Abstract
Aim
Silk-tropoelastin alloys, composed of recombinant human tropoelastin and regenerated Bombyx mori silk fibroin, are an emerging, versatile class of biomaterials endowed with tunable combinations of physical and biological properties. Electrodeposition of these alloys provides a programmable means to assemble functional gels with both spatial and temporal controllability.
Materials & methods
Tropoelastin-modified silk was prepared by enzymatic coupling between tyrosine residues. Hydrogel coatings were electrodeposited using two wire electrodes.
Results & discussion
Mechanical characterization and in vitro cell culture revealed enhanced adhesive capability and cellular response of these alloy gels as compared with electrogelled silk alone.
Conclusion
These electro-depositable silk-tropoelastin alloys constitute a suitable coating material for nanoparticle-based drug carriers and offer a novel opportunity for on-demand encapsulation/release of nanomedicine.
Keywords: dityrosine bond, electrodeposition, enzymatic coupling, protein alloy, silk, sol–gel, tissue adhesive, tropoelastin
Advanced protein alloys of regenerated silk fibroin, exhibiting mechanical strength and structural durability, and recombinant human tropoelastin, possessing resilience and stimuli responsive capabilities, have been shown to interact strongly via complementary characteristics [1–3]. Compositions of silk-tropoelastin alloys and methods of sol-gel processing have been researched to achieve a range of mechanical properties and biological functions [1–5]. For example, optimized silk/tropoelastin ratios for promoting neurite outgrowth of primary cortical neuronal cells (E18) has been determined using a set of autoclave- gelled, compositionally graded protein alloy films. The physical interactions between silk and tropoelastin across hierarchical levels as well as the composition-dependent surface features, including surface charge, roughness and stiffness, have been investigated and linked to neural cell substrate responses [2].
The distinctive electrostatic potentials of silk and tropoelastin proteins (with isoelectric points of 4.2 ≪ 7.0 and 11.2 ≫ 7.0, respectively) [6,7], together with their unique electrochemical nature and possible conformational interactions under electric fields, make electrodeposition an intriguing method to generate silk-tropoelastin hydrogels on demand. Over the past few years an electrochemically reversible, sol–gel transition has been discovered in regenerated Bombyx mori silk [8–12]. When passing a low current of electricity (typically <1 mA) through the solution, silk molecules with abundant carboxylate groups (~3% of COO−) respond to the decrease in local pH by undergoing protonation (to form COOH). Electrogel forms at the H+ ion-releasing anode surfaces. In an earlier example, electrodeposition paints with protonated ammonium groups were shown to recognize the local pH increase at the cathode. These polymer electrolytes switched from soluble to an insoluble state, and subsequently formed a precipitation layer on the surface [13]. Direct blending of silk with tropoelastin (equal volume at 2 wt%), however, failed to show an electrochemical response (up to an applied electric field strength of ~2 × 103 V/m), as the total charges on the two types of proteins cancelled each other out to a significant extent.
In this work, we have developed a chemical modification method for preparation of silk-tropoelastin conjugates with different composition ratios, and report the first demonstration of electrodeposition of thin-layered hydrogels made of these protein alloys. Fluorescence measurements, 1H-NMR (proton nuclear magnetic resonance) spectroscopy, infrared spectroscopy and gel electrophoresis (SDS-PAGE) were used to confirm the enzymatic coupling between tyrosine bonds. Mechanical and biological properties of the alloy gels were characterized using atomic force microscopy (AFM) based nanoindentation, Instron and in vitro cultures of endothelial cells and fibroblasts. Altogether, the data suggest that the electrodeposited protein alloys, responsive to environmental cues and capable of promoting desired cellular activities, stand out as a multifunctional hydrogel coating material suitable for integration with nanoparticle-based drug delivery systems.
Materials & methods
Materials
Cocoons of B. mori silkworm silk were supplied by Tajima Shoji Co., Ltd (Sumiyoshicho, Naka-ku, Yokohama, Japan). Sodium carbonate (Na2CO3, 99%), lithium bromide (LiBr, 99%), hydrogen peroxide (H2O2, 30%), horseradish peroxidase (HRP, Type VI, salt-free) and fluorescamine (98%) were purchased from Cambridge Bioscience (Bar Hill, Cambridge, UK). Sulfosuccinimidyl acetate (Sulfo-NHS-acetate, 99%) and borate buffer (1M, pH 8.5) were obtained from Pierce Biotechnology (IL, USA). All chemicals were used without further purification. Briefly, silk cocoons were cut into small pieces and boiled in 0.02 M Na2CO3 solution for 30 min to remove the sericin. The degummed silk fibers were then dissolved in a 9.3 M aqueous solution of LiBr followed by dialysis against distilled water [14]. After centrifugation and filtration, the obtained regenerated silk fibroin (denoted as S) stock solution (6 wt%) was stored at 4°C for further use. Recombinant human tropoelastin isoform SHELΔ26A (Synthetic Human Elastin without domain 26A) corresponding to amino acid residues 27–724 of GenBank entry AAC98394 (gi 182020) was expressed and purified from bacteria as previously described [15].
Acetylation of tropoelastin
Tropoelastin (denoted as E) was solubilized in 10 mM phosphate-buffered saline (PBS; pH 7.4) to 1 mg/ml and a 25-fold molar excess of sulfosuccinimidyl acetate was added to the solution following the method reported by Wu et al. [7]. The reaction was incubated at room temperature for 3 h before the excess sulfosuccinimidyl acetate was removed by dialysis against distilled water. Typically, 85–90% of the lysine residues in tropoelastin were acetylated, as determined from a fluorescence assay using fluorescamine [16]. The positively charged tropoelastin chains were thus converted to neutral (and denoted as E′).
Preparation of tropoelastin-modified silk via enzymatic oxidation of tyrosine
Silk and hydrophobically modified tropoelastin were mixed at mass ratios of S:E′ = 1:2 and 2:1 forming reaction solutions in 0.25 M borate buffer (pH 8.5) with a total protein concentration of 4.3 mg/ml. Pure unmodified tropoelastin and silk were used as controls. Then to catalyze dityrosine formation, 10 µl of a 25 mg/ml stock solution of horseradish peroxidase was added to each of the solutions, followed by 10 µl, 10 mM hydrogen peroxide [17]. The reaction was incubated at room temperature for 4 h and the four final products were denoted as E100, SE′33, SE′67 and S100, respectively. Successful covalent coupling was confirmed by gel electrophoresis (SDS-PAGE) and fluorescence detection with a microplate spectrofluorometer (Molecular Devices, CA, USA).
Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectra of tropoelastin, acetylated tropoelastin, blended and enzymatically coupled silk/tropoelastin, noted as E, E′, S/E′ and SE′, respectively, were obtained using a JASCO FTIR 6200 spectrometer (JASCO, Hachioji, Tokyo, Japan) equipped with a Smart MIRacle™ horizontal attenuated total reflection Ge crystal accessory (Pike Technologies, Inc., WI, USA) in the spectral region of 400–4000 cm−1. The air-dried samples were pressed into potassium bromide (KBr) pellets prior to data collection.
1H-NMR spectrometry
The 1H-NMR spectra of E100, SE′33 and S100 were recorded on a Bruker (MA, USA) Advance 500 MHz spectrometer.
Electrodeposition of silk/tropoelastin alloy gels
The electrodeposition experiments were performed using a previously described geometry adapted to a miniature size. A 200 µl aliquot of tropoelastin or silktropoelastin alloy was placed in a 500 µl Eppendorf tube. Two parallel metal wires were immersed in the aqueous solution of the proteins. Electrodeposition was initiated by applying a constant voltage of 13 V (electric field of ~4 V/mm) across the electrodes using a DC power supply (Agilent E3612A DC power supply, Agilent Technologies, Inc., CA, USA). During the 1 min electrodeposition process, protein gel(s) formed at the solution/electrode interfaces.
AFM-based nanoindentation tests
Protein alloy gels were electrodeposited on aluminum foil sheets from solutions at a concentration of 10 mg/ml. The E100 gel formed on the anode and the S100, SE′33 and SE′67 gels formed on the cathodes were then transferred onto glass slides and air-dried, followed by autoclaving treatment to immobilize them. The films were rehydrated in 1× PBS buffer. Force measurements were performed on these samples in PBS at pH 7.4 using a MFP-3D bio-AFM (Asylum Research, CA, USA) equipped with triangular silicon nitride cantilever probes with nominal tip radius of 42 nm and a nominal spring constant approximately 20 pN/m, as determined from thermal calibration [18].
Instron adhesion tests
Tensile adhesion tests were performed on electrodeposited gels of silk-tropoelastin conjugates with an Instron 3366 (MA, USA) testing frame equipped with a 100 N capacity load cell. Twenty microliter of each of the test solutions was placed on a smooth 7 mm titanium disc and a second 7 mm titanium disc was placed on top of the solution. Hydrogel samples were electrodeposited in situ, allowed to dry overnight and any overflow was cleaned prior to tensile testing. The titanium discs were affixed to the Instron using pneumatic clamps and the loading rate was fixed at 0.5 mm/min.
UV-visible spectroscopy
The inverse temperature phase transitions of the tropoelastin and of the silk-tropoelastin conjugates were characterized by monitoring the absorbance of an aqueous protein solution of 0.5 mg/ml at 300 nm on an Aviv 14DS UV-Vis spectrophotometer equipped with a Peltier temperature controller (Aviv Biomedical, NJ, USA). Turbidity profiles were obtained both as a function of temperature, as the protein solutions were heated at a rate of 3°C/min and as a function of time, as the protein solutions were rapidly heated to 36°C from room temperature. The temperature at which the half-maximal turbidity occurred in the continuous heating process was defined as the transition temperature.
Scanning electron microscopy
Thermally precipitated SE′67 were cross-linked with glutaraldehyde, followed by progressive dehydration in a graded series of ethanol solutions (30, 50, 75, 95% and twice in 100%, 30 min at each concentration). The samples were subsequently dried by critical point drying with a liquid CO2 dryer (AutoSamdri-815, Tousimis Research Corp., MD, USA). Prior to imaging using a scanning electron microscopy (SEM; Zeiss UltraPlus SEM or Zeiss Supra 55 VP SEM, Carl Zeiss SMT Inc., MA, USA) at a voltage of 2–3 kV, the samples were coated with a thin layer (10 nm thick) of Pt/Pd using a sputter coater (208HR, Cressington Scientific Instruments Inc., PA, USA).
Cell culture & F-actin staining
Lab-derived human epidermal fibroblast (HEF) cells were cultured in high glucose DMEM medium (Sigma Aldrich, MO, USA), supplemented with 10% fetal calf serum (Gibco Invitrogen, NY, USA), 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin (Gibco Invitrogen). Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza and cultured in EGM™-2 BulletKits™ media (ME, USA). Both cell types were cultured in T-175 tissue culture flasks, maintained at 37°C, 5% CO2 humidified atmosphere and harvested with 0.25% trypsin-EDTA prior to seeding. HEFs and HUVECs for all experiments were used between passages 3 through 5.
For the visualization and comparison of cell adhesion on different materials, protein gels were electrodeposited on aluminum foil sheets. The E100 gel formed on the anode and the S100, SE′33 and SE′67 gels formed on the cathodes were then transferred onto cover glasses (Ted Pella, CA, USA), followed by autoclaving treatment for immobilization. HEFs and HUVECs were seeded on the gel films at a seeding density of 5 × 104/cm2 of films and then cultivated for 48 h. Cells were then fixed with 4% paraformaldehyde followed by permeabilization with 0.1% Triton X-100 in PBS. F-actin and nuclei were stained with Alexa Fluor 488 phalloidin (Gibco Invitrogen) and 4′,6-diamidino- 2-phenyl-indole, dihydrochloride (DAPI; Gibco Invitrogen), respectively. Stained samples were imaged using a fluorescence microscope (Leica DM, IL, USA) with FITC and Texas Red filters. Cell area was determined using ImageJ software (NIH, MD, USA).
Alamar Blue™ (AB) assay (Gibco Invitrogen) was used to assess metabolic activity of HUVECs. At 1, 3, 5, 7 and 11 days postseeding a 2-h AB assay was carried out on all samples. AB was added to EGM-2 medium at a concentration of 10% (v/v). At each AB assay time point wells were washed with 1 ml PBS and 1 ml of the AB/EGM-2 mixture was added to each well. One milliliter AB/EGM-2 mixture was placed into each of six empty wells as a negative control. After 2 h, a 100 µl sample of the AB/EGM-2 mixture was removed and the absorbance at 560 and 590 nm measured in a 96-well assay plate using a Spectramax M2 Multimode Microplate Reader (Molecular Devices, CA, USA).
Results
Enzymatic conjugation of hydrophobically modified recombinant human tropoelastin to regenerated silk fibroin
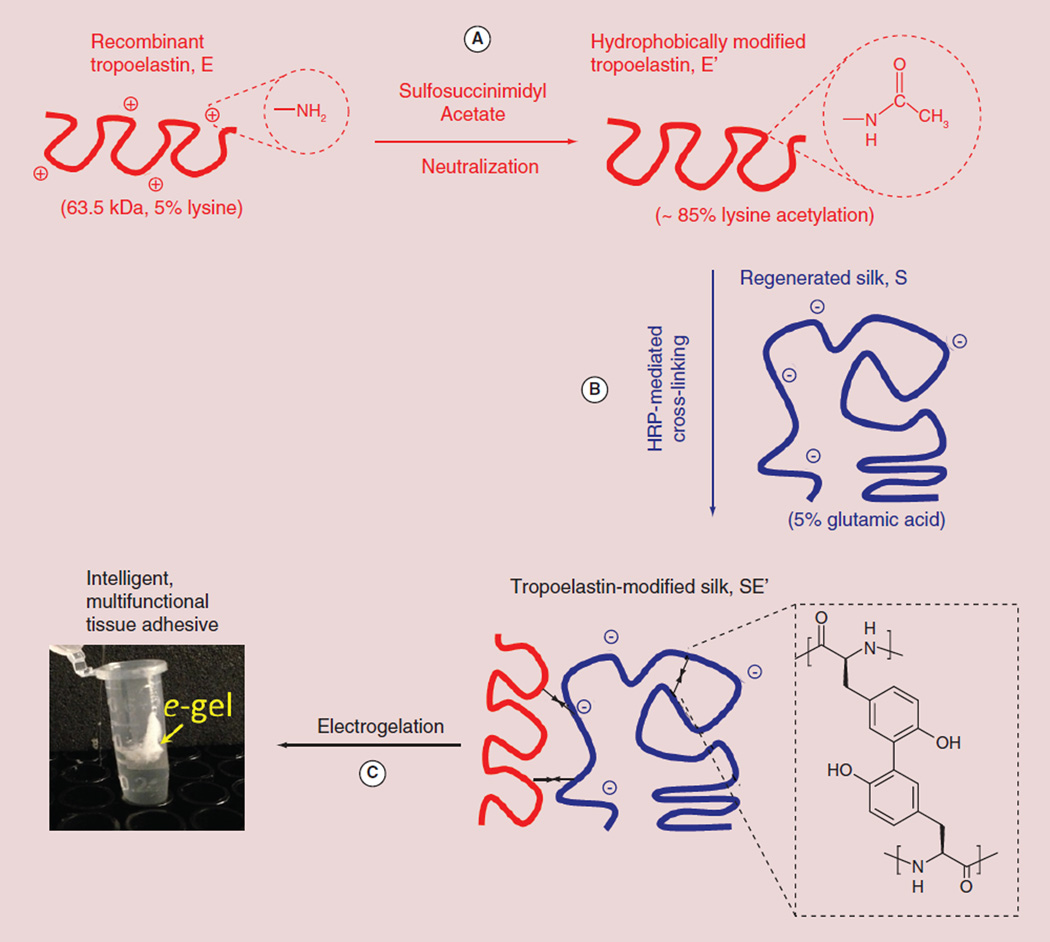
Figure 1 shows a schematic of the chemical modification approach to synthesizing electrochemically responsive tropoelastin-silk conjugates. To prevent electrostatic complexation between the oppositely charged tropoelastin and silk molecules, a reduction in the net electrical charge is required; thus the lysine residues on recombinant tropoelastin (denoted as E) [15,19] were first neutralized with sulfosuccinimidyl acetate (Figure 1A) according to a previously reported method [7]. A fluorescamine assay was used to estimate the degree of acetylation of the hydrophobically modified tropoelastin (denoted as E′) [16]. Approximately 85–90 % of the initially free amine groups were found to be acetylated. The E′ (of ~2% tyrosine) [15] was subsequently coupled with regenerated silk (denoted as S, of ~5% tyrosine) [6] via a well-understood horseradish peroxidase-mediated dityrosine coupling reaction (Figure 1B) [17]. Despite the lack of molecular specificity and topology control, the proposed covalent cross-linking between silk and tropoelastin was anticipated to facilitate the electrodeposition of protein alloy gels near the electrode surface, similar to the ‘electrogelation’ behavior of silk alone [8]. Figure 1C displays a photograph of the initial demonstration of a gel from tropoelastin-modified silk (denoted as SE′) deposited on the anode.
Figure 1. The process of tropoelastin modification of regenerated silk fibroin for gel electrodeposition.
(A) Neutralization of positively charged lysine residues on tropoelastin was achieved by acetylation with sulfosuccinimidyl acetate. Approximately 85% of the lysine amine groups were successfully blocked as determined using a fluorescamine assay. (B) Hydrophobically modified tropoelastin was covalently coupled with regenerated silk by peroxidase-mediated dityrosine cross-linking. (C) When an electric current (~5 mA) was delivered to a tropoelastin/silk solution, the protein alloy formed a hydrogel coating at the electrode surface(s). For color figures, please see online at www.futuremedicine.com/doi/full/10.2217/NNM.14.230
Identification of dityrosine formation in the tropoelastin-silk conjugates
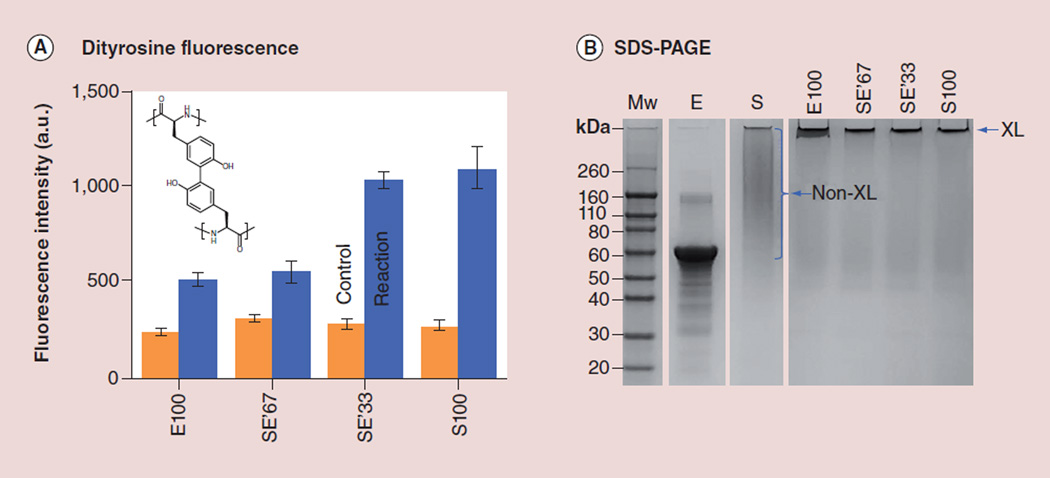
Three tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0%, denoted SE′67, SE′33 and S100, respectively) were prepared according to the chemical method illustrated in Figure 1. For the ‘tropoelastin’ without silk (denoted as E100), enzymatic couplings between tyrosine residues were directly performed on the unmodified tropoelastin to preserve its abundance of positive charges for electrochemical response. With excitation at 320 nm, fluorescence emission at 415 nm (Figure 2A) characteristic of dityrosine [17] underwent increases in all four specimens (blue bars) as compared with the controls (orange bars, uncatalyzed protein/enzyme mixtures). Figure 2B shows representative SDS-PAGE (10% acrylamide) carried out on a batch of tropoelastin–silk conjugation reactions. The molecular weights of E100, SE′67, SE′33 and S100 all increased as a result of dityrosine bond formation, and these conjugates failed to enter the gel.
Figure 2. Identification of dityrosine in tropoelastin-modified silk.
(A) Enzymatic couplings of the tyrosine residues between the unmodified tropoelastin (of 2% tyrosine) or between the lysine-acetylated tropoelastin and the regenerated silk (of 5% tyrosine) were confirmed by the presence of dityrosine fluorescence (excitation at 320 nm and emission at 415 nm). The enzymatic reactions of silk and tropoelastin at four different ratios, E100, SE′67, SE′33 and S100, were studied. (B) The SDS-PAGE image shows that the tropoelastin band in the control sample, E (no H2O2 added), disappeared in all of the three reaction samples, E100, SE′67 and SE′33. All of the peroxidase-mediated reactions (last four lanes) have high MWs and retained in the wells of the gel. E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; E100: Tropoelastin’ without silk; FTIR: Fourier transform infrared; NMR: Nuclear magnetic resonance; S: Silk fibroin; SE′67, SE′33, S100: Tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0%, denoted, respectively); XL: Cross-linked.
Identification of the tropoelastin-silk conjugates by 1H-NMR & FTIR spectroscopy
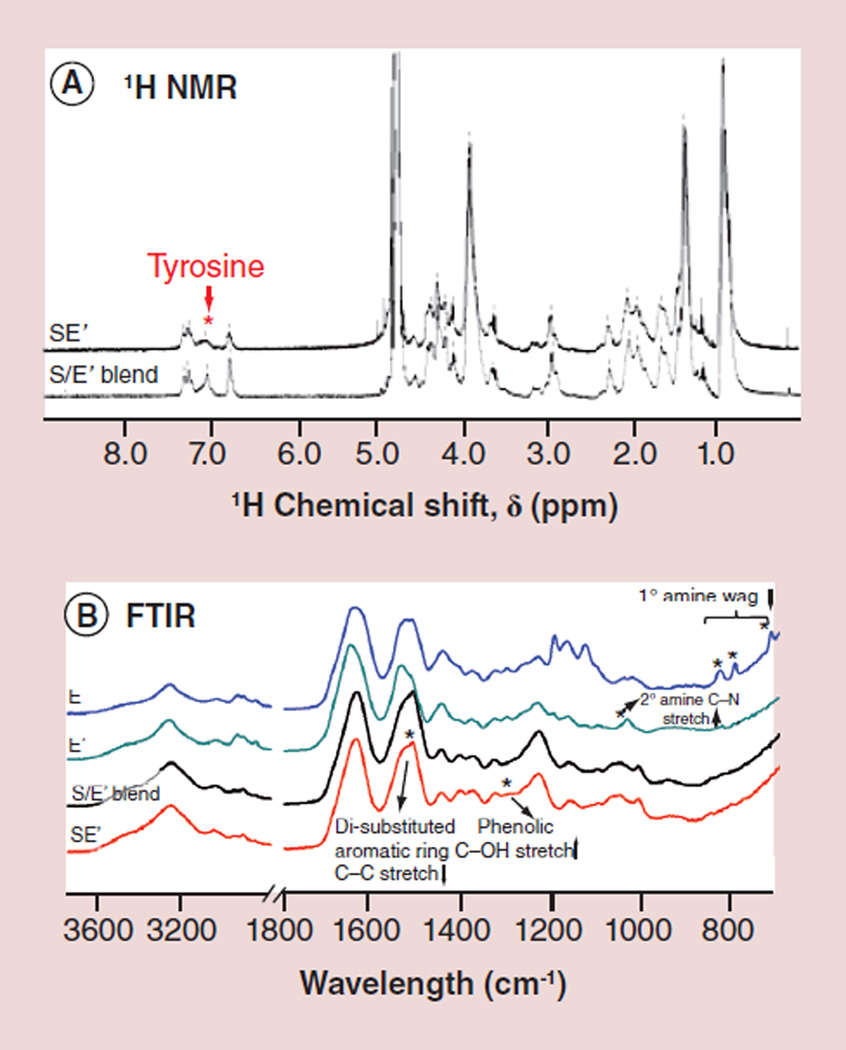
The presence of dityrosine bonds in the tropoelastin-silk conjugates, as opposed to the initial tyrosine residues, was further corroborated by 1H-NMR and FTIR spectra (Figure 3). Figure 3A reveals a significant decrease of the tyrosine-associated NMR peak at approximately 7.1 ppm after the peroxidase-mediated reaction which converted tyrosine in both silk and tropoelastin into dityrosine. The infrared signatures of the proteins were monitored throughout the chemical modification process (Figure 3B). A broad N-H wagging absorption at 700–900 cm−1 attributed to the primary amine groups of lysine residues on tropoelastin disappeared after the acetylation reaction, as accompanied by the appearance of a C–N stretching vibration peak at 1034 cm−1 attributed to secondary amine groups [20,21]. After enzymatic couplings between S and E′, an increase in the phenolic C–O stretching band was observed at 1290 cm−1 and a decrease in the di-substituted aromatic ring C–C stretching band associated with tyrosine at 1512 cm−1, both suggesting the formation of dityrosine bonds [22,23].
Figure 3. 1H-nuclear magnetic resonance and Fourier transform infrared characterizations of tropoelastinmodified silk fibroin.
(A) A decrease of the tyrosine-associated peak (~7.1 ppm) was observed in the 1H-NMR spectra as the peroxidase-mediated reaction was performed in the silk/tropoelastin blend. (B) FTIR spectra reveal the disappearance of the N-H wagging peaks (between 700 and 900 cm−1) of the primary amine and the appearance of the C–N stretching peak (at 1034 cm−1) of secondary amine after lysine acetylation (E′). The peak at 1512 cm−1 in both S/E′ blend and SE′, characteristic of the di-substituted aromatic ring C–C stretching in tyrosine, decreased after the formation of di-tyrosine bonds between silk and tropoelastin and the phenolic C–O stretching peak at 1290 cm−1 increased.
E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; FTIR: Fourier transform infrared; NMR: Nuclear magnetic resonance; S: Silk fibroin.
Mechanical, adhesive and thermal characteristics of the tropoelastin-silk conjugates
Young’s moduli of the binary protein alloys with various compositions between silk and tropoelastin (Figure 4A) were determined using AFM-based nanoindentation. The inset of Figure 4A shows a representative force-distance curve of one of the autoclaved hybrid gels immersed in 10 mM PBS (1× PBS). The softness of silk-tropoelastin conjugates, containing up to 67% silk, was found to be comparable to cross-linked pure tropoelastin, exhibiting elastic moduli on the order of 10 kPa. By contrast, the modulus of cross-linked pure silk was about two orders of magnitude higher, approximately 1 MPa, an indication of a harder material.
Figure 4. Mechanical, adhesive and thermal characteristics of tropoelastin-modified silk e-gels.
(A) Nanoindentation tests in PBS. Inset: reprehensive approach (blue line) and retraction (red) force curves of SE′67 during the adhesion event. (B) Instron tensile adhesion tests in humidified air. (C) Temperature-dependent turbidity profiles of E, E100, SE′67 and SE′33, monitored by the increase in absorbance at 300 nm. (D) Time-dependent turbidity profiles of E and SE′67 over a 2 min period at 36°C as heated from room temperature. Inset: scanning electron microscopy micrograph of thermally precipitated SE′67.
E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; E100: Tropoelastin’ without silk; S: Silk fibroin; SE′67, SE′33, S100: Tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0% denoted, respectively).
PBS: Phospahte-buffered saline.
The tensile adhesion strength of silk-tropoelastin conjugates was also studied using an Instron universal testing machine (Figure 4B). The measured stress-at-break varied in terms of the presence of tropoelastin, all around 300 kPa for SE′33, SE′67 and E100, at least 100% higher than that of S100, approximately 120 kPa. The tropoelastin constituent was expected to confer resilience and, therefore, dictate the viscoelastic energy dissipation capability of the protein alloys. The threshold percent composition for tropoelastin to show the adhesion strength, beyond the limit of its silk counterpart, was clearly lower than 33%.
Tropoelastin, the precursor of elastin, is known to undergo a coacervation process to aggregate as multimers at human body temperature [24]. The thermal responses of the protein alloys were, therefore, investigated in Figure 4C & D. The turbidity profiles of tropoelastin and dityrosine-cross-linked tropoelastin, E and E100, in 1 mg/ml aqueous solutions showed a sharp decrease in the transition temperature from 37 to around 33°C after the molecular weight increase as a result of cross-linking (Figure 4C). The introduction of the silk constituent led to an increase in molecular interactions between the protein chains.
No apparent thermal response was observed on both SE′67 and SE′33 as monitored by absorbance at 300 nm (Figure 4C). The time-dependent turbidity profiles of E and SE′67 solutions, when heated from room temperature to 36°C, are shown in Figure 4D. Sedimentation of SE′67 occurred during the thermal incubation as exemplified by a small bump at approximately 55 s on the turbidity curve (arrow). A representative SEM micrograph of the thermally precipitated SE′67 alloy is shown in the inset of Figure 4D.
Electrodeposition of the silk-tropoelastin hybrid gels
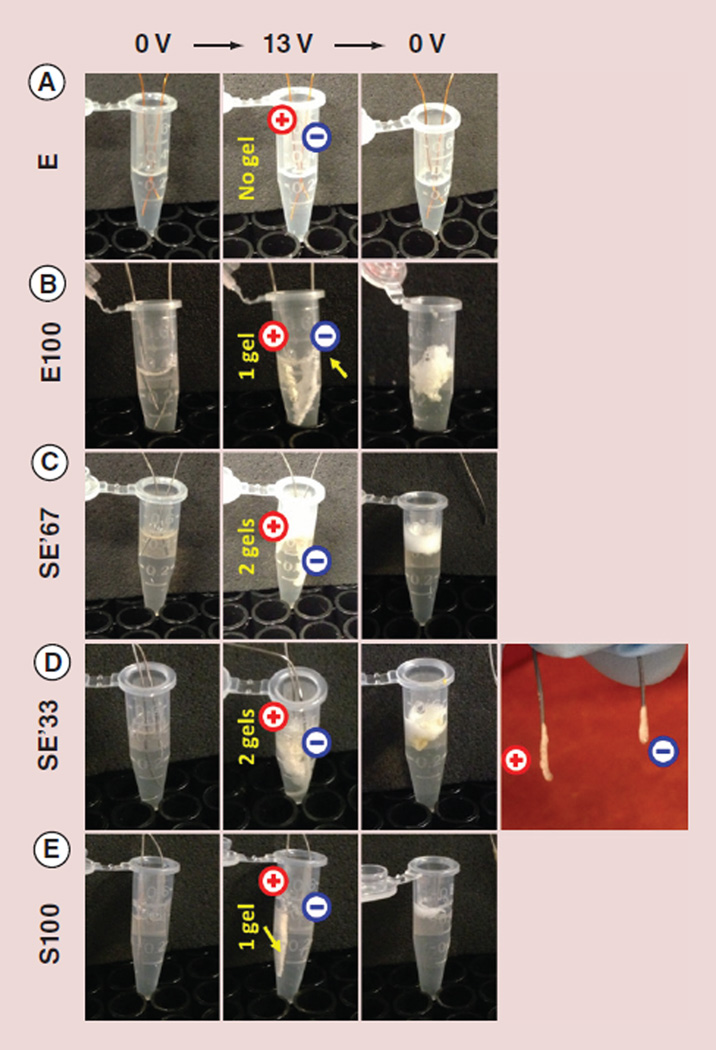
Figure 5 and Table 1 summarize the electrodeposited gels prepared from silk-tropoelastin alloys. Two parallel wire electrodes were immersed in a 0.5 ml Eppendorf tube, each containing 200 µl protein solution. When a DC electric field of 13 V was applied to the electrode pair over a period of 12 min, gel deposition was not observed in the unmodified tropoelastin E solution (Figure 5A), even at a concentration as high as 50 mg/ml. However, within seconds of applying voltage, protein gels were deposited on the electrode(s) from 10 mg/ml solutions of E100, SE′67, SE′33 and S100 (Figure 5B–E). As tropoelastin contains approximately 5% lysine residues bearing positively charged side chains, E100 underwent cathodic deposition only (Figure 5B). The molecular weight increase in tropoelastin as a result of cross-linking was a factor promoting electrodeposition/electrogelation. Silk, on the other hand, is comprised of approximately 3% glutamic acid residues and has an overall negative charge. Only anodically deposited gel formed in S100 (Figure 5E). Within seconds of the application of voltage, two gels formed, each on the surface of the two electrodes, from the silk-tropoelastin alloy solutions, SE′67 and SE′33 (Figure 5C & D). The formation of two electrodeposited gels, similar to electroseparation, could be due to a distinction in the net charge between different populations of the silk-tropoelastin conjugates. For example, the silk-dominated conjugates would electro-migrate toward the anode under an electric field, while the tropoelastin-dominated conjugates toward the cathode. The two conjugate populations appeared to both have very high molecular masses that did not enter the SDS-PAGE gel, where no separation of protein bands was shown in Figure 2B.
Figure 5. Electrodeposition of protein alloy gels.
Electrodeposited gel was not observed in (A) the unmodified tropoelastin (E, 50 mg/ml) but in (B) the di-tyrosine cross-linked tropoelastin (E100; 10 mg/ml). As the tropoelastin, containing 5% lysine residues, was overall positively charged, a gel was electrodeposited only at the surface of the cathode. The two tropoelastin-modified silk samples, (C) SE′67 and (D) SE′33, formed thin layers of gel on both of the electrodes. (E) The di-tyrosine cross-linked silk (S100) formed only an anodically electrodeposited gel, since the isoelectric point of the regenerated silk was dominated by its 3% of glutamic acid residues.
E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; E100: Tropoelastin’ without silk; S: Silk fibroin; SE′67, SE′33, S100: Tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0% denoted, respectively).
Table 1.
Electrodeposition of protein alloy gels on cathode or anode electrodes.
| Sample number | Protein alloys | Gel appearance | |
|---|---|---|---|
| Cathode (−) electrodeposition | Anode (+) electrodeposition | ||
| 1 | E | No | No |
| 2 | E100 | Yes | No |
| 3 | SE′67 | Yes | Yes |
| 4 | SE′33 | Yes | Yes |
| 5 | S100 | No | Yes |
The appearance (Yes) or absence (No) of the electrodeposited gels from the five protein solutions tested in Figure 5 was summarized. E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; E100: Tropoelastin’ without silk; FTIR: Fourier transform infrared; NMR: Nuclear magnetic resonance; S: Silk fibroin; SE′67, SE′33, S100: Tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0%, denoted, respectively).
Biocompatibility & bioactivities of the tropoelastin-silk conjugates
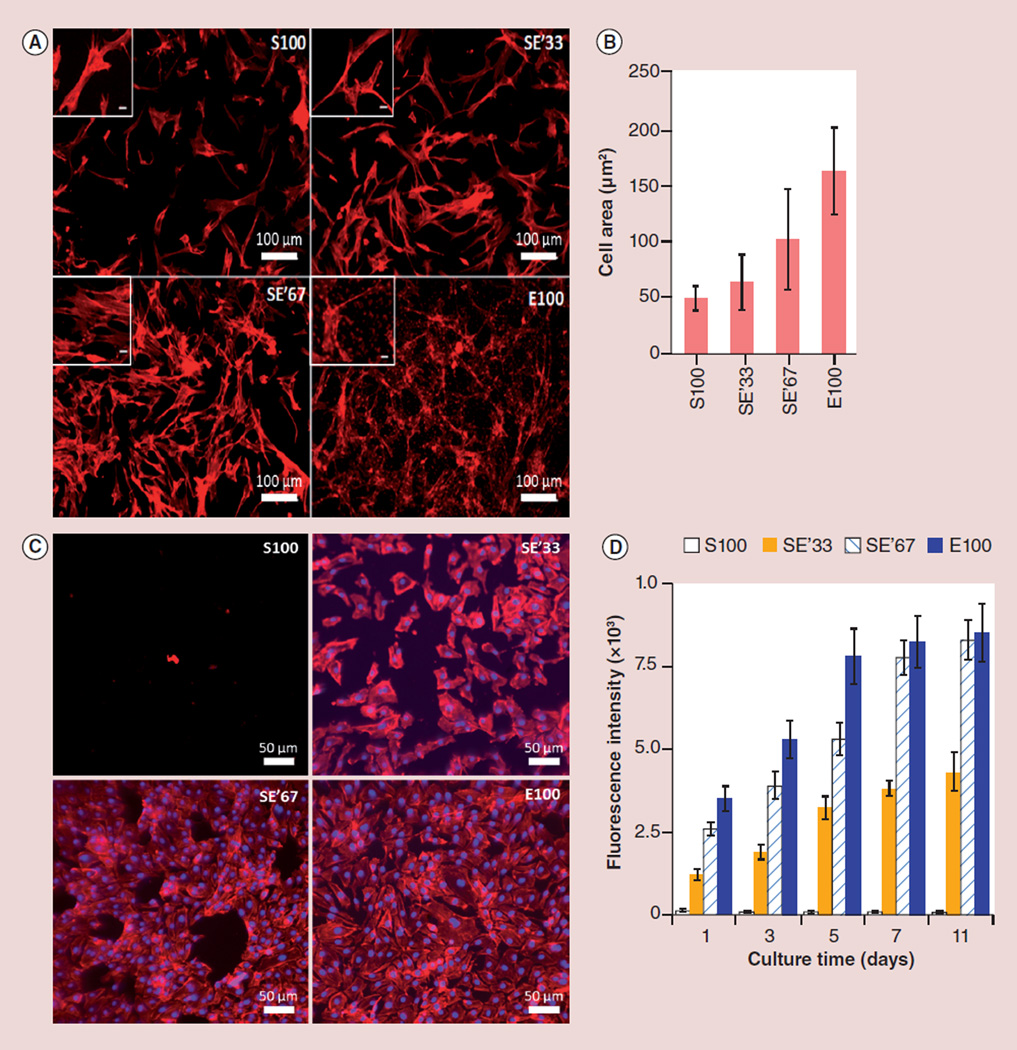
In order to investigate the effect of silk/tropoelastin ratios on cellular responses, electrodeposited protein alloys (S100, SE′33, SE′67 and E100) were placed in 12-well plates and seeded with HEFs or HUVECs. Both cell types were cultured in vitro, respectively. HEFs attached on tropoelastin surfaces within 30 min and 1 h on silk films. Four days post-cell seeding, HEFs grown on films containing the tropoelastin constituents, SE′33, SE′67 and E100, displayed a more clustered morphology and larger cell area compared with pure silk as observed by fluorescence microscopy (Figure 6A & B). This finding is consistent with a previous report that cell adhesion to tropoelastin was mediated via the C-terminal GRKRK motif and integrin αVβ3 [25].
Figure 6. Cell response to electrodeposited silk-tropoelastin alloy gels.
(A) Phalloidin staining of fibroblasts on different silk/tropoelastin gel surfaces at day 4 post-seeding. (B) Fibroblast cell area measurements after 4 days of cultivation. E100 vs S100: p < 0.01. (C) Phalloidin and DAPI staining of endothelial cells at day 4 post-seeding. (D) Endothelial cell viability characterized by using Alamar Blue™ assay kit (Gibco Invitrogen, NY, USA). n = 4–5 from two independent experiments. Significance was tested by one way ANOVA test. SE′33 vs S100, SE′67 vs S100 and E100 vs S100: all p < 0.01.
DAPI: 4′,6-diamidino-2-phenyl-indole, dihydrochloride; E: Tropoelastin; E′: Positively charged tropoelastin converted to neutral; E100: Tropoelastin’ without silk; S: Silk fibroin; SE′67, SE′33, S100: Tropoelastin-silk conjugates with decreasing ratios of acetylated tropoelastin to silk (from 67 to 33 and to 0% denoted, respectively).
The metabolic activity of HUVECs on various films was determined by the Alamar Blue assay (Figure 6D). HUVECs were not active on S100 films, while all the tropoelastin-containing samples, including SE′33, SE′67 and E100 films, supported high cell activity over the culture period. This suggests that an increase of the tropoelastin constituent in silk/tropoelastin protein alloys significantly promoted cell attachment and growth as compared with silk alone. The endothelialization of HUVECs was characterized by DAPI and F-actin staining (Figure 6C). Cell nuclei (DAPI) were stained to determine the number of growing cells, while F-actin staining determined cell morphology. The HUVECs rapidly adhered and subsequently spread on SE′33, SE′67 and E100. Furthermore, while cell proliferation was observed on all the three tropoelastin-containing samples, films contain higher ratio of tropoelastin tend to facilitate the formation of a better integrated endothelium (Figure 6C). This result confirmed that, in addition to enhanced cell attachment, the tropoelastin components in the protein alloys had a positive impact on accelerated HUVEC endothelialization [26].
Discussion
Silk gels, commonly used for their programmable strength and durability, have demonstrated their advantages and promising potential for biomedical applications, for example, controlled drug release and tissue regeneration scaffolds. In contrast to the rigid silk gels, dictated by β-sheet nanocrystals induced during sonication or vortexing, electrodeposited silk gels are dominated by random coil, α-helix and β-turn structures. These electrodeposited gels appeared to be highly resilient, and exhibited strong adhesion to various surfaces, for example, metals, wood, plastics [12]. For bioadhesive polymers, typically experiencing large shear deformations in various physiological environments, a high capacity to dissipate viscoelastic energy is both necessary and desired. The incorporation of tropoelastin constituents into silk was found to further enhance the adhesiveness of the alloy proteins and the electrodeposited gels.
In the electrodeposition process, where dramatic local pH changes near the electrode surface(s) triggered protein gelation, gel coatings were formed in a spatially and temporally selective manner. Multiple therapeutics, including but not limited to small molecule antibiotics and hemostatic agents, enzymatic proteins and probiotic microorganisms, can be encapsulated in the electrodeposited gels [27–29]. Simultaneous engineering of the net charge as well as the molecular weight of the protein polymers was useful to control electro-deposition. The electric charge carried by the protein polymers determined their electromigration direction under the electric field. Increased molecular size, on the other hand, promoted gel network formation at the electrochemical interfaces. Physical or chemical cross-links can be introduced into the electrodeposited gels via post-treatments [10] to achieve controlled degradation and tunable release kinetics.
Recombinant human tropoelastin, displaying a wide range of biological activities, is especially suitable for mediation of the cell responses to biomaterials [2,4–5,25–26]. Our results confirmed that the addition of tropoelastin constituents to silk-tropoelastin alloys promoted the attachment of HEFs and the endothelialization by HUVECs. Re-endothelialization is a crucial aspect of wound healing after vascular injury and similarly after stenting [26]. Furthermore, cellular binding domains as well as other specific biofunctional motifs can be easily integrated into the assembled gels through genetic engineering techniques [30,31]. The electrodeposited protein alloy coatings, as a versatile biomaterial platform, could serve as building blocks of nanoparticle-based drug carriers. Their on-demand self-assembly capabilities and tissue adhesive characteristics can aid cells in their adhesion and functional recovery, with the potential to address a variety of needs especially in wound management.
Conclusion
To explore a wide range of tunable integrative properties in complementary binary protein alloys – silk and tropoelastin – carrying opposite charges, a chemical modification strategy combining neutralization of tropoelastin and enzymatic coupling between silk and tropoelastin was developed to engineer the overall electrical charge and to increase the molecular weight. The multi-step synthesis was monitored spectroscopically using FTIR and 1H-NMR. The formation of dityrosine bonds were also confirmed directly by an increase in fluorescence intensity and indirectly by a presence of a high molecular-weight band in SDS-PAGE. The remarkable viscoelastic contributions tropoelastin conferred a superior adhesion performance to the silk-tropoelastin conjugates, in a compositionally dependent manner, as revealed by both AFM-based nanoindentation and Instron-based tensile adhesion tests. Enhanced proliferation of endothelial cells and fibroblasts and increased amounts of biologically active tropoelastin in the protein alloys were highly correlated. Protein aggregates and a distinction in electrical charge of the protein dispersions were found to impact on their electrodeposition process and the position of the electrodeposited gels. As an intelligent biomaterials platform, electrodeposited gels prepared from silk-tropoelastin protein alloys provide a novel, promising and versatile solution for multifunctional coating material in nanoparticle-based drug delivery systems. The enhanced tissue adhesion, desired cellular attachment and growth and programmable gel matrix degradation are all critical for enhanced loading and controlled release of nanomedicine.
Future perspective
Achieving higher targeting efficacy as well as better controlled, sustained drug release lies at the heart of the development of next-generation nanomedicines. We envision that the advancing frontier of protein-based intelligent materials will soon equip the nanosized drug carriers and other types of drug delivery systems with unprecedented levels of functionality by enabling stimuli-responsive self-assembled structures fine-tuned across multiple length scales. In particular, smart proteins capable of reversible sol-gel switching under electro- chemical triggers have demonstrated promise due to their unique digitally programmable nature. Further expansion of this ‘protein biomaterials’ toolkit will shed fresh light on remote monitoring and dynamic control of nanomedicines and other therapeutic agents delivered to human body.
Executive summary.
Background
Protein alloys composed of regenerated silk fibroin and recombinant human tropoelastin represent an emerging class of biomaterials that provides unprecedented opportunities to tailor physical and biological functions desired for biomedical applications.
Electrochemical deposition is a promising method to generate functional sol-gel films in a spatially and temporally controlled manner. The structural and functional interactions between silk and tropoelastin, with distinctive electrostatic and electrochemical natures, during an electrodeposition process remain unexplored.
Materials & methods
The responsiveness to electrochemical cues was preserved in silk-tropoelastin alloys through chemical tuning of the protein net electric charge. To minimize direct electrostatic self-assembly between oppositely charged silk and tropoelastin upon mixing, lysine residues on tropoelastin were first acetylated.
Silk and neutralized tropoelastin were covalently cross-linked to achieve high molecular weight aggregates through the formation of peroxidase-catalyzed dityrosine bonds.
Silk-tropoelastin conjugation was characterized using SDS-PAGE gel, fluorescence, 1H-NMR (proton nuclear magnetic resonance spectrometry) and Fourier transform infrared spectroscopy. Physical performances of these silk-tropoelastin alloys were studied by atomic force microscopy, Instron and turbidity tests, and their ability to support cell growth were evaluated by in vitro cell cultures.
Results
Tropoelastin conferred to protein alloys enhanced adhesion, likely due to its remarkable viscoelastic energy dissipation capability.
Gel was electrodeposited on the cathodic electrode from a solution of enzymatically cross-linked tropoelastin but not from solutions of unmodified tropoelastin, an indication of a preference for increased molecular weights in the electrogelation process.
Enzymatically cross-linked silk was electrodeposited on only the anodic electrode, while silk-tropoelastin conjugates on both electrodes, verifying that the electromigration of protein could be controlled by engineering the net electric charge.
The inclusion of tropoelastin in the protein alloys was shown to promote both proliferation and differentiation of human epidermal fibroblasts and human umbilical vein endothelial cells, cultured on the gels.
Conclusion
Electrodeposited gels made from silk-tropoelastin protein alloys show a great potential for use in diverse biocoating platforms, such as tissue adhesives for wound repair and hydrogel substrates.
Future perspective
Smart protein polymers that are capable of self-assembling across multiple hierarchical orders into functional hydrogel materials has shed light on the challenges of effective nanomedicine drug delivery systems. Both the ability of these biopolymers to respond to external stimuli, for example, electrochemical triggers, and the way they respond are being engineered to better meet the needs of next-generation therapeutics, diagnosis, medical devices and digital health.
Acknowledgements
The authors thank R Elia for helping with the Instron testing and J Rnjak and C Ghezzi for their advice on recombinant tropoelastin materials.
We thank funding agencies for support of these studies (NIH EB014283, AR005593, AR061988, P41 EB002520, Australian Research Council, National Health & Medical Research Council). AS Weiss is the Scientific Founder of Elastagen Pty Ltd. We thank the NIH (R01 EB014283 and R01 AR061988) for support of these studies. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN). CNS is part of the Faculty of Arts and Sciences at Harvard University.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
•of interest; •• of considerable interest
- 1. Liu HJ, Wise SG, Rnjak-Kovacina J, et al. Biocompatibility of silk-tropoelastin protein polymers. Biomaterials. 2014;35(19):5138–5147. doi: 10.1016/j.biomaterials.2014.03.024. • Innovative protein alloys derived from silk and tropoelastin.
- 2.Hu X, Tang-Schomer MD, Huang WW, et al. Charge-tunable autoclaved silk-tropoelastin protein alloys that control neuron cell responses. Adv. Funct. Mater. 2013;23(31):3875–3884. doi: 10.1002/adfm.201202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghezzi CE, Rnjak-Kovacina J, Weiss AS, Kaplan DL. Multifunctional silk-tropoelastin biomaterial systems. Isr. J. Chem. 2013;53(9–10):777–786. doi: 10.1002/ijch.201300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu XA, Wang XL, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31(32):8121–8131. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu X, Park SH, Gil ES, Xia XX, Weiss AS, Kaplan DL. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials. 2011;32(34):8979–8989. doi: 10.1016/j.biomaterials.2011.08.037. • Protocols of silk regeneration and human tropoelastin expression.
- 6.Wray LS, Hu X, Gallego J, et al. Effect of processing on silk-based biomaterials: reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B. 2011;99B(1):89–101. doi: 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WJ, Vrhovski B, Weiss AS. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J. Biol. Chem. 1999;274(31):21719–21724. doi: 10.1074/jbc.274.31.21719. [DOI] [PubMed] [Google Scholar]
- 8. Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL. Electrogelation for protein adhesives. Adv. Mater. 2010;22(6):711–715. doi: 10.1002/adma.200902643. •• Electrogelation of regenerated silk.
- 9.Kojic N, Panzer MJ, Leisk GG, Raja WK, Kojic M, Kaplan DL. Ion electrodiffusion governs silk electrogelation. Soft Matter. 2012;8(26):6897–6905. doi: 10.1039/C2SM25783A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y, Xia X, Shang K, et al. Tuning chemical and physical crosslinks in silk electrogels for morphological analysis and mechanical reinforcement. Biomacromolecules. 2013;14(8):2629–2635. doi: 10.1021/bm4004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Q, Huang YL, Li MZ, et al. Silk fibroin electrogelation mechanisms. Acta Biomater. 2011;7(6):2394–2400. doi: 10.1016/j.actbio.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yucel T, Kojic N, Leisk GG, Lo TJ, Kaplan DL. Non-equilibrium silk fibroin adhesives. J. Struct. Biol. 2010;170(2):406–412. doi: 10.1016/j.jsb.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray KM, Liba BD, Wang YF, et al. Electrodeposition of a biopolymeric hydrogel: potential for one-step protein electroaddressing. Biomacromolecules. 2012;13(4):1181–1189. doi: 10.1021/bm3001155. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood DN, Preda RC, Yucel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SL, Vrhovski B, Weiss AS. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin. Gene. 1995;154(2):159–166. doi: 10.1016/0378-1119(94)00848-m. [DOI] [PubMed] [Google Scholar]
- 16.Chung LA. A fluorescamine assay for membrane protein and peptide samples with non-amino-containing lipids. Anal. Biochem. 1997;248(2):195–201. doi: 10.1006/abio.1997.2137. [DOI] [PubMed] [Google Scholar]
- 17.Qin GK, Lapidot S, Numata K, et al. Expression, cross-linking, and characterization of recombinant chitin binding resilin. Biomacromolecules. 2009;10(12):3227–3234. doi: 10.1021/bm900735g. [DOI] [PubMed] [Google Scholar]
- 18.Spedden E, White JD, Naumova EN, Kaplan DL, Staii C. Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys. J. 2012;103(5):868–877. doi: 10.1016/j.bpj.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrhovski B, Jensen S, Weiss AS. Coacervation characteristics of recombinant human tropoelastin. Eur. J. Biochem. 1997;250(1):92–98. doi: 10.1111/j.1432-1033.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 20.Qiu WG, Teng WB, Cappello JY, Wu X. Wet-spinning of recombinant silk-elastin-like protein polymer fibers with high tensile strength and high deformability. Biomacromolecules. 2009;10(3):602–608. doi: 10.1021/bm801296r. [DOI] [PubMed] [Google Scholar]
- 21.Popescu MC, Vasile C, Craciunescu O. Structural analysis of some soluble elastins by means of FT-IR and 2D IR correlation spectroscopy. Biopolymers. 2010;93(12):1072–1084. doi: 10.1002/bip.21524. [DOI] [PubMed] [Google Scholar]
- 22.Ayala I, Perry JJP, Szczepanski J, et al. Hydrogen bonding in human manganese superoxide dismutase containing 3-fluorotyrosine. Biophys. J. 2005;89(6):4171–4179. doi: 10.1529/biophysj.105.060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattinen ML, Kruus K, Buchert J, Nielsen JH, Andersen HJ, Steffensen CL. Laccase-catalyzed polymerization of tyrosine-containing peptides. FEBS J. 2005;272(12):3640–3650. doi: 10.1111/j.1742-4658.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- 24.Toonkool P, Jensen SA, Maxwell AL, Weiss AS. Hydrophobic domains of human tropoelastin interact in a context-dependent manner. J. Biol. Chem. 2001;276(48):44575–44580. doi: 10.1074/jbc.M107920200. [DOI] [PubMed] [Google Scholar]
- 25.Bax DV, Rodgers UR, Bilek MMM, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin αVβ3. J. Biol. Chem. 2009;284(42):28616–28623. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tersteeg C, Roest M, Mak-Nienhuis EM, et al. A fibronectin-fibrinogen-tropoelastin coating reduces smooth muscle cell growth but improves endothelial cell function. J. Cell. Mol. Med. 2012;16(9):2117–2126. doi: 10.1111/j.1582-4934.2011.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XQ, Wenk E, Matsumoto A, et al. Silk microspheres for encapsulation and controlled release. J. Control. Release. 2007;117(3):360–370. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Wenk E, Wandrey AJ, Merkle HP, Meinel L. Silk fibroin spheres as a platform for controlled drug delivery. J. Control. Release. 2008;132(1):26–34. doi: 10.1016/j.jconrel.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JE, Pritchard E, Hu X, et al. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc. Natl Acad. Sci. USA. 2012;109(30):11981–11986. doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Numata K, Hamasaki J, Subramanian B, Kaplan DL. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. J. Control. Release. 2010;146(1):136–143. doi: 10.1016/j.jconrel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal G, Blanchi T, Mieszawska AJ, et al. Enhanced cellular adhesion on titanium by silk functionalized with titanium binding and RGD peptides. Acta Biomater. 2013;9(1):4935–4943. doi: 10.1016/j.actbio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]