures of immortalized GnRH (GT1) cells [113, 114] (see Fig. 5b), embryonic GnRH neurons [115], adult hypothalamic cultures [116, 117], and hypothalamic explants [87]. Episodic electrical activity, a correlate to secretion in this system, has also been reported from recordings of individual GnRH neurons in brain slices (see Fig. 5c) and in dissociated GnRH neurons [27, 118, 119]. These findings collectively suggest that the low-frequency rhythm observed for GnRH secretion is indeed endogenous to individual GnRH neurons.
As for the cellular mechanism of the GnRH pulse generator, there are several possibilities. Autocrine or paracrine feedback of GnRH is one candidate mechanism. GnRH neurons express GnRH-receptors [93, 117, 120], and exogenous GnRH (or GnRH agonists) has both positive and/or negative feedback effects on GnRH secretion [117, 121, 122] and electrical activity [93]. These findings support a model in which GnRH could provide autocrine or paracrine feedback involving GnRH receptors to modulate secretory pulse frequency through a G-protein-coupled-receptor signaling cascade [85, 123]. Alternatively, cycles of gene transcription and translation could occur within cells, as it has been shown that the transcription and translation of GnRH mRNA occur in a pulsatile fashion in GT1 cells [11, 124], and the expression of circadian clock genes modulates the frequency of GnRH pulses [125, 126]. However, GnRH pulses are maintained on a short-term basis of a few hours even when transcription and translation are blocked [124, 127]. Yet another candidate mechanism involves cyclic adenosine monophosphate (cAMP) opening cyclic-nucleotide-gated (CNG)-channels to cause depolarization, increased electrical activity, and GnRH secretion [128]. Rising cAMP levels also subsequently activate a protein kinase-A pathway that in turn reduces cAMP, thus closing the CNG-channels and reducing secretion [128, 129]. There is both support for [130–133] and against this mechanism [134, 135]. Finally, KNDy neurons have emerged recently as a putative external driver of pulsatility in GnRH neurons as reviewed here [136–139]. Although each of these candidate mechanisms shows promise, further evidence is required to determine which mechanism or mechanisms are involved in mediating the underlying oscillatory process responsible for generating GnRH pulses.
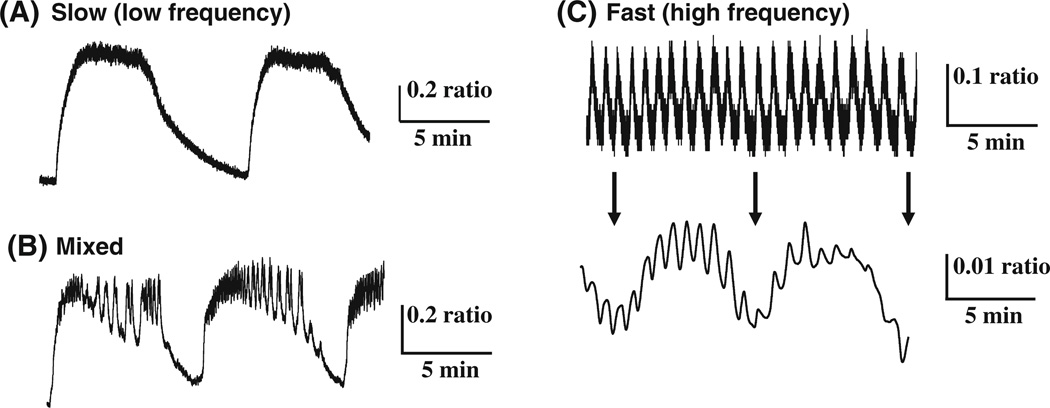
In islets, the established period of pulsatile insulin secretion measured in vivo ranges from 5 to 10 min and has been reported for many species [76, 140–142]. The two initial studies of rhythms on this timescale showed that there were clusters of more intense electrical bursting in isolated islets that occurred on the order of ~5 min [143, 144]. Subsequent reports using a variety of techniques have shown that islets produce a wide variety of oscillatory patterns, which may be slow (Fig. 6a), a mixture of fast and slow (b), or fast (c) [145]. Note that even some fast patterns still can display a small, underlying slow component, as shown in (Fig. 6c, lower panel).
Fig. 6.
Examples of the multiple modes of oscillations in [Ca2+]i, including purely slow oscillations (a), a mixture of fast and slow (b), and fast (c, top) with an underlying slow oscillations revealed (c, bottom) by signal averaging (Nunemaker and Satin, unpublished example)
As summarized in Table 1, it is now well established that islets generate rhythms in electrical activity, [Ca2+]i, and a variety of metabolic processes that have a period of ~3–5 min, in addition to more rapid bursting activity. Based on observations from isolated beta-cells as well as intact islets, low-frequency oscillations appear to be endogenous to individual beta-cells. Low-frequency islet oscillations have also been shown to closely correlate with oscillations in insulin secretion in vitro [146–148] and in vivo [149], suggesting that islet oscillations are important for physiological insulin secretion. Also, isolated human islets have been reported to display slow oscillations in response to glucose for calcium [150, 151] and insulin release [152].
Table 1.
A summary of slow oscillatory cellular processes
| Rhythmic process | Period (min) | Glucose (mM) | Beta-cells or islets tested | Reference (1st author, year, citation) |
|---|---|---|---|---|
| Electrical activity | 3–4 | 10, 15 | Beta-cell | Henquin, 1982 [143] |
| 4.6 | 11 | Islet | Cook, 1983 [144] | |
| 1–6 | 11 | Islet | Manning-Fox, 2006 [202] | |
| Intracellular calcium | 2–6 | 11 | Beta-cell | Grapengiesser, 1988 [203] |
| 1–5 | 11 or 16.7 | Islet | Valdeomillos, 1989 [204] | |
| Mitochondrial membrane potential | N/A | 11 | Beta-cell | Krippeit-Drews, 2000 [205] |
| N/A | 10 | Islet | Kindmark, 2001 [206] | |
| 4.5, 3.0 | 11 | Islet, beta-cell | Nunemaker, 2004 [207] | |
| ~3–6 | 6, 12 | Beta-cells in islets | Katzman, 2004 [208] | |
| Oxygen consumption | 5.3 | 20 | Beta-cell | Longo, 1991 [159] |
| 3.3 | 10 | Islet | Jung, 1999 [209] | |
| ~4 | 3, 11 | Islet | Ortsater, 2000 [210] | |
| 3.1 | 15 | Beta-cell (HIT) | Porterfield, 2000 [211] | |
| NAD(P)H | ~5 | 10 | Islet | Luciani, 2006 [212] |
| ~5 | 11 | Islet | Nunemaker, 2006 [213] | |
| Glycolytic enzyme activity | Merrins, 2013 [160] | |||
| Glucose consumption | 3.1 | 10 | Islet | Jung, 2000 [214] |
| ATP/ADP | 3.6 | 10 | Beta-cell (HIT) | Deeney, 2001 [215] |
| KATP-current | 2.5–4 | 0, 3 | Beta-cell | Dryselius, 1994 [188] |
| (1–4) | 10 | Beta-cell | Larsson, 1996 [189] | |
| 3.2 | 11 | Beta-cell | Ren, 2013 [155] |
First reports and/or detailed studies of oscillations are listed; apologies for any omissions
It has been proposed that the low-frequency rhythms of islets are driven by endogenous oscillations in glycolysis mediated by PFKM, which in turn, drive oscillations in downstream mitochondrial activity, ATP/ADP, KATP-channel conductance, electrical activity, and ultimately insulin secretion [153–155]. It has long been known that yeast exhibits endogenous oscillations in glycolytic activity on the order of minutes [156, 157], but confirming similar glycolytic oscillations in individual beta-cells has been difficult. While there is ample indirect evidence for the glycolytic model [153, 158, 159], the first direct evidence of oscillatory glycolysis in pancreatic beta-cells has only recently been reported using a FRET-based reporter based on pyruvate kinase M2 activity as the glycolytic product of PFKM, fructose-1,6-bisphosphate as an allosteric activator of this key enzyme [160]. These data confirm a glycolytic mechanism that produces oscillations on the order of ~5 min, the same period of insulin pulses produced in healthy humans.
Electrical bursting (period in seconds)
Rhythms in beta-cells were first identified by electrophysiological recordings of dissected mouse islets. In 1968, Dean and Matthews showed that mouse islets produce rhythmic bursts of repeated action potentials exhibiting periods of ~ 15 s in response to elevated glucose [161]. Subsequent studies determined that these rhythms involved the coordinated activity of calcium and potassium ion currents [162–166] and that their frequency and duration are glucose-dependent [167, 168]. An example of the rhythmic activity of a mouse islet is shown for both electrical activity (Fig. 7a) and by corresponding changes in [Ca +]i (Fig. 7b). These rhythms in electrical activity have been shown to induce oscillations in [Ca +]i [169, 170], and both electrical activity and [Ca +]i have been directly correlated with insulin secretion [146–148, 171, 172].
Fig. 7.
Bursting in islets, (a, b) A simultaneous recording of electrical activity (a) and [Ca2+]i (b) illustrates islet bursting (Zhang and Satin, unpublished example). The periodic firing of action potentials results in corresponding changes in [Ca2+]i, which is also reflective of insulin secretion, c A description of the components of a burst.
This phenomenon of rhythmic electrical activity is termed “bursting.” A burst is described as a train of action potentials that occur during a “plateau” or “active phase,” followed by a period of relative quiescence called the “silent phase” (see Fig. 7c). The period of burst firing is measured as the time interval from the start of one burst to the start of the next burst and is also called the “burst interval” or “activity cycle.” In beta-cells, bursts are also characterized by their “plateau fraction,” the relative time spent in the active phase divided by the total burst interval. Plateau fraction is particularly useful for comparing burst firing patterns in beta-cells at different glucose concentrations. The duration of time spent in the plateau phase progressively increases with glucose concentration, while the time spent in the silent phase is correspondingly reduced. Because secretion in excitable cells is closely linked to electrical activity and calcium influx [173], the plateau fraction yields a reasonable estimate of the relative rates of insulin secretion for islets because it is proportional to mean beta-cell calcium concentration [148, 163, 171, 172].
Although the low-frequency rhythm that produces pulsatile GnRH secretion clearly plays an integral role in maintaining reproductive function, much faster rhythms (periods ~5–60 s) have also been observed. Burst firing from unidentified neurons thought to be GnRH neurons based on their low-frequency rhythms was first reported in electrophysiological studies of sheep [18]. Spontaneous burst firing of action potentials was later described in detail in GT1 cells [174] and in GnRH neurons in brain slices [118, 119, 175], as shown in Fig. 8a. Burst firing has also been observed in acutely dissociated GnRH neurons [118, 176, 177] (Fig. 8b), strongly suggesting that burst firing is an intrinsic property of the GnRH neuron.
Fig. 8.
a Burst firing from GnRH neurons within a 200-µm coronal brain slice. Adapted from Nunemaker et al. [218] with permission of the Endocrine Society, ©2002. b Burst firing from an isolated GnRH neuron (Nunemaker and Moenter, unpublished example)
By testing the effects of a number of ion channel blockers in cultures of GT1 cells, a critical role for Na+-channels in generating bursts of action potentials and for L-type Ca2+-channels in generating [Ca2+]i oscillations was established [174, 178]. Potential modulators of burst firing properties were also identified, including potassium-channels, T-type calcium-channels, and [Ca2+]i stores [174, 178–180]. These findings led to a model suggesting that amplitude and/or frequency modulation of burst firing could underlie the secretory patterns in GnRH neurons [174, 180]. The presence of many of the same ion channels expressed in GT1 cells has been subsequently confirmed in native GnRH neurons [175, 176, 181–183] as well as additional channels that could participate in burst firing, as reviewed in [184].
The link between low- and high-frequency rhythms
For burst patterns resulting from oscillations in plasma membrane potential to interact with slow oscillations in intracellular processes as described above, a mechanism is needed which links plasma membrane ion fluxes to biochemical oscillations in the cytoplasm. The KATP-channel can readily provide this link, as first proposed by Cook and Hales [185]. KATP-channels were first shown to be metabolically sensitive in beta-cells by demonstrating the direct effects of exogenous ATP on single-channel activity in inside-out patches [185, 186] or by exposing beta-cells to bath glucose while recording KATP-channel activity in cell-attached patches [187]. Spontaneous changes in single-channel KATP-conductance in situ were later observed to occur at similar intervals as low-frequency metabolic oscillations [188, 189] (more recently for whole cells [155]), implying that the activity of KATP-channels may be responsive to endogenous changes in beta-cell fuel metabolism.
Although the KATP-channel appears to be the dominant link between metabolic processes and the plasma membrane of the beta-cell, low-frequency islet rhythms in electrical activity persist in islets from SUR1−/−knockout mice, which lack functional KATP-channels [190]. This suggests metabolic oscillations must also influence other electrical mechanisms. A number of other ion channels are thought to be metabolically sensitive in beta-cells, such as chloride-channels [191], L-type Ca2+-channels [192], and Kslow-channels [193, 194], several of which might in theory be able to function as redundant or secondary mechanisms in the event of the loss of the KATP-channel. It is also possible that other K-channels that play minor roles when KATP-expression is significant become dominant when KATP-channels are lost, although strong evidence for this is currently lacking (but see [195]. The majority of evidence, nevertheless, points to the KATP-channel as the dominant player in coupling rhythmic processes in the pancreatic beta-cell under physiological conditions. Indeed, mutations have been identified in the genes encoding the KATP-channel subunits SUR1 and Kir6.2 and these produce gain or loss of function for KATP-current that have been linked to neonatal diabetes or congenital hypoglycemia, respectively [196].
For GnRH neurons, an analogous mechanism for linking intracellular processes to changes in the membrane potential is far less settled. Without a clear understanding of what constitutes the GnRH pulse generator that drives circhoral secretory episodes, it is difficult to speculate as to what links these rhythms to bursts of electrical activity. Bursts of electrical activity in GnRH neurons have been shown to cluster more frequently or less frequently in patterns on the order of many minutes, leading to conjecture that gradual shifts in membrane potential toward or away from excitability may underlie this phenomenon [119]. Supporting this concept, a small subset of GnRH neurons appears to display a distinct subthreshold membrane potential oscillation with a period on the order of ~20 min [197]. Because these neurons are recorded in slices, not in isolation, it is not possible to discern whether these subthreshold oscillations are intrinsic to the GnRH neurons, driven by inputs, or a combination of the two possibilities.
Frequency versus amplitude coding
Although GnRH neurons and pancreatic beta-cells may generate rhythms in a seemingly analogous fashion, one interesting difference between the two systems is how their rhythmic hormone release is modulated. GnRH neurons govern reproductive function primarily by altering the frequency of GnRH pulses to code for different responses from the pituitary. This is interesting in that proper reproductive function in females requires numerous processing stages of the follicle during each ovulatory cycle; this requires a precise balance of hormones that are specific to each stage [38, 198]. GnRH neurons thus promote continuous changes in the steroidal milieu throughout the ovulatory cycle, which may be most effectively communicated by modulating the frequency of GnRH pulses in response to steroid feedback and cycle stage.
The pancreas, in contrast to GnRH neurons, generates pulses at a fairly steady frequency, and responds to increased glucose or to other fuel stimuli mostly by increasing the amplitude of insulin pulses produced, as demonstrated by in vivo studies [74, 199, 200]. This use of pulse amplitude modulation instead of frequency modulation could be indicative of a different mechanism of action and purpose [201]. Rather than promoting complex shifts in the hormone milieu, the role of islets is to secrete appropriate amounts of insulin on a minute-to-minute basis in order to maintain blood glucose within a tight range. The advantage of amplitude rather than frequency coding in this system is not known, although perhaps the targets of insulin action, liver, muscle, and fat tissue, may be better able to respond appropriately to changes in pulse amplitude.
Conclusions
Several common themes have emerged from this comparison of the GnRH and insulin endocrine systems. First, rhythmic activity takes on several forms, including a low-frequency rhythm synonymous with secretory pulses and also a higher-frequency burst pattern, which may facilitate secretory efficiency and/or communication with downstream targets. Second, these rhythms appear to be functionally connected. Third, secretory rhythms from GnRH neurons and pancreatic beta-cells are functionally important to their respective systems, as evidenced by the disorders and disease states that can result from the loss of pulsatile activity. The GnRH neuronal network and the pancreatic islet may thus serve as models for study of other endocrine systems.
Acknowledgments
Supported by NIH Grants RO1DK46409 to LSS and RO1DK089182 to CSN.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Craig S. Nunemaker, Division of Endocrinology and Metabolism, Department of, Medicine, University of Virginia, P.O. Box 801413, Charlottesville, VA 22901, USA, nunemaker@virginia.edu
Leslie S. Satin, Pharmacology Department, University of Michigan Medical School, 5128 Brehm Tower, Ann Arbor, MI 48105, USA lsatin@umich.edu Brehm Diabetes Research Center, University of Michigan, Medical School, 5128 Brehm Tower, Ann Arbor, MI 48105, USA.
References
- 1.Steriade M, Amzica F. Sleep oscillations developing into seizures in corticothalamic systems. Epilepsia. 2003;44(Suppl 12):9–20. doi: 10.1111/j.0013-9580.2003.12006.x. [DOI] [PubMed] [Google Scholar]
- 2.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 3.Roper P, Callaway J, Armstrong W. Burst initiation termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical electrical and calcium fluorescence study. J. Neurosci. 2004;24:4818–4831. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 5.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 6.Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J. Biol. Rhythm. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- 7.Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS. Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity. Regul. Pept. 2003;111:1–11. doi: 10.1016/s0167-0115(02)00305-1. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen TA. Chronobiological features of dream production. Sleep Med Rev. 2004;8:403–424. doi: 10.1016/j.smrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Voss U. Functions of sleep architecture and the concept of protective fields. Rev. Neurosci. 2004;15:33–46. doi: 10.1515/revneuro.2004.15.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Patzak A. Short-term rhythms of the cardiorespiratory system and their significance in neonatology. Chronobiol. Int. 1999;16:249–268. doi: 10.3109/07420529909116856. [DOI] [PubMed] [Google Scholar]
- 11.Nunez L, Faught WJ, Frawley LS. Episodic gonadotropin-releasing hormone gene expression revealed by dynamic monitoring of luciferase reporter activity in single, living neurons. Proc. Natl. Acad. Sci. USA. 1998;95:9648–9653. doi: 10.1073/pnas.95.16.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kippert F. Cellular signalling and the complexity of biological timing: insights from the ultradian clock of schizosaccharomyces pombe. Philos. Trans. R. Soc. Lond.B. 2001;356:1725–1733. doi: 10.1098/rstb.2001.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polonsky KS, Sturis J, Van Cauter E. Temporal profiles and clinical significance of pulsatile insulin secretion. Horm. Res. 1998;49:178–184. doi: 10.1159/000023168. [DOI] [PubMed] [Google Scholar]
- 14.Simon C, Brandenberger G. Ultradian oscillations of insulin secretion in humans. Diabetes. 2002;51(Suppl 1):S258–S261. doi: 10.2337/diabetes.51.2007.s258. [DOI] [PubMed] [Google Scholar]
- 15.Levy JC. Insulin signalling through ultradian oscillations. Growth Horm. IGF Res. 2001;11(Suppl A):S17–S23. doi: 10.1016/s1096-6374(01)80004-6. [DOI] [PubMed] [Google Scholar]
- 16.Rossmanith WG. The impact of sleep on gonadotropin secretion. Gynecol. Endocrinol. 1998;12:381–389. doi: 10.3109/09513599809012840. [DOI] [PubMed] [Google Scholar]
- 17.Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J. Neuroendocrinol. 2005;17:119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JC, Pelletier J. Multiunit activity in the anterior median eminence and adjacent areas of the hypothalamus of the ewe in relation to LH secretion. Neuroendocrinology. 1981;32:217–224. doi: 10.1159/000123162. [DOI] [PubMed] [Google Scholar]
- 19.Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Medeau V, Kevorkian JP. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab. 2008;34(Suppl 2):S43–S48. doi: 10.1016/S1262-3636(08)73394-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 2009;56:729–737. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz O, Brock B, Hollingdal M, Juhl CB, Porksen N. High-frequency insulin pulsatility and type 2 diabetes: from physiology and pathophysiology to clinical pharmacology. Diabetes Metab. 2002;28:4S14–4S20. [PubMed] [Google Scholar]
- 22.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–337. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog. Brain Res. 2010;181:111–126. doi: 10.1016/S0079-6123(08)81007-2. [DOI] [PubMed] [Google Scholar]
- 24.Silverman AJ. Distribution of luteinizing hormone-releasing hormone (LHRH) in the guinea pig brain. Endocrinology. 1976;99:30–41. doi: 10.1210/endo-99-1-30. [DOI] [PubMed] [Google Scholar]
- 25.Silverman AJ, Antunes JL, Ferin M, Zimmerman EA. The distribution of luteinizing hormone-releasing hormone (LHRH) in the hypothalamus of the rhesus monkey. Light microscopic studies using immunoperoxidase technique. Endocrinology. 1977;101:134–142. doi: 10.1210/endo-101-1-134. [DOI] [PubMed] [Google Scholar]
- 26.Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy as estimated with push-pull cannulae. Endocrinology. 1982;111:1439–1448. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- 27.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front. Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 28.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 29.Hauffa BP. Clinical implications of pulsatile hormone signals. Growth Horm. IGF Res. 2001;11(Suppl A):S1–S8. doi: 10.1016/s1096-6374(01)80002-2. [DOI] [PubMed] [Google Scholar]
- 30.McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin. Reprod. Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- 31.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 32.Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology. 1983;112:2076–2080. doi: 10.1210/endo-112-6-2076. [DOI] [PubMed] [Google Scholar]
- 33.Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- 34.Shupnik MA. Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol. Reprod. 1996;54:279–286. doi: 10.1095/biolreprod54.2.279. [DOI] [PubMed] [Google Scholar]
- 35.Reame N, Sauder SE, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J. Clin. Endocrinol. Metab. 1984;59:328–337. doi: 10.1210/jcem-59-2-328. [DOI] [PubMed] [Google Scholar]
- 36.Cook CB, Nippoldt TB, Kletter GB, Kelch RP, Marshall JC. Naloxone increases the frequency of pulsatile luteinizing hormone secretion in women with hyperprolactinemia. J. Clin. Endocrinol. Metab. 1991;73:1099–1105. doi: 10.1210/jcem-73-5-1099. [DOI] [PubMed] [Google Scholar]
- 37.Mauvais-Jarvis P, Bricaire C. Pathophysiology of polycystic ovary syndrome. J. Steroid Biochem. 1989;33:791–794. doi: 10.1016/0022-4731(89)90494-9. [DOI] [PubMed] [Google Scholar]
- 38.Marshall JC, Eagleson CA, McCartney CR. Hypothalamic dysfunction. Mol. Cell. Endocrinol. 2001;183:29–32. doi: 10.1016/s0303-7207(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 39.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab. 1986;2:163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- 40.Henderson JR. Why are the islets of langerhans? Lancet. 1969;2:469–470. doi: 10.1016/s0140-6736(69)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of langerhans in the rat. Diabetes. 1982;31:883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- 42.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development. 2012;139:2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahren B. Islet nerves in focus—defining their neurobiological and clinical role. Diabetologia. 2012;55:3152–3154. doi: 10.1007/s00125-012-2727-6. [DOI] [PubMed] [Google Scholar]
- 45.Weir GC, Bonner-Weir S. Islets of langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J. Clin. Invest. 1990;85:983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brissova M, Powers AC. Architecture of pancreatic islets. In: Seino S, Bell GI, editors. Pancreatic Beta Cell in Health and Disease. Japan: Springer; 2008. pp. 3–11. [Google Scholar]
- 48.Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia. 1983;24:231–237. doi: 10.1007/BF00282705. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz O, Arnfred J, Nielsen OH, Beck-Nielsen H, Orskov H. Glucose uptake and pulsatile insulin infusion: euglycaemic clamp and [3-3H]glucose studies in healthy subjects. Acta Endocrinol. 1986;113:559–563. doi: 10.1530/acta.0.1130559. [DOI] [PubMed] [Google Scholar]
- 50.Paolisso G, Sgambato S, Torella R, Varricchio M, Scheen A, D’Onofrio F, Lefebvre PJ. Pulsatile insulin delivery is more efficient than continuous infusion in modulating islet cell function in normal subjects and patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 1988;66:1220–1226. doi: 10.1210/jcem-66-6-1220. [DOI] [PubMed] [Google Scholar]
- 51.Paolisso G, Sgambato S, Gentile S, Memoli P, Giugliano D, Varricchio M, D’Onofrio F. Advantageous metabolic effects of pulsatile insulin delivery in noninsulin-dependent diabetic patients. J. Clin. Endocrinol. Metab. 1988;67:1005–1010. doi: 10.1210/jcem-67-5-1005. [DOI] [PubMed] [Google Scholar]
- 52.Verdin E, Castillo M, Luyckx AS, Lefebvre PJ. Similar metabolic effects of pulsatile versus continuous human insulin delivery during euglycemic hyperinsulinemic glucose clamp in normal man. Diabetes. 1984;33:1169–1174. doi: 10.2337/diab.33.12.1169. [DOI] [PubMed] [Google Scholar]
- 53.Kerner W, Bruckel J, Zier H, Arias P, Thun C, Moncayo R, Pfeiffer EF. Similar effects of pulsatile and constant intravenous insulin delivery. Diabetes Res. Clin. Pract. 1988;4:269–274. doi: 10.1016/s0168-8227(88)80028-7. [DOI] [PubMed] [Google Scholar]
- 54.Bratusch-Marrain PR, Komjati M, Waldhausl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35:922–926. doi: 10.2337/diab.35.8.922. [DOI] [PubMed] [Google Scholar]
- 55.Komjati M, Bratusch-Marrain P, Waldhausl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology. 1986;118:312–319. doi: 10.1210/endo-118-1-312. [DOI] [PubMed] [Google Scholar]
- 56.Koopmans SJ, Sips HC, Krans HM, Radder JK. Pulsatile intravenous insulin replacement in streptozotocin diabetic rats is more efficient than continuous delivery: effects on glycaemic control insulin-mediated glucose metabolism and lipolysis. Diabetologia. 1996;39:391–400. doi: 10.1007/BF00400670. [DOI] [PubMed] [Google Scholar]
- 57.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 58.Matveyenko AV, Veldhuis JD, Butler PC. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and beta-cell mass to age-related insulin resistance in rats. Am. J. Physiol. Endocrinol. Metab. 2008;295:E832–E841. doi: 10.1152/ajpendo.90451.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matveyenko AV, Liuwantara D, Gurlo T, Kirakossian D, Dalla MC, Cobelli C, White MF, Copps KD, Volpi E, Fujita S, Butler PC. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 2012;61:2269–2279. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 61.Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 62.Ristow M, Carlqvist H, Hebinck J, Vorgerd M, Krone W, Pfeiffer A, Muller-Wieland D, Ostenson CG. Deficiency of phosphofructo-1-kinase/muscle subtype in humans is associated with impairment of insulin secretory oscillations. Diabetes. 1999;48:1557–1561. doi: 10.2337/diabetes.48.8.1557. [DOI] [PubMed] [Google Scholar]
- 63.Hollingdal M, Juhl CB, Pincus SM, Sturis J, Veldhuis JD, Polonsky KS, Porksen N, Schmitz O. Failure of physiological plasma glucose excursions to entrain high-frequency pulsatile insulin secretion in type 2 diabetes. Diabetes. 2000;49:1334–1340. doi: 10.2337/diabetes.49.8.1334. [DOI] [PubMed] [Google Scholar]
- 64.Song SH, Rhodes CJ, Veldhuis JD, Butler PC. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology. 2003;144:3399–3405. doi: 10.1210/en.2003-0056. [DOI] [PubMed] [Google Scholar]
- 65.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N. Engl. J. Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 66.Nyholm B, Porksen N, Juhl CB, Gravholt CH, Butler PC, Weeke J, Veldhuis JD, Pincus S, Schmitz O. Assessment of insulin secretion in relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus: evidence of early beta-cell dysfunction. Metabolism. 2000;49:896–905. doi: 10.1053/meta.2000.6737. [DOI] [PubMed] [Google Scholar]
- 67.Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byrne MM, Sturis J, Clement K, Vionnet N, Pueyo ME, Stoffel M, Takeda J, Passa P, Cohen D, Bell GI. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J. Clin. Invest. 1994;93:1120–1130. doi: 10.1172/JCI117064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Velho G, Byrne MM, Clement K, Sturis J, Pueyo ME, Blanche H, Vionnet N, Fiet J, Passa P, Robert JJ, Polonsky KS, Froguel P. Clinical phenotypes, insulin secretion and insulin sensitivity in kindreds with maternally inherited diabetes and deafness due to mitochondrial tRNALeu (UUR) gene mutation. Diabetes. 1996;45:478–487. doi: 10.2337/diab.45.4.478. [DOI] [PubMed] [Google Scholar]
- 70.Andersen UB, Dige-Petersen H, Frandsen EK, Ibsen H, Volund A. Basal insulin-level oscillations in normotensive individuals with genetic predisposition to essential hypertension exhibit an irregular pattern. J. Hypertens. 1997;15:1167–1173. doi: 10.1097/00004872-199715100-00015. [DOI] [PubMed] [Google Scholar]
- 71.Van Cauter EV, Polonsky KS, Blackman JD, Roland D, Sturis J, Byrne MM, Scheen AJ. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian Cortisol rhythmicity. J. Clin. Endocrinol. Metab. 1994;79:1797–1805. doi: 10.1210/jcem.79.6.7989487. [DOI] [PubMed] [Google Scholar]
- 72.Zarkovic M, Ciric J, Penezic Z, Trbojevic B, Drezgic M. Effect of weight loss on the pulsatile insulin secretion. J. Clin. Endocrinol. Metab. 2000;85:3673–3677. doi: 10.1210/jcem.85.10.6919. [DOI] [PubMed] [Google Scholar]
- 73.Porksen N. Early changes in beta-cell function and insulin pulsatility as predictors for type 2 diabetes. Diabetes Nutr. Metab. 2002;15:9–14. [PubMed] [Google Scholar]
- 74.Bergsten P. Pathophysiology of impaired pulsatile insulin release. Diabetes. Metab. Res. 2000;16:179–191. doi: 10.1002/1520-7560(200005/06)16:3<179::aid-dmrr115>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 75.Porksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes. 2002;51(Suppl 1):S245–S254. doi: 10.2337/diabetes.51.2007.s245. [DOI] [PubMed] [Google Scholar]
- 76.Stagner JI, Samols E, Weir GC. Sustained oscillations of insulin, glucagon, and somatostatin from the isolated canine pancreas during exposure to a constant glucose concentration. J. Clin. Invest. 1980;65:939–942. doi: 10.1172/JCI109750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gylfe E, Ahmed M, Bergsten P, Dansk H, Dyachok O, Eberhardson M, Grapengiesser E, Hellman B, Lin JM, Sundsten T, Tengholm A, Vieira E, Westerlund J. Signaling underlying pulsatile insulin secretion. Ups. J. Med. Sci. 2000;105:35–51. doi: 10.1517/03009734000000054. [DOI] [PubMed] [Google Scholar]
- 78.Sha L, Westerlund J, Szurszewski JH, Bergsten P. Amplitude modulation of pulsatile insulin secretion by intrapancreatic ganglion neurons. Diabetes. 2001;50:51–55. doi: 10.2337/diabetes.50.1.51. [DOI] [PubMed] [Google Scholar]
- 79.Yao NK, Chang LW, Lin BJ, Kuo TS. Dynamic aspects for interislet synchronization of oscillatory insulin secretions. Am. J. Physiol. 1997;272:E981–E988. doi: 10.1152/ajpendo.1997.272.6.E981. [DOI] [PubMed] [Google Scholar]
- 80.Sturis J, VanCauter E, Blackman JD, Polonsky KS. Entrainment of pulsatile insulin secretion by oscillatory glucose infusion. J. Clin. Invest. 1991;87:439–445. doi: 10.1172/JCI115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pedersen MG, Bertram R, Sherman A. Intra- and inter-islet synchronization of metabolically driven insulin secretion. Bio-phys. J. 2005;89:107–119. doi: 10.1529/biophysj.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nunemaker CS, Zhang M, Wasserman DH, McGuin-ness OP, Powers AC, Bertram R, Sherman A, Satin LS. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54:3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 83.Nunemaker CS, Dishinger JF, Dula SB, Wu R, Merrins MJ, Reid KR, Sherman A, Kennedy RT, Satin LS. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+](i) and insulin rhythms in mouse islets. PLoS One. 2009;4:e8428. doi: 10.1371/journal.pone.0008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Witkin JW, Silverman AJ. Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides. 1985;6:263–271. doi: 10.1016/0196-9781(85)90050-6. [DOI] [PubMed] [Google Scholar]
- 85.Witkin JW. Synchronized neuronal networks: the GnRH system. Microsc. Res. Tech. 1999;44:11–18. doi: 10.1002/(SICI)1097-0029(19990101)44:1<11::AID-JEMT3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 86.Pompolo S, Rawson JA, Clarke IJ. Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain Res. 2001;904:1–12. doi: 10.1016/s0006-8993(01)02372-1. [DOI] [PubMed] [Google Scholar]
- 87.Woller M, Nichols E, Herdendorf T, Tutton D. Release of luteinizing hormone-releasing hormone from enzymatically dispersed rat hypothalamic explants is pulsatile. Biol. Reprod. 1998;59:587–590. doi: 10.1095/biolreprod59.3.587. [DOI] [PubMed] [Google Scholar]
- 88.Rasmussen DD. Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuro-endocrinology. 1993;58:511–518. doi: 10.1159/000126584. [DOI] [PubMed] [Google Scholar]
- 89.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 2013;33:12689–12697. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez FJ, Moretto M, Merchenthaler I, Negro-Vilar A. Nitric oxide is involved in the genesis of pulsatile LHRH secretion from immortalized LHRH neurons. J. Neuroendocrinol. 1997;9:647–654. doi: 10.1046/j.1365-2826.1997.t01-1-00618.x. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Fuentes AJ, Hu L, Krsmanovic LZ, Catt KJ. Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: role in pulsatile neurosecretion. Mol. Endocrinol. 2004;18:1808–1817. doi: 10.1210/me.2003-0321. [DOI] [PubMed] [Google Scholar]
- 93.Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145:728–735. doi: 10.1210/en.2003-0562. [DOI] [PubMed] [Google Scholar]
- 94.Hrabovszky E, Liposits Z. Afferent neuronal control of type-I gonadotropin releasing hormone neurons in the human. Front. Endocrinol. 2013;4:130. doi: 10.3389/fendo.2013.00130. Lausanne. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karsch FJ, Malpaux B, Wayne NL, Robinson JE. Characteristics of the melatonin signal that provide the photoperiodic code for timing seasonal reproduction in the ewe. Reprod. Nutr. Dev. 1988;28:459–472. doi: 10.1051/rnd:19880311. [DOI] [PubMed] [Google Scholar]
- 96.Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am. J. Physiol. 1996;270:E1–E19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- 97.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 99.Savari O, Zielinski MC, Wang X, Misawa R, Millis JM, Witkowski P, Hara M. Distinct function of the head region of human pancreas in the pathogenesis of diabetes. Islets. 2013;5:226–228. doi: 10.4161/isl.26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng XB, Wen JP, Yang J, Yang Y, Ning G, Li XY. GnRH secretion is inhibited by adiponectin through activation of AMP-activated protein kinase and extracellular signal-regulated kinase. Endocrine. 2011;39:6–12. doi: 10.1007/s12020-010-9375-8. [DOI] [PubMed] [Google Scholar]
- 101.Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut adipose hormones, and reproduction. Hum. Reprod. Update. 2013;20:153–174. doi: 10.1093/humupd/dmt033. [DOI] [PubMed] [Google Scholar]
- 102.Martin LJ. Implications of adiponectin in linking metabolism to testicular function. Endocrine. 2013 doi: 10.1007/s12020-013-0102-0. [DOI] [PubMed] [Google Scholar]
- 103.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism. 2011;60:1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Witchel SF, Recabarren SE, Gonzalez F, Diamanti-Kandarakis E, Cheang KI, Duleba AJ, Legro RS, Homburg R, Pasquali R, Lobo RA, Zouboulis CC, Kelestimur F, Fruzzetti F, Futterweit W, Norman RJ, Abbott DH. Emerging concepts about prenatal genesis aberrant metabolism and treatment paradigms in polycystic ovary syndrome. Endocrine. 2012;42:526–534. doi: 10.1007/s12020-012-9701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calabrese A, Zhang M, Serre-Beinier V, Caton D, Mas C, Satin LS, Meda P. Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes. 2003;52:417–424. doi: 10.2337/diabetes.52.2.417. [DOI] [PubMed] [Google Scholar]
- 106.Bavamian S, Klee P, Britan A, Populaire C, Caille D, Cancela J, Charollais A, Meda P. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 2007;9(Suppl 2):118–132. doi: 10.1111/j.1463-1326.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 107.Cigliola V, Chellakudam V, Arabieter W, Meda P. Connexins and beta-cell functions. Diabetes Res. Clin. Pract. 2013;99:250–259. doi: 10.1016/j.diabres.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 108.Eddlestone GT, Goncalves A, Bangham JA, Rojas E. Electrical coupling between cells in islets of langerhans from mouse. J. Membr. Biol. 1984;77:1–14. doi: 10.1007/BF01871095. [DOI] [PubMed] [Google Scholar]
- 109.Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61:1700–1707. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. doi: 10.1210/endo-87-5-850. [DOI] [PubMed] [Google Scholar]
- 111.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 112.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res. Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 113.Wetsel WC, Valenca MM, Merchenthaler I, Liposits Z, Lopez FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc. Natl. Acad. Sci. USA. 1992;89:4149–4153. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de la Martinez EG, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc. Natl. Acad. Sci. USA. 1992;89:1852–1855. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140:1432–1441. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- 116.Krsmanovic LZ, Stojilkovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc. Natl. Acad. Sci. USA. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Navarro CE, Chen HC, Stojilkovic SS, Catt KJ. Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology. 1999;140:1423–1431. doi: 10.1210/endo.140.3.6588. [DOI] [PubMed] [Google Scholar]
- 118.Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J. Neurosci. 2002;22:2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144:823–831. doi: 10.1210/en.2002-220585. [DOI] [PubMed] [Google Scholar]
- 120.Krsmanovic LZ, Stojilkovic SS, Mertz LM, Tomic M, Catt KJ. Expression of gonadotropin-releasing hormone receptors and autocrine regulation of neuropeptide release in immortalized hypothalamic neurons. Proc. Natl. Acad. Sci. USA. 1993;90:3908–3912. doi: 10.1073/pnas.90.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. doi: 10.1159/000127011. [DOI] [PubMed] [Google Scholar]
- 122.DePaolo LV, King RA, Carrillo AJ. In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology. 1987;120:272–279. doi: 10.1210/endo-120-1-272. [DOI] [PubMed] [Google Scholar]
- 123.Krsmanovic LZ, Hu L, Leung PK, Feng H, Catt KJ. The hypothalamic GnRH pulse generator: multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009;20:402–408. doi: 10.1016/j.tem.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vazquez-Martinez R, Shorte SL, Faught WJ, Leaumont DC, Frawley LS, Boockfor FR. Pulsatile exocytosis is functionally associated with GnRH gene expression in immortalized GnRH-expressing cells. Endocrinology. 2001;142:5364–5370. doi: 10.1210/endo.142.12.8551. [DOI] [PubMed] [Google Scholar]
- 125.Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J. Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tonsfeldt KJ, Chappell PE. Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol. Cell. Endocrinol. 2012;349:3–12. doi: 10.1016/j.mce.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pitts GR, Nunemaker CS, Moenter SM. Cycles of transcription and translation do not comprise the gonadotropin-releasing hormone pulse generator in GT1 cells. Endocrinology. 2001;142:1858–1864. doi: 10.1210/endo.142.5.8137. [DOI] [PubMed] [Google Scholar]
- 128.Vitalis EA, Costantin JL, Tsai PS, Sakakibara H, Par-uthiyil S, Iiri T, Martini JF, Taga M, Choi AL, Charles AC, Weiner RI. Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc. Natl. Acad. Sci. USA. 2000;97:1861–1866. doi: 10.1073/pnas.040545197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wetsel WC, Eraly SA, Whyte DB, Mellon PL. Regulation of gonadotropin-releasing hormone by protein kinase-A and -C in immortalized hypothalamic neurons. Endocrinology. 1993;132:2360–2370. doi: 10.1210/endo.132.6.8504741. [DOI] [PubMed] [Google Scholar]
- 130.El-Majdoubi M, Weiner RI. Localization of olfactory cyclic nucleotide-gated channels in rat gonadotropin-releasing hormone neurons. Endocrinology. 2002;143:2441–2444. doi: 10.1210/endo.143.6.8951. [DOI] [PubMed] [Google Scholar]
- 131.de la Martinez EG, Choi AL, Weiner RI. Signaling pathways involved in GnRH secretion in GT1 cells. Neuroendocrinology. 1995;61:310–317. doi: 10.1159/000126853. [DOI] [PubMed] [Google Scholar]
- 132.Charles A, Weiner R, Costantin J. cAMP modulates the excitability of immortalized H = hypothalamic (GT1) neurons via a cyclic nucleotide gated channel. Mol. Endocrinol. 2001;15:997–1009. doi: 10.1210/mend.15.6.0653. [DOI] [PubMed] [Google Scholar]
- 133.Paruthiyil S, eL Majdoubi M, Conti M, Weiner RI. Phosphodiesterase expression targeted to gonadotropin-releasing hormone neurons inhibits luteinizing hormone pulses in transgenic rats. Proc. Natl. Acad. Sci .USA. 2002;99:17191–17196. doi: 10.1073/pnas.012678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology. 2008;149:3500–3511. doi: 10.1210/en.2007-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology. 2008;149:279–290. doi: 10.1210/en.2007-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv. Exp. Med. Biol. 2013;784:297–323. doi: 10.1007/978-1-4614-6199-9_14. [DOI] [PubMed] [Google Scholar]
- 139.Steiner RA. Kisspeptin: past present, and prologue. Adv. Exp. Med. Biol. 2013;784:3–7. doi: 10.1007/978-1-4614-6199-9_1. [DOI] [PubMed] [Google Scholar]
- 140.Goodner CJ, Walike BC, Koerker DJ, Ensinck JW, Brown AC, Chideckel EW, Palmer J, Kalnasy L. Insulin glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977;195:177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 141.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N. Engl. J. Med. 1979;301:1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 142.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 2000;85:4491–4499. doi: 10.1210/jcem.85.12.7043. [DOI] [PubMed] [Google Scholar]
- 143.Henquin JC, Meissner HP, Schmeer W. Cyclic variations of glucose-induced electrical activity in pancreatic B cells. Pflugers Arch. 1982;393:322–327. doi: 10.1007/BF00581418. [DOI] [PubMed] [Google Scholar]
- 144.Cook DL. Isolated islets of langerhans have slow oscillations of electrical activity. Metab. Clin. Exp. 1983;32:681–685. doi: 10.1016/0026-0495(83)90124-5. [DOI] [PubMed] [Google Scholar]
- 145.Bertram R, Sherman A, Satin LS. Metabolic and Electrical Oscillations: Partners in Controlling Pulsatile Insulin Secretion. Am. J. Physiol. Endocrinol, Metab. 2007 doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 146.Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 1994;269:8749–8753. [PubMed] [Google Scholar]
- 147.Gilon P, Henquin JC. Distinct effects of glucose on the synchronous oscillations of insulin release and cytoplasmic Ca2+ concentration measured simultaneously in single mouse islets. Endocrinology. 1995;136:5725–5730. doi: 10.1210/endo.136.12.7588329. [DOI] [PubMed] [Google Scholar]
- 148.Barbosa RM, Silva AM, Tome AR, Stamford JA, Santos RM, Rosario LM. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. J. Physiol. 1998;510(Pt 1):135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bergsten P, Westerlund J, Liss P, Carlsson PO. Primary in vivo oscillations of metabolism in the pancreas. Diabetes. 2002;51:699–703. doi: 10.2337/diabetes.51.3.699. [DOI] [PubMed] [Google Scholar]
- 150.Kindmark H, Kohler M, Arkhammar P, Efendic S, Lars-son O, Linder S, Nilsson T, Berggren PO. Oscillations in cytoplasmic free calcium concentration in human pancreatic islets from subjects with normal and impaired glucose tolerance. Di-abetologia. 1994;37:1121–1131. doi: 10.1007/BF00418376. [DOI] [PubMed] [Google Scholar]
- 151.Martin F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium. 1996;20:409–414. doi: 10.1016/s0143-4160(96)90003-2. [DOI] [PubMed] [Google Scholar]
- 152.Song SH, Kjems L, Ritzel R, Mclntyre SM, Johnson ML, Veldhuis JD, Butler PC. Pulsatile insulin secretion by human pancreatic islets. J. Clin. Endocrinol. Metab. 2002;87:213–221. doi: 10.1210/jcem.87.1.8181. [DOI] [PubMed] [Google Scholar]
- 153.Tornheim K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes. 1997;46:1375–1380. doi: 10.2337/diab.46.9.1375. [DOI] [PubMed] [Google Scholar]
- 154.Bertram R, Satin L, Zhang M, Smolen P, Sherman A. Calcium and glycolysis mediate multiple bursting modes in pancreatic islets. Biophys. J. 2004;87:3074–3087. doi: 10.1529/biophysj.104.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ren J, Sherman A, Bertram R, Goforth PB, Nunemaker CS, Waters CD, Satin LS. Slow oscillations of KATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations. Am. J. Physiol. Endocrinol. Metab. 2013;305:E805–E817. doi: 10.1152/ajpendo.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chance B, Estabrook RW, Ghosh A. Damped sinusoidal oscillations of cytoplasmic reduced pyridine nucleotide in yeast cells. Proc. Natl. Acad. Sci. USA. 1964;51:1244–1251. doi: 10.1073/pnas.51.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Laxman S, Tu BP. Systems approaches for the study of metabolic cycles in yeast. Curr. Opin. Genet. Dev. 2010;20:599–604. doi: 10.1016/j.gde.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Corkey BE, Tornheim K, Deeney JT, Glennon MC, Parker JC, Matschinsky FM, Ruderman NB, Prentki M. Linked oscillations of free Ca2+ and the ATP/ADP ratio in permeabilized RINm5F insulinoma cells supplemented with a glycolyzing cell-free muscle extract. J. Biol. Chem. 1988;263:4254–4258. [PubMed] [Google Scholar]
- 159.Longo EA, Tornheim K, Deeney JT, Varnum BA, Tillotson D, Prentki M, Corkey BE. Oscillations in cytosolic free Ca2+, oxygen consumption and insulin secretion in glucose-stimulated rat pancreatic islets. J. Biol. Chem. 1991;266:9314–9319. [PubMed] [Google Scholar]
- 160.Merrins MJ, Van Dyke AR, Mapp AK, Rizzo MA, Satin LS. Direct measurements of oscillatory glycolysis in pancreatic islet beta-cells using novel fluorescence resonance energy transfer (FRET) biosensors for pyruvate kinase M2 activity. J. Biol. Chem. 2013;288:33312–33322. doi: 10.1074/jbc.M113.508127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dean PM, Matthews EK. Electrical activity in pancreatic islet cells. Nature. 1968;219:389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- 162.Dean PM, Matthews EK. Electrical activity in pancreatic islet cells: effect of ions. J. Physiol. (Lond.) 1970;210:265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meissner HP, Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351:195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- 164.Atwater I, Dawson CM, Ribalet B, Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J. Physiol. (Lond.) 1979;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- 165.Ribalet B, Beigelman PM. Calcium action potentials and potassium permeability activation in pancreatic beta-cells. Am. J. Physiol. 1980;239:C124–C133. doi: 10.1152/ajpcell.1980.239.3.C124. [DOI] [PubMed] [Google Scholar]
- 166.Ribalet B, Beigelman PM. Effects of divalent cations on beta-cell electrical activity. Am. J. Physiol. 1981;241:C59–C67. doi: 10.1152/ajpcell.1981.241.1.C59. [DOI] [PubMed] [Google Scholar]
- 167.Dean PM, Matthews EK. Glucose-induced electrical activity in pancreatic islet cells. J. Physiol. (Lond.) 1970;210:255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beigelman PM, Ribalet B. Beta-cell electrical activity in response to high glucose concentration. Diabetes. 1980;29:263–265. doi: 10.2337/diab.29.4.263. [DOI] [PubMed] [Google Scholar]
- 169.Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- 170.Zhang M, Goforth P, Bertram R, Sherman A, Satin L. The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophys. J. 2003;84:2852–2870. doi: 10.1016/S0006-3495(03)70014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scott AM, Atwater I, Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- 172.Ammala C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J. Physiol. (Lond.) 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of langerhans. Endocrine. 2000;13:251–262. doi: 10.1385/ENDO:13:3:251. [DOI] [PubMed] [Google Scholar]
- 174.Charles AC, Hales TG. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. J. Neurophysiol. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 175.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 176.Nunemaker CS, DeFazio RA, Moenter SM. Calcium current subtypes in GnRH neurons. Biol. Reprod. 2003;69:1914–1922. doi: 10.1095/biolreprod.103.019265. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y, Garro M, Dantzler HA, Taylor JA, Kline DD, Kuehl-Kovarik MC. Age affects spontaneous activity and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4938–4947. doi: 10.1210/en.2008-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Control of action potential-driven calcium influx in GT1 neurons by the activation status of sodium and calcium channels. Mol. Endocrinol. 1999;13:587–603. doi: 10.1210/mend.13.4.0261. [DOI] [PubMed] [Google Scholar]
- 179.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons. Biochem. Cell Biol. 2000;78:359–370. [PubMed] [Google Scholar]
- 180.Costantin JL, Charles AC. Modulation of Ca(2+) signaling by K(+) channels in a hypothalamic neuronal cell line (GT1-1) J. Neurophysiol. 2001;85:295–304. doi: 10.1152/jn.2001.85.1.295. [DOI] [PubMed] [Google Scholar]
- 181.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol. Endocrinol. 2002;16:2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 183.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 184.Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res. 2010;1364:10–24. doi: 10.1016/j.brainres.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 186.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 187.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 188.Dryselius S, Lund PE, Gylfe E, Hellman B. Variations in ATP-sensitive K+ channel activity provide evidence for inherent metabolic oscillations in pancreatic beta-cells. Biochem. Bio-phys. Res. Commun. 1994;205:880–885. doi: 10.1006/bbrc.1994.2746. [DOI] [PubMed] [Google Scholar]
- 189.Larsson O, Kindmark H, Brandstrom R, Fredholm B, Berggren PO. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic beta cell. Proc. Natl. Acad. Sci. USA. 1996;93:5161–5165. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dufer M, Haspel D, Krippeit-Drews P, Aguilar-Bryan L, Bryan J, Drews G. Oscillations of membrane potential and cytosolic Ca(2+) concentration in SUR1(−/−) beta cells. Diabetologia. 2004;47:488–498. doi: 10.1007/s00125-004-1348-0. [DOI] [PubMed] [Google Scholar]
- 191.Kinard TA, Satin LS. An ATP-sensitive cl- channel current that is activated by cell swelling cAMP, and glyburide in insulin-secreting cells. Diabetes. 1995;44:1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- 192.Smith PA, Rorsman P, Ashcroft FM. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989;342:550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- 193.Gopel SO, Kanno T, Barg S, Eliasson L, Galvanovskis J, Renstrom E, Rorsman P. Activation of Ca(2+)-dependent K(+) channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J. Gen. Physiol. 1999;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goforth PB, Bertram R, Khan FA, Zhang M, Sherman A, Satin LS. Calcium-activated K+ channels of mouse beta-cells are controlled by both store and cytoplasmic Ca2+: experimental and theoretical studies. J. Gen. Physiol. 2002;120:307–322. doi: 10.1085/jgp.20028581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ravier MA, Nenquin M, Miki T, Seino S, Henquin JC. Glucose controls cytosolic Ca2+ and insulin secretion in mouse islets lacking adenosine triphosphate-sensitive K+ channels owing to a knockout of the pore-forming subunit Kir6.2. Endocrinology. 2009;150:33–45. doi: 10.1210/en.2008-0617. [DOI] [PubMed] [Google Scholar]
- 196.Huopio H, Shyng SL, Otonkoski T, Nichols CG. K(ATP) channels and insulin secretion disorders. Am. J. Physiol. Endocrinol. Metab. 2002;283:E207–E216. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- 197.Chu Z, Tomaiuolo M, Bertram R, Moenter SM. Two types of burst firing in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2012;24:1065–1077. doi: 10.1111/j.1365-2826.2012.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marshall JC, Griffin ML. The role of changing pulse frequency in the regulation of ovulation. Hum. Reprod. 1993;8(Suppl 2):57–61. doi: 10.1093/humrep/8.suppl_2.57. [DOI] [PubMed] [Google Scholar]
- 199.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes. 1983;32:617–621. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- 200.Porksen NK, Munn SR, Steers JL, Schmitz O, Veldhuis JD, Butler PC. Mechanisms of sulfonylurea’s stimulation of insulin secretion in vivo: selective amplification of insulin secretory burst mass. Diabetes. 1996;45:1792–1797. doi: 10.2337/diab.45.12.1792. [DOI] [PubMed] [Google Scholar]
- 201.Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 202.Manning FJE, Gyulkhandanyan AV, Satin LS, Wheeler MB. Oscillatory membrane potential response to glucose in islet beta-cells: a comparison of islet-cell electrical activity in mouse and rat. Endocrinology. 2006;147:4655–4663. doi: 10.1210/en.2006-0424. [DOI] [PubMed] [Google Scholar]
- 203.Grapengiesser E, Gylfe E, Hellman B. Glucose-induced oscillations of cytoplasmic Ca2+ in the pancreatic beta-cell. Biochem. Biophys. Res. Commun. 1988;151:1299–1304. doi: 10.1016/s0006-291x(88)80503-5. [DOI] [PubMed] [Google Scholar]
- 204.Valdeolmillos M, Santos RM, Contreras D, Soria B, Rosario LM. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of langerhans. FEBS Lett. 1989;259:19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- 205.Krippeit-Drews P, Dufer M, Drews G. Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 2000;267:179–183. doi: 10.1006/bbrc.1999.1921. [DOI] [PubMed] [Google Scholar]
- 206.Kindmark H, Kohler M, Brown G, Branstrom R, Larsson O, Berggren PO. Glucose-induced oscillations in cytoplasmic free Ca2+ concentration precede oscillations in mitochondrial membrane potential in the pancreatic beta-cell. J. Biol. Chem. 2001;276:34530–34536. doi: 10.1074/jbc.M102492200. [DOI] [PubMed] [Google Scholar]
- 207.Nunemaker CS, Satin LS. Comparison of metabolic oscillations from mouse pancreatic beta cells and islets. Endocrine. 2004;25:61–67. doi: 10.1385/ENDO:25:1:61. [DOI] [PubMed] [Google Scholar]
- 208.Katzman SM, Messerli MA, Barry DT, Grossman A, Harel T, Wikstrom JD, Corkey BE, Smith PJ, Shirihai OS. Mitochondrial metabolism reveals a functional architecture in intact islets of langerhans from normal and diabetic psammomys obesus. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1090–E1099. doi: 10.1152/ajpendo.00044.2004. [DOI] [PubMed] [Google Scholar]
- 209.Jung SK, Aspinwall CA, Kennedy RT. Detection of multiple patterns of oscillatory oxygen consumption in single mouse islets of langerhans. Biochem. Biophys. Res. Commun. 1999;259:331–335. doi: 10.1006/bbrc.1999.0784. [DOI] [PubMed] [Google Scholar]
- 210.Ortsater H, Liss P, Lund PE, Akerman KE, Bergsten P. Oscillations in oxygen tension and insulin release of individual pancreatic ob/ob mouse islets. Diabetologia. 2000;43:1313–1318. doi: 10.1007/s001250051528. [DOI] [PubMed] [Google Scholar]
- 211.Porterfield DM, Corkey RF, Sanger RH, Tornheim K, Smith PJ, Corkey BE. Oxygen consumption oscillates in single clonal pancreatic beta-cells (HIT) Diabetes. 2000;49:1511–1516. doi: 10.2337/diabetes.49.9.1511. [DOI] [PubMed] [Google Scholar]
- 212.Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J. Physiol. (Lond.) 2006;572:379–392. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys. J. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jung SK, Kauri LM, Qian WJ, Kennedy RT. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca(2+) in single islets of langerhans. J. Biol. Chem. 2000;275:6642–6650. doi: 10.1074/jbc.275.9.6642. [DOI] [PubMed] [Google Scholar]
- 215.Deeney JT, Kohler M, Kubik K, Brown G, Schultz V, Tornheim K, Corkey BE, Berggren PO. Glucose-induced metabolic oscillations parallel those of Ca(2+) and insulin release in clonal insulin-secreting cells. A multiwell approach to oscillatory cell behavior. J. Biol. Chem. 2001;276:36946–36950. doi: 10.1074/jbc.M105056200. [DOI] [PubMed] [Google Scholar]
- 216.Wildt L, Hiiusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 217.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol. Proced. Online. 2003;5:53–62. doi: 10.1251/bpo46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143(6):2284–2292. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]