Abstract
Muscle degeneration is a prevalent disease, particularly in aging societies where it has a huge impact on quality of life and incurs colossal health costs. Suitable donor sources of smooth muscle cells are limited and minimally invasive therapeutic approaches are sought that will augment muscle volume by delivering cells to damaged or degenerated areas of muscle. For the first time, we report the use of highly porous microcarriers produced using thermally induced phase separation (TIPS) to expand and differentiate adipose-derived mesenchymal stem cells (AdMSCs) into smooth muscle-like cells in a format that requires minimal manipulation before clinical delivery. AdMSCs readily attached to the surface of TIPS microcarriers and proliferated while maintained in suspension culture for 12 days. Switching the incubation medium to a differentiation medium containing 2 ng/mL transforming growth factor beta-1 resulted in a significant increase in both the mRNA and protein expression of cell contractile apparatus components caldesmon, calponin, and myosin heavy chains, indicative of a smooth muscle cell-like phenotype. Growth of smooth muscle cells on the surface of the microcarriers caused no change to the integrity of the polymer microspheres making them suitable for a cell-delivery vehicle. Our results indicate that TIPS microspheres provide an ideal substrate for the expansion and differentiation of AdMSCs into smooth muscle-like cells as well as a microcarrier delivery vehicle for the attached cells ready for therapeutic applications.
Introduction
Cell therapy as a form of regenerative medicine is being explored for an increasing number of clinical conditions involving bone,1 cartilage,2 and cardiomyopathies.3 Urethral sphincter deficiency resulting in incontinence is a condition that is also likely to benefit from this type of therapy.4–8 Smooth muscle provides an important function for a number of physiological processes. This includes, for example, peristaltic movements within the gastrointestinal tract,9 regulation of blood pressure,10 and maintaining ventilation of the airways.11 The smooth muscle component provides important tonic or phasic contraction to these physiological processes.12 Therefore, damage, degeneration, or loss of smooth muscle through injury and disease results in significant morbidity and mortality. Technologies that will support smooth muscle cell-based therapy are currently limited and none is currently at a stage ready for clinical translation.
Treatment of coronary and peripheral artery disease using small-molecule therapy and gene approaches has largely failed to date13 and the focus has now shifted toward cell-based approaches, with delivery of cells to injured vasculature being potentially useful for the restoration of structure and function. This has been demonstrated by Allaire et al., who used endovascular delivery of vascular smooth muscle cells to improve the stability of aortic injuries.14 However, one of the major limitations of cell-based regenerative medicine therapy targeting smooth muscle is the lack of donor tissue suitable for harvesting cells. The use of stem cells as a source of smooth muscle for therapeutic purposes has generated interest. Cell types being investigated include embryonic stem cells, adult endothelial progenitor cells, mesenchymal stem cells (MSCs), and induced pluripotent stem cells.15,16
MSCs isolated from bone marrow and adipose tissue can differentiate into osteoblasts,17 chondrocytes,18 adipocytes,19 and cardiomyocytes20 using biomaterials as a scaffold. However, limitations with bone marrow-derived MSC isolation are donor-site morbidity, pain, and inpatient recovery after biopsy collection.21 For a large proportion of the population, visceral fat is a readily available tissue that contains an abundance of MSCs that can be differentiated into a variety of tissues.22
Chemical, physical, and biological cues can be used to drive the stem cell fate. The suitability of three-dimensional scaffolds for culture and differentiation of MSCs is determined by their physical properties.23 This includes surface topography, microstructure, and mechanical properties, which will influence cell adhesion, proliferation, and differentiation.24 The use of spherical microcarriers combined with suspension bioreactors is a widely used technique to expand anchorage-dependent cells.24–26 The microcarriers provide a large surface area for cell adhesion and growth in a homogeneous and controlled environment.
Moreover, the use of microcarriers may provide a more favorable environment for the expansion of cell numbers in vitro and their subsequent in vivo delivery for therapeutic purposes. Cell expansion on two-dimensional (2D) substrates requires their chemical and/or physical detachment from the culture vessel before delivery. This process disrupts cell adhesion to extracellular matrix proteins and may damage the cell membranes resulting in changes to the structure and function of the cells.27–29 The detrimental effects caused by this process are likely to reduce the efficacy of the delivered cell product and contribute to poor clinical outcomes.
Limitations associated with smooth muscle cell therapy and approaches to in vitro cell culture have highlighted the need for improvements in this area before this type of therapy can be successfully translated into a clinical application for humans. We have previously developed a novel type of biodegradable polymer microcarrier intended for minimally invasive treatment of incontinence and sphincter-sparing treatment of fistulas that can be used for large-scale expansion of cells.29–32 The microcarriers, composed of a biocompatible and degradable material, poly(D,L-lactide-co-glycolide) (PLGA), are prepared using a thermally induced phase separation (TIPS) technique, resulting in a highly porous structure that facilitates controlled degradation compared with solid microcarriers. We have demonstrated the manufacture of TIPS microcarriers to be a scalable process that can be performed under GMP conditions, providing a clinically ready product.
The objective of the current study was to investigate the use of TIPS microspheres for the in vitro expansion of MSCs and their differentiation into smooth muscle-like cells that might ultimately be used for cell therapy.
Materials and Methods
Preparation of PLGA TIPS microcarriers
PLGA TIPS microcarriers were prepared as previously described.29 Briefly, 75:25 PLGA (Purasorb PDLG7507; Purac Biomaterials) was dissolved in dimethylcarbonate (DMC, 1:25 [w/v]; Sigma-Aldrich) using magnetic stirring overnight. Microcarriers were fabricated from the polymer solution using a Nisco Encapsulator Unit Var D (Nisco Engineering), fitted with a stainless steel sapphire-tipped nozzle with a 150-μm orifice. A polymer solution was fed into the encapsulator unit by a syringe pump (Pump 11; Harvard Apparatus), connected through a silicone tube, at a constant rate of 3 mL min−1. The vibration frequency of the nozzle was kept at 1.80 kHz and the amplitude of frequency at 100%. Liquid polymer droplets were ejected into a polyethylene beaker containing 400 mL of liquid nitrogen. The frozen droplets were allowed to equilibrate in the liquid nitrogen and transferred to a 50-mL Falcon™ tube (BD Biosciences). Samples were lyophilized using an Edwards MicroModulyo freeze dryer (ThermoFisher Scientific) for 24 h to allow the sublimation of residual DMC. For the experiments conducted in the current study, PLGA TIPS microcarriers were sieved to produce batches with a size range of 150–425 μm.
Attachment and growth of adipose-derived MSC on PLGA TIPS microcarriers
PLGA TIPS microcarriers were prepared as outlined above and preincubated under vacuum in a culture medium (Alpha Minimal Essential Medium [α-MEM; Gibco] supplemented with 10% fetal bovine serum [FBS; Gibco-Invitrogen], 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 μg mL−1amphotericin B [Sigma-Aldrich]; termed the proliferation medium), as previously described.29 Human adipose-derived mesenchymal stem cells (AdMSCs, StemPro; Invitrogen) were seeded on the TIPS microcarriers at a density of 2000 cells/cm2 of equivalent microsphere surface area in spinner flasks (MicroCarrier Spinner Flask Composite 100 mL, 1965-00100 [BellCo Glass], precoated with Sigmacote [Sigma-Aldrich] to prevent cell attachment to the flasks) containing 50 mL of proliferation medium. TIPS microcarriers in the spinner flasks were stirred by a polytetrafluoroethylene paddle driven by an external magnetic device (Variomag Magnetic Stirrer Biosystem Direct) in a 37°C and 5% CO2 humidified incubator. For loading cells onto the TIPS microcarriers, the stirring regimen consisted of a 2-min stirring at 45 rpm followed by resting for 30 min over a period of 18 h. After 18 h of loading, the cellularized TIPS microcarriers were allowed to settle and the medium was replaced with 100 mL of fresh proliferation medium. Subsequently, the stirring was set at a continuous rate of 45 rpm. The cellularized TIPS microcarriers were incubated in the proliferation medium for 12 days, after which the medium was changed to either the α-MEM supplemented with 2 ng mL−1 TGF-β1 (PeproTech), 10% FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 μg mL−1 amphotericin B (termed the smooth muscle cell differentiation medium) or the α-MEM supplemented with 10% FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 μg mL−1 amphotericin B (termed the control medium) and incubated for a further 7 or 14 days. Fifty percent of the culture medium was removed and replaced with a fresh medium every 3 days.
Quantification of adipose-derived MSC growth on PLGA TIPS microcarriers
The expansion of AdMSCs cultured on TIPS microcarriers was quantified at 1, 4, 8, 12, and 18 days after cell seeding. At each time point, an equal volume of microcarriers (100 μL) was removed from the spinner flasks and the number of cells on the microcarriers determined using the CellTiter 96 Aqueous nonradioactive cell proliferation assay (Promega). The reaction product was transferred to a 96-well plate and the optical density measured using a microplate reader at 490 nm. The absorbance measurement for each sample was converted to the number of viable cells using a standard curve derived from AdMSCs cultured on a 96-well plate. This figure was then used to calculate the number of cells per microcarrier in each sample. The number of TIPS microcarriers in each sample was counted directly using light microscopy.
Quantitative polymerase chain reaction for smooth muscle cell markers
Aliquots of cell-seeded TIPS microcarriers were lysed with buffer RLT (Qiagen) containing 1% (v/v) β-mercaptoethanol (Sigma) at 12, 19 (7 days postdifferentiation), and 26 days (14 days postdifferentiation) of culture. Total RNA was isolated from the lysed cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. The total RNA was eluted with 30 μL RNase-free water. The RNA concentration was quantified at an absorbance of 260 nm using Tecan Infinite M200 (Tecan Group).
Two hundred nanograms of extracted RNA from each sample was reverse transcribed into single-stranded cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) following the manufacturer's instructions. The RNA samples were subjected to reverse transcription reaction at 25°C for 10 min followed by 37°C for 120 min using a PTC-100 Programmable thermal controller (MJ Research, Inc.).
Real-time PCR 7300 System (Applied Biosystems) was used to amplify cDNA for each sample using human TaqMan Gene Expression Assays (Hs99999905-m1, GAPDH; Hs00154543_m1, CNN1; Hs00263989_m1, CALD1; Hs00224610_m1, MYH11) (Applied Biosystems). A total reaction volume of 25 μL was used comprising 1.25 μL primers, 5 μL of single-stranded cDNA, 12.5 μL of TaqMan Mastermix (Applied Biosystems), and 6.25 μL of RNase-free water conducted in a 96-well reaction plate. Thermocycling conditions consisted of exposure to 50°C for 2 min followed by 95°C for 10 min and the subsequent 40 cycles denaturation step at 95°C for 15 s followed by 60°C for 1 min. Measurement of quantitative polymerase chain reaction (qPCR) products was performed using the 7300 SDS software relative to samples collected on day 12 and normalized to the housekeeping gene, GAPDH.
In-cell Western for smooth muscle cell markers
TIPS microcarriers with AdMSCs attached to the surface were aliquoted into 96-well plates (25±5 microcarriers per well) and washed in phosphate-buffered saline (PBS) followed by fixation in 4% formaldehyde in PBS for 30 min. Cells were permeabilized by washing five times in 0.1% triton X-100 in PBS for 5 min per wash. Nonspecific binding was blocked using the Odyssey blocking buffer (Li-Cor Biosciences) for 1 h at room temperature. The samples were incubated with anti-caldesmon (1:1000), anti-calponin (1:1000), and anti-smooth muscle myosin heavy chain (1:1000) antibodies (Sigma-Aldrich) at 4°C overnight. Samples were then washed extensively in PBS containing 0.1% Tween 20 five times for 5 min per wash. Samples were then incubated with the IR Dye 800 CW secondary antibody (1:800) with the CellTag™ 700 stain (1:500; Li-Cor Biosciences) in the Odyssey blocking buffer for 1 h at room temperature. Samples were extensively washed in PBS containing Tween 20 for 5 min per wash. After a final wash, all liquid was removed and the plate was scanned on the Odyssey Classic Infrared Imaging System (Li-Cor Biosciences) using both 700 and 800 nm detection channels at a 169 nm resolution, medium quality with a focus offset of 4.0 mm. Quantitative in-cell Western analysis was performed using Image Studio Lite version 3.1 (Li-Cor Biosciences).
Immunocytochemistry for smooth muscle cell markers
Cellularized TIPS microcarriers were fixed in 4% formaldehyde in PBS for 30 min. The cells were permeabilized in 0.02% Triton X-100 (Sigma-Aldrich) in PBS for 10 min followed by incubation in 1% bovine serum albumin (Sigma-Aldrich) in PBS for 30 min to block nonspecific binding. The samples were incubated with anti-α-SMA (1:1000), anti-calponin (1:1000), or anti-caldesmon (1:1000) (Sigma-Aldrich) for 1 h, washed extensively in PBS, followed by incubation in an anti-mouse secondary antibody (1:200, Alexa-Fluor 488; Molecular Probes) for 1 h. Isotype control antibodies (mouse IgG1 and IgG2a; Sigma-Aldrich) were used as control. The samples were stained with a vector shield mounting medium for fluorescence with DAPI (Vector Laboratories) and mounted on glass microscope slides. The samples were viewed using a Leica TCS SP8 confocal microscope system (Leica).
TIPS microcarrier degradation in the presence of smooth muscle cells
TIPS microcarriers were prepared as outlined in the Preparation of PLGA TIPS Microcarriers section and incubated in a culture medium consisting of Eagle's Minimal Essential Medium (EMEM; Sigma-Aldrich) supplemented with 10% FBS (Gibco-Invitrogen), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 0.25 μg mL−1amphotericin B (Sigma-Aldrich), and 1% nonessential amino acids (Sigma-Aldrich). Primary cultures of smooth muscle cells (porcine aortic [P354–05]; European Collection of Cell Cultures) were seeded on the microcarriers, using the regimen outlined in the Attachment and growth of adipose-derived MSC on PLGA TIPS microcarriers section, at a density of 2000 cells/cm2 of microsphere surface area in 50 mL of medium in spinner flasks coated with Sigmacote (Sigma-Aldrich). Subsequently, the stirring was set at a continuous rate of 45 rpm. PAOSMC-seeded PLGA TIPS microcarriers were incubated in the above medium for 35 days. The control group consisted of PLGA microcarriers without cells. During the culture period, 50% of the culture medium was refreshed every 3 days.
Following 35 days culture, PLGA microcarriers±cells were washed in PBS. To detach cells, the microcarriers were incubated in 0.1% sodium dodecyl sulfate (SDS; Invitrogen) in deionized water for 120 min in a sonicator water bath. The samples were transferred into deionized water and sonicated for 15 min followed by 10-min sonication in 1% (v/v) Triton X-100 (Sigma-Aldrich) in deionized water. The samples were washed in deionized water and dried in a vacuum oven. The absence of residual DNA in the microcarriers was determined using the DNeasy Blood & Tissue Kit (Quiagen) to ensure complete removal of cellular debris. Microcarrier degradation was evaluated by calculating the percentage change in mass loss.
The diameter of dry PLGA microcarriers at day 0 and 35 (decellularized and control samples) was measured from digital images taken with a photomicroscope (Olympus BX 50) connected to a video camera (CoolSNAP-Pro Colour; Media Cybernetics, Inc.). Image processing software (Image-Pro Plus v. 4.5.0.19; Media Cybernetics, Inc.) was used to determine the mean diameter of the microcarriers (n=25 per group).
Ultrastructural analysis of TIPS microcarriers
Scanning electron microscopy (SEM) was used to assess the surface topography of PLGA microcarriers. Cellularized microcarriers were fixed in 2.5% glutaraldehyde (Sigma-Aldrich) in PBS for 15 min, followed by dehydration through increasing concentrations of ethanol (70%, 80%, 90%, 100%; Sigma-Aldrich). The samples were transferred to hexamethyldisilazane (Sigma-Aldrich), mounted on aluminum stubs using adhesive carbon tabs, and sputter coated with gold/palladium (Polaron E5000). Samples were viewed under a JEOL JSM-5410 LV SEM (JEOL) at 20 keV.
Statistical analysis
All experiments were repeated in replicates of five, unless otherwise stated. The data were analyzed using the unpaired two-tail Student's t-test and one-way analysis of variance with Dunnett's multiple comparison test using GraphPad Prism v.4.00 statistical software (GraphPad Software). The data are presented as mean±standard deviation. A p-value of <0.05 indicated statistical significance.
Results
AdMSC growth on TIPS microcarriers
AdMSCs were seeded on TIPS microcarriers and cultured for a total of 18 days. The absorbance measurements derived from the CellTiter assay performed on aliquots of microcarriers at different time intervals increased significantly between days 1 and 4 (p<0.05), corresponding with a 35% increase in the number of viable cells per microcarrier, and between days 8 and 12 (p<0.01) corresponding with a 58% increase in the number of viable cells per microcarrier (Fig. 1). The assay absorbance measurements reached a peak on day 12 followed by a plateauing on day 18.
FIG. 1.
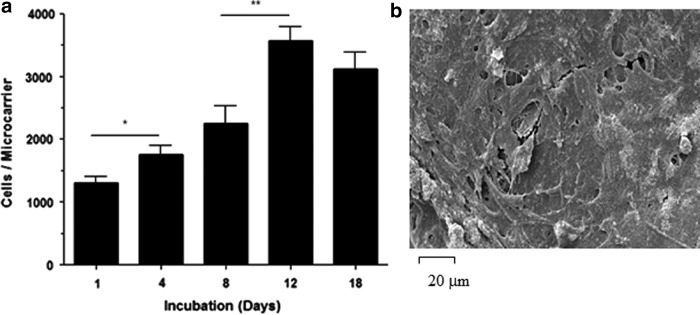
Cell attachment to the TIPS microcarriers. (a) The number of viable AdMSCs attached to the surface of PLGA TIPS microcarriers increased up to day 12, after which the number plateaued indicating that confluence has been attained. (b) Scanning electron microscopy (SEM) of the microcarriers at day 12 confirms the confluence of AdMSCs on the microcarrier surface. AdMSCs, adipose-derived mesenchymal stem cells; PLGA, poly(D,L-lactide-co-glycolide); TIPS, thermally induced phase separation (*p<0.05, **p<0.01).
Expression of smooth muscle cell markers
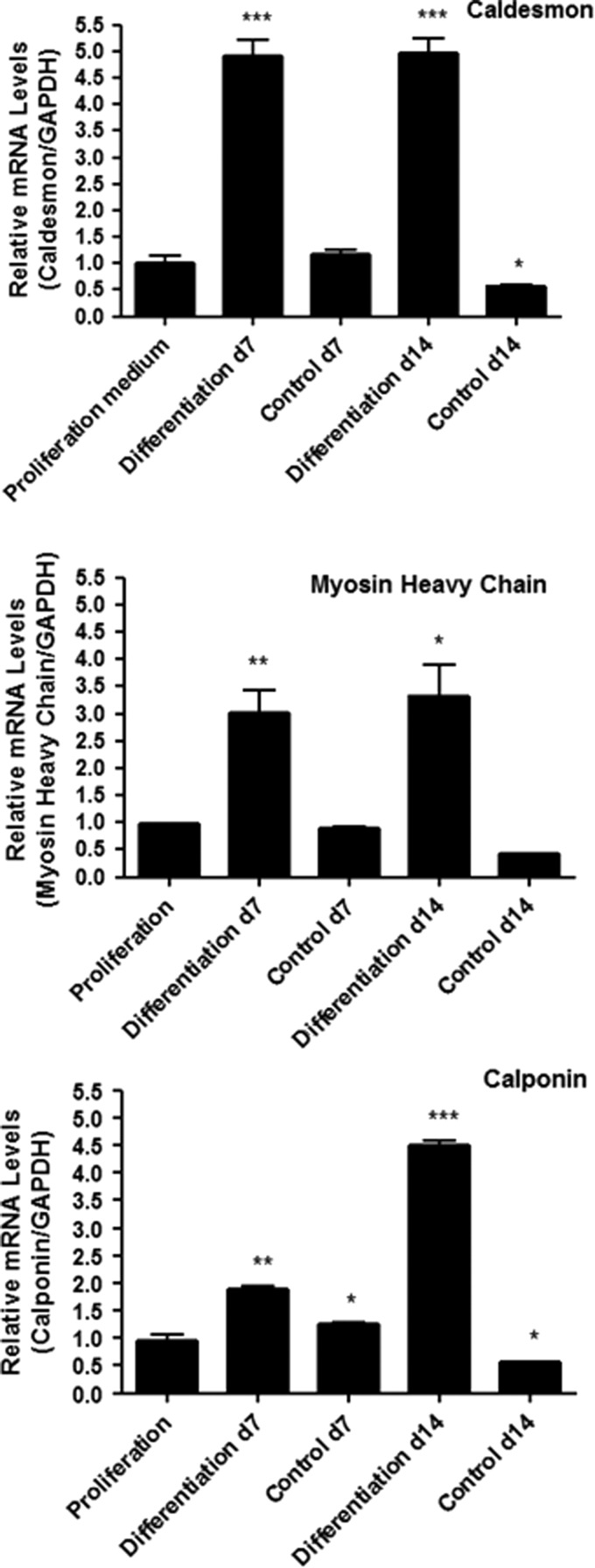
TIPS microcarriers seeded with AdMSCs were cultured in a proliferation medium for 12 days, after which the medium was removed and replaced with either a smooth muscle cell differentiation medium or control medium for a further 7 and 14 days before PCR was performed using relative quantification. All data were normalized to the housekeeping gene and compared to the threshold cycle (CT) value of the calibrator obtained at the beginning of the differentiation process (day 12 postseeding). The expression of caldesmon, calponin, and myosin heavy chain was increased significantly after 7 and 14 days of culture in the differentiation medium (Fig. 2 and Table 1). Cells cultured on TIPS microcarriers incubated in the control medium did not express increased levels of these markers at either time point.
FIG. 2.
Expression levels of caldesmon, calponin, and myosin heavy chain mRNA at 7 and 14 days culture in a differentiation or control medium relative to expression after 12 days of incubation in a proliferation medium (*p<0.05, **p<0.01, ***p<0.001).
Table 1.
Change in mRNA Expression After 7 and 14 Days Incubation in Differentiation Medium Relative to Cells Incubated in Proliferation Medium for 12 Days
| Change in expression at day 7 | Change in expression at day 14 | |||
|---|---|---|---|---|
| Control | Differentiation | Control | Differentiation | |
| Caldesmon | ns | p<0.001 | ns | p<0.001 |
| Calponin | ns | p<0.01 | ns | p<0.0001 |
| Myosin heavy chain | ns | p<0.01 | ns | p<0.05 |
ns, not significant.
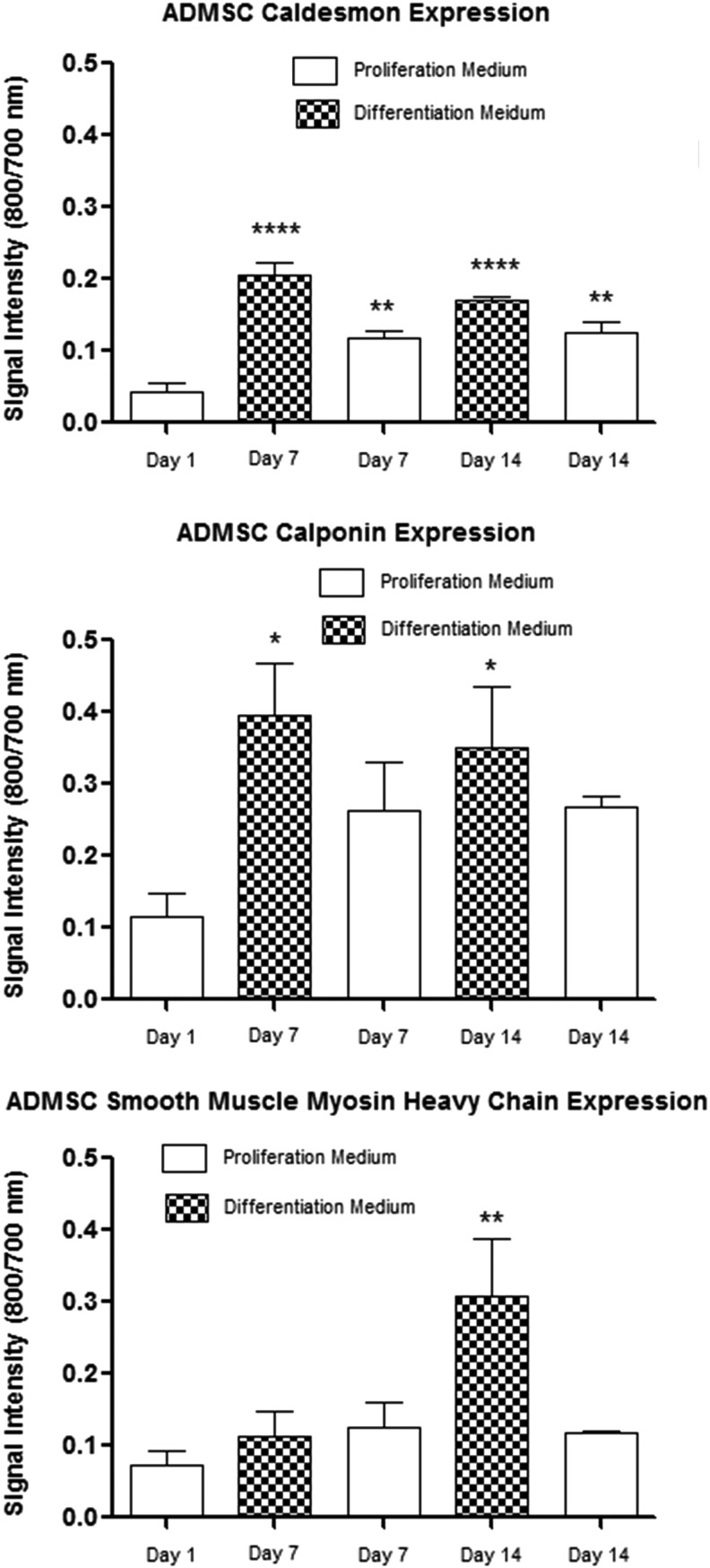
In-cell Western protein analysis was performed using immunofluorescence quantification of the target protein in a cell population. Protein detection was normalized to the cell number for well-to-well population variation. Calponin expression was increased at days 7 and 14 after incubation in the differentiation medium compared with the expression level at day 1 (p<0.05), but not after incubation in the proliferation medium at day 7 and 14 (Fig. 3). Caldesmon expression was increased at days 7 and 14 in both the proliferation (p<0.01) and differentiation medium (p<0.001) compared with the expression level at day 1, with higher levels of expression in cells incubated in the differentiation medium. The expression of myosin heavy chain was significantly increased only at day 14 after incubation in the differentiation medium compared with the expression level at day 1 (p<0.01).
FIG. 3.
In-cell Western data showing the expression levels of caldesmon, calponin, and myosin heavy chain protein at 7 and 14 days culture in a differentiation or control proliferation medium compared with the expression at day 1 in a proliferation medium (n=4) (*p<0.05, **p<0.01, ***p<0.001).
After 12 days of culture in the proliferation medium for cellularization, AdMSCs expressed α-smooth muscle actin, but calponin and caldesmon were not visible by immunocytochemistry. Incubation in the differentiation medium for a further 7 and 14 days resulted in the induced expression of calponin and caldesmon (Fig. 4).
FIG. 4.
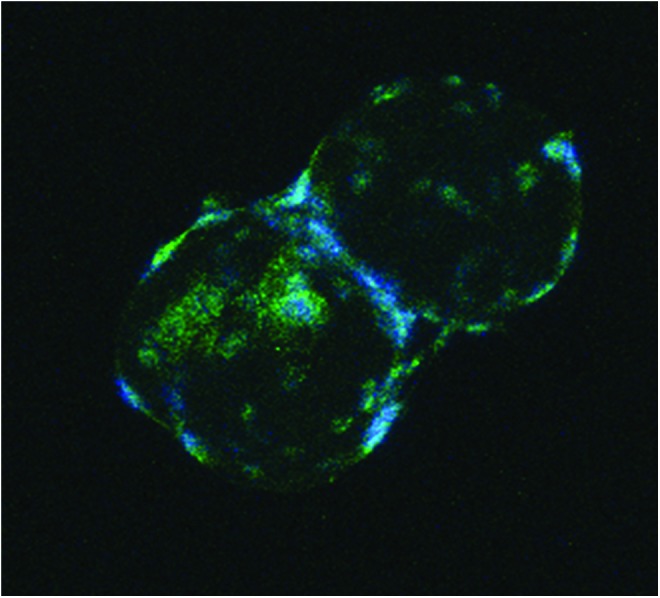
Immunocytochemistry showing expression of calponin in cells attached to PLGA TIPS microcarriers cultured in a differentiation medium for 14 days. Color images available online at www.liebertpub.com/tec
Degradation of TIPS microcarriers in the presence of smooth muscle cells
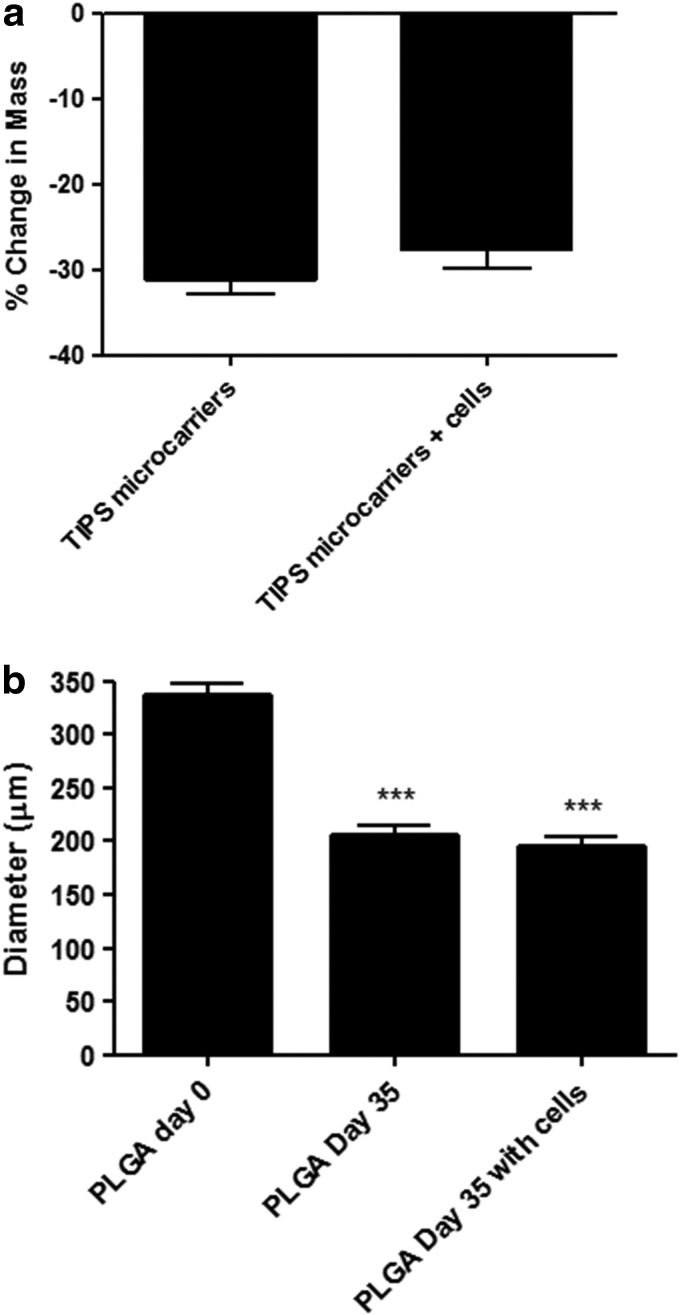
TIPS microspheres prepared for use as microcarriers in the current study exhibited characteristic structural features. This included a highly porous surface, with the noncircular pores measuring 1–5 μm, often appearing to be arranged in a chevron-like pattern (Fig. 5a). The effect of smooth muscle cell growth and metabolic activity on the degradation and structural features of TIPS microcarriers in spinner flask culture systems was assessed over a period of 35 days and compared with noncellularized microcarriers. The diameter of PLGA microcarriers under both conditions was significantly reduced after 35 days compared with the starting diameter of nondegraded microcarriers (p<0.001; Fig. 6). The growth of cells on the surface of the microcarriers resulted in no significant difference in the diameter of TIPS microcarriers after 35 days compared with noncellularized microcarriers degraded for the same amount of time.
FIG. 5.
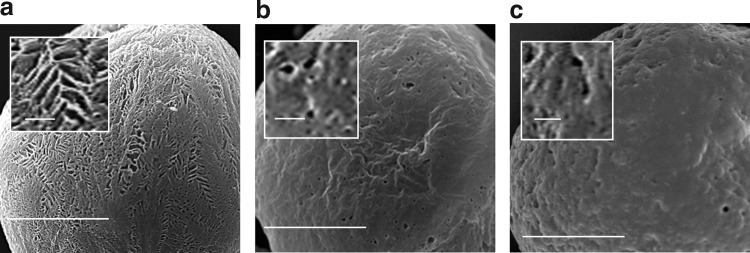
SEM images showing the surface of TIPS microcarriers (insets show the surface at higher magnification). (a) Nondegraded TIPS microcarrier preincubation. (b) TIPS microcarrier after incubation for 35 days in the absence of cells. (c) TIPS microcarrier after incubation for 35 days in the presence of cells. The cells were removed before imaging (scale bars: main figures, 50 μm; inset, 5 μm).
FIG. 6.
Degradation of TIPS microcarriers in the presence of smooth muscle cells. (a) Percentage change in the mass of PLGA TIPS microcarriers relative to the starting mass after 35 days incubation±cellularization with smooth muscle cells. (b) Reduction in the mean diameter of PLGA TIPS microcarriers relative to the starting diameter after 35 days incubation±cellularization with smooth muscle cells (***p<0.001).
The dry mass of TIPS microcarriers±cells was also significantly reduced (p<0.0001) after 35 days incubation in the spinner flask system. There was no significant difference in mass loss between TIPS microcarriers seeded with cells and those without cells.
Surface ultrastructural features of the microcarriers were changed after incubation for 35 days in the spinner flasks. Most notably, the surface of the microcarriers no longer contained pores arranged in a chevron-like pattern. Instead, the pores had become more circular and less abundant, with the surface also appearing to be smoother (Fig. 5b). This change in structural features did not appear to be influenced by the presence of cells cultured on the surface of the microcarriers, as revealed after decellularization (Fig. 5c).
Discussion
Sourcing sufficient quantities of smooth muscle cells and their delivery in an optimal format for therapeutic purposes poses a challenge that is hindering the development of new therapies for a wide range of diseases. The current study demonstrates the feasibility of attaching, expanding, and differentiating AdMSCs into smooth muscle-like cells on the surface of clinically ready TIPS microcarriers. It is envisaged that delivery of cellularized TIPS microcarriers directly to target muscle will avoid detrimental effects associated with cell detachment procedures used with conventional cell culture techniques. Furthermore, this approach has the additional benefit of being minimally invasive with targeted delivery to the site requiring regeneration. Once delivered into damaged or dysfunctional smooth muscle, we have shown that the cells are likely to migrate from the microcarriers and integrate with host tissue.29
Our previous studies have demonstrated that in vitro smooth muscle cells rapidly attach to the surface of the PLGA TIPS microcarriers and increase in number when cultured in suspension bioreactors.29 This approach offers advantages for the expansion of smooth muscle cells in vitro before in vivo delivery of cellularized microcarriers. However, suitable donor sites for harvesting smooth muscle cells are not readily available. To address this need, other groups have investigated the ability to transdifferentiate skeletal muscle-derived stem cells into smooth muscle cells using growth factor-immobilized poly caprolactone/Pluronic F127 porous microbeads.33 While the inclusion of bioactive components into the microcarrier system was shown to have a positive effect on smooth muscle cell differentiation, it would require careful consideration from a regulatory perspective if it were to be implanted in vivo. The current study aimed to explore the ability of TIPS microcarriers to facilitate the expansion of AdMSCs and their differentiation into smooth muscle-like cells, as well as evaluating the effect of smooth muscle cellularization on microcarrier degradation, which could have an impact on the clinical use of the proposed therapeutic system.
Cell expansion on the microcarriers increased up to day 12 incubation, after which the number of cells plateaued, which coincided with cell confluence on the surface of the microcarriers. TIPS microcarriers with confluent layers of cells were transferred into the differentiation medium at day 12 and incubated for a further 7 or 14 days to evaluate induction of AdMSC differentiation into smooth muscle-like cells. Differentiation was achieved primarily by the inclusion of 2 ng/mL TGF-β1 in the differentiation culture medium. A similar approach has previously been used to induce differentiation of MSCs into smooth muscle-like cells when cultured on 2D tissue culture surfaces.34,35 Differentiation following TGF-β1 binding results in signaling through the Smad pathway, causing inhibition of cell proliferation and induction of contractile proteins in smooth muscle cells.34–36
Incubation of the TIPS microcarriers in the differentiation medium resulted in the induced expression of the three myogenic markers—caldesmon, calponin, and myosin heavy chains. These markers are intermediate to late markers of smooth muscle cell differentiation, unlike α-smooth muscle actin, which is considered to be an early marker of smooth muscle differentiation and, importantly, is not limited only to expression in smooth muscle cells.35 The mRNA and protein expression for these contractile apparatus markers was significantly higher for cells attached to the microcarriers and incubated in TGFβ-1 compared with the microcarriers incubated in the control medium when quantified by QPCR and in-cell Western, indicating that TGFβ-1 enhanced the differentiation process. The expression of myosin heavy chain was only significantly expressed in cells incubated in the differentiation medium for 14 days (p<0.01). In our study, cells attached to the TIPS microspheres may be in an intermediate stage of smooth muscle differentiation, with upregulation of caldesmon and calponin at 7 days, and myosin heavy chain, a late smooth muscle contractile marker at day 14. Our findings are supported by a study by Narita et al., where MSC differentiation into smooth muscle-like cells using a defined differentiation medium containing TGFβ-1 caused mRNA upregulation of calponin and caldesmon, but not smooth muscle myosin heavy chain.37 Surprisingly, immunocytochemistry revealed the expression of caldesmon and calponin in cells incubated in the control medium at day 7 and 14 postincubation in the proliferation medium. While this result was unexpected, spontaneous differentiation in the absence of TGFβ-1 might have occurred due to the confluent density of the cells on the surface of the microcarriers, or through the effect of autocrine/paracrine factors released by the cells, or the shear effect of incubating cells in the spinner flasks, as described elsewhere.38,39 Myosin heavy chain was not detected by immunocytochemistry at day 14.
The PLGA TIPS microcarriers used in the current study initially had a highly porous surface structure, typical of microspheres produced using the TIPS manufacturing process.29,31,40 Relative to solid microcarriers, TIPS microcarriers of an equal size contain approximately 80–90% less polymer material.40 This is advantageous as less degradation products are produced as the microcarriers degrade to be replaced by host tissue.
The open porous structure also allows surface erosion to occur rather than an autocatalytic process that is associated with solid PLGA microspheres due to entrapment and accumulation of acidic degradation products. In vitro degradation of TIPS microcarriers in the current indicates a change in the pore structure over time; however, pores were still visible at day 35. Porosity of the TIPS microspheres will influence the shortest linear distance through the open porous structure is a key factor, which will affect oxygen and nutrient diffusion rates to cells infiltrated within the interconnected pores and central cavity.41
Degradation of the microcarriers in the current study was also assessed using fully differentiated smooth muscle cells cultured on the surface of microcarriers for up to 35 days. This approach was used to provide an indication of the likely effects of microcarrier degradation following their implantation into smooth muscle tissues. While the fully differentiated smooth muscle cells were derived from porcine aorta, they provided a useful surrogate cell type for testing the degradation activity of smooth muscle cells on the microcarrier. Both cellularized and control noncellularized microcarriers exhibited a significant decrease in their diameters after 35 days of dynamic culture in the spinner flasks. The presence of cells on the microcarrier surface did not produce any observable change to the degradation process of the PLGA microcarriers, indicating that degradation of the microcarriers is likely to occur in a predictable manner. To substantiate these findings and to fulfill the requirements of regulatory authorities, preclinical safety studies will be required, which will initially investigate the implantation and degradation of noncellularized microcarriers into target smooth muscle tissues.
We have previously shown that the porosity of TIPS microspheres prepared from the same polymer composition to that used in the current study will allow cell infiltration inside the microspheres.29 We have also shown that TIPS microspheres retain an open scaffold structure when implanted in vivo, which facilitates rapid tissue infiltration and integration at the site of implantation, thus reducing the possibility of migration from the target site.40
Results from the current study indicate that it is possible to achieve smooth muscle-like cells from AdMSCs when incubated on TIPS microcarriers. Further studies will be necessary to prove that the differentiated cells are capable of providing phasic and tonic contractile properties similar to native smooth muscle and that they can restore damaged or lost smooth muscle function.
We have shown that it is feasible to use TIPS microcarriers to produce a stem cell-derived smooth muscle cell construct, which avoids the need to harvest donor smooth muscle cells, provides a minimally invasive approach for smooth muscle cell-based delivery, and addresses an unmet need for regenerative medicine.
Acknowledgments
This project was supported by generous grants from the Henry Smith Charity, Action Medical Research, Research into Ageing (Small Incontinence Award), and the Sir Halley Stewart Trust. The authors appreciate the technical support of Nicola Mordan and Mohamed Parkar, UCL Eastman Dental Institute. This work was undertaken at UCL/UCLH, which receives funding from the Department of Health's NIHR as a Comprehensive Biomedical Research Centre.
Disclosure Statement
The authors do not have any conflicts of interest.
References
- 1.Peister A., Woodruff M.A., Prince J.J., Gray D.P., Hutmacher D.W., and Guldberg R.E.Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res 7,17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinatier C., Bouffi C., Merceron C., Gordeladze J., Brondello J.M., Jorgensen C., Weiss P., Guicheux J., and Noel D.Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther 4,318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotkar A.J., and Balinsky W.Stem cells in the treatment of cardiovascular disease—an overview. Stem Cell Rev 8,494, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Huard J., Yokoyama T., Pruchnic R., Qu Z., Li Y., Lee J.Y., Somogyi G.T., de Groat W.C., and Chancellor M.B.Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther 9,1617, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Lecoeur C., Swieb S., Zini L., Riviere C., Combrisson H., Gherardi R., Abbou C., and Yiou R.Intraurethral transfer of satellite cells by myofiber implants results in the formation of innervated myotubes exerting tonic contractions. J Urol 178,332.2007 [DOI] [PubMed] [Google Scholar]
- 6.Mitterberger M., Pinggera G.M., Marksteiner R., Margreiter E., Plattner R., Klima G., and Strasser H.Functional and histological changes after myoblast injections in the porcine rhabdosphincter. Eur Urol 52,1736, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Frudinger A., Kolle D., Schwaiger W., Pfeifer J., Paede J., and Halligan S.Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut 59,55, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Kang S.W., Jeon O., and Kim B.S.Poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for cartilage tissue engineering. Tissue Eng 11,438, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Goyal R.K., and Chaudhury A.Physiology of normal esophageal motility. J Clin Gastroenterol 42,610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurley A., Pires P.W., Bender S.B., Aronovitz M., Zhao M.J., Metzger D., Chambon P., Hill M.A., Dorrance A.M., Mendelsohn M.E., and Jaffe I.Z.Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18,1429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fryer A.D., and Jacoby D.B.Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med 158,S154, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Webb R.C.Smooth muscle contraction and relaxation. Adv Physiol Educ 27,201, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Leeper N.J., Hunter A.L., and Cooke J.P.Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation 122,517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allaire E., Muscatelli-Groux B., Guinault A.M., Pages C., Goussard A., Mandet C., Bruneval P., Melliere D., and Becquemin J.P.Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann Surg 239,417, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung C., and Sinha S.Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J Mol Cell Cardiol 51,651, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Merkulova-Rainon T., Broquères-You D., Kubis N., Silvestre J.S., and Lévy B.I.Towards the therapeutic use of vascular smooth muscle progenitor cells. Cardiovasc Res 95,205, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Oughlis S., Lessim S., Changotade S., Poirier F., Bollotte F., Peltzer J., Felgueiras H., Migonney V., Lataillade J.J., and Lutomski D.The osteogenic differentiation improvement of human mesenchymal stem cells on titanium grafted with polyNaSS bioactive polymer. J Biomed Mater Res A 101,582, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Richardson S.M., Curran J.M., Chen R., Vaughan-Thomas A., Hunt J.A., Freemont A.J., and Hoyland J.A.The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials 27,4069, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Song W., Lu H., Kawazoe N., and Chen G.Adipogenic differentiation of individual mesenchymal stem cell on different geometric micropatterns. Langmuir 27,6155, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Yang M.C., Wang S.S., Chou N.K., Chi N.H., Huang Y.Y., Chang Y.L., Shieh M.J., and Chung T.W.The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials 30,3757, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Tholpady S.S., Katz A.J., and Ogle R.C.Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat Rec A Discov Mol Cell Evol Biol 272,398, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Mazo M., Arana M., Pelacho B., and Prosper F.Mesenchymal stem cells and cardiovascular disease: a bench to bedside roadmap. Stem Cells Int 2012,175979, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilian K.A., Bugarija B., Lahn B.T., and Mrksich M.Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 107,4872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight T., Basu J., Rivera E.A., Spencer T., Jain D., and Payne R.Fabrication of a multi-layer three-dimensional scaffold with controlled porous micro-architecture for application in small intestine tissue engineering. Cell Adh Migr 7,267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schop D., Janssen F.W., Borgart E., de Bruijn J.D., and van Dijkhuizen-Radersma R.Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med 2,126, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Zhu X.H., Wang C.H., and Tong Y.W.Growing tissue-like constructs with Hep3B/HepG2 liver cells on PHBV microspheres of different sizes. J Biomed Mater Res B Appl Biomater 82,7, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gilbert T.W., Sellaro T.L., and Badylak S.F.Decellularization of tissues and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Rossi F.M., and Putnins E.E.Ex vivo expansion of rat bone marrow mesenchymal stromal cells on microcarrier beads in spin culture. Biomaterials 28,3110, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Ahmadi R., Mordan N., Forbes A., and Day R.M.Enhanced attachment, growth and migration of smooth muscle cells on microcarriers produced using thermally induced phase separation. Acta Biomater 7,1542, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Blaker J.J., Knowles J.C., and Day R.M.Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater 4,264, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Blaker J.J., Pratten J., Ready D., Knowles J.C., Forbes A., and Day R.M.Assessment of antimicrobial microspheres as a prospective novel treatment targeted towards the repair of perianal fistulae. Aliment Pharmacol Ther 28,614, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Foong K.S., Patel R., Forbes A., and Day R.M.Anti-tumor necrosis factor-alpha-loaded microspheres as a prospective novel treatment for Crohn's disease fistulae. Tissue Eng Part C Methods 16,855, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Oh S.H., Kim I.G., Lee J.Y., Lee J.Y., and Lee H.J.Bioactive porous beads as an injectable urethral bulking agent: their in vitro evaluation on smooth muscle cell differentiation. Tissue Eng Part A 17,655, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Jeon E.S., Moon H.J., Lee M.J., Song H.Y., Kim Y.M., Bae Y.C., Jung J.S., and Kim J.H.Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci 119(Pt 23),4994, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Harris L.J., Abdollahi H., Zhang P., McIlhenny S., Tulenko T.N., and DiMuzio P.J.Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res 168,306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beamish J.A., He P., Kottke-Marchant K., and Marchant R.E.Molecular regulation of contractile smooth muscle cell phenotype: implication for vascular tissue engineering. Tissue Eng Part B 16,467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narita Y., Yamawaki A., Kagami H., Ueda M., and Ueda Y.Effects of transforming growth factor-beta1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Curr Cardiol Rev 333,49, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Leung H.W., Chen A., Choo A.B., Reuveny S., and Oh S.K.Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng Part C Methods 17,165, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Hsiai T.K., and Wu J.C.Hemodynamic forces regulate embryonic stem cell commitment to vascular progenitors. Curr Cardiol Rev 4,269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshaw H., Georgiou G., Blaker J.J., Forbes A., Knowles J.C., and Day R.M.Assessment of polymer/bioactive glass-composite microporous spheres for tissue regeneration applications. Tissue Eng Part A 15,1451, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Silva M.M., Cyster C.G., Barry L.A., Yang X.B., and Rose F.R.The effect of anisotropic architecture on cell and tissue infiltration into tissue engineering scaffolds. Biomaterials 27,5909, 2006 [DOI] [PubMed] [Google Scholar]