Abstract
Objective: To assess the impact of body mass index (BMI) on perioperative and renal functional outcomes in patients undergoing minimally invasive partial nephrectomy (MIPN).
Materials and Methods: In our IRB-approved, prospectively maintained clinical database, we identified 1206 patients who underwent kidney surgery from 2002 to 2013. Estimated glomerular filtration rate (eGFR) was obtained at baseline and each follow-up visit. From this group, patients who underwent MIPN with more than 12 months of follow-up were selected. Patients were separated into 4 cohorts based on BMI: normal weight (<25 kg/m2), preobese (25–30 kg/m2), obese class 1 (30–35 kg/m2), and obese class ≥2 (>35 kg/m2). Change in eGFR was compared across demographic and clinical variables through linear and logistic regression models.
Results: A total of 235 patients met inclusion criteria with median follow-up of 29 months (interquartile range [IQR] 19, 45). There were no differences in demographic, perioperative, or pathologic features between BMI groups. While controlling for gender, race, Charlson comorbidity score, tumor size, and ischemia time, obese class 1 (odds ratio [OR] 4.68, p=0.019), obese class ≥2 (OR 4.27, p=0.033), and age (OR 1.06, p=0.014) were associated with increased risk of CKD stage ≥3; however, higher baseline eGFR (OR 0.91, p<0.001) was associated with a reduced risk of CKD stage ≥3. While controlling for the same variables, increasing BMI was associated with a significant absolute reduction in eGFR at 1 year (0.38 mL/minute/1.73 m2 reduction in GFR per 1 kg/m2 increase in BMI, p=0.009).
Conclusions: MIPN is technically feasible in obese patients with similar perioperative outcomes to nonobese patients. BMI is an independent risk factor for worsening kidney function following MIPN.
Introduction
Obesity is a major healthcare epidemic afflicting an estimated one-third of the population in the United States and nearly a quarter of the population in the United Kingdom, Canada, Chile, Hungary, Australia, and New Zealand.1 Across the globe, the World Health Organization (WHO) has estimated that 1.5 billion people are overweight, of whom 500 million are obese. Obesity is a known risk factor for several cancers, and excess weight has been estimated to be causal in approximately 20% of malignancies.2 In men and women, a 5 kg/m2 increase in body mass index (BMI) was found to be strongly associated with increased risk of kidney cancer (Relative Risk [RR] 1.24 and 1.34 respectively, p<0.0001).3 Furthermore, cancer-specific mortality is higher for overweight and obese men and women with kidney cancer, with a near linear relationship with increasing BMI.4 Therefore, effective diagnosis and management of kidney cancer in patients with excess weight is paramount.
The majority of patients with kidney cancer present with small renal masses (tumor size <4 cm), and nephron-sparing surgery is considered a standard of care since many of these patients have concomitant risk factors, which pose a current and future threat to renal function. Furthermore, loss of renal function significantly correlates with increased risk of death, cardiovascular events, and rates of hospitalization.5 Obesity is a primary risk factor for cardiovascular disease, diabetes mellitus, and hypertension, and all of these risk factors are associated with chronic kidney disease (CKD).6
In a multi-institutional retrospective cohort study of 1,228 patients undergoing partial nephrectomy (PN) for renal tumors, neither BMI nor operative approach (open vs minimally invasive) were associated with progression to CKD stage ≥3; however, a majority of patients underwent open PN (72%).7 Robot-assisted surgery is being increasingly utilized for the management of renal masses, recapitulating the surgical steps of open PN except the ability to administer cold ischemia. Several single-institution reports have highlighted the feasibility of robotic PN in obese patients with minimal impact on short-term kidney function.8–10 However, the impact on long-term renal function in obese patients undergoing robotic PN has not been further elucidated. Furthermore, obesity makes minimally invasive partial nephrectomy (MIPN) more challenging, as increasing BMI and intra-abdominal fat (IAF) are associated with increased risk of postoperative complications following MIPN.11 Herein, our aim is to assess the impact of BMI on perioperative and renal functional outcomes in patients undergoing MIPN.
Materials and Methods
Case selection
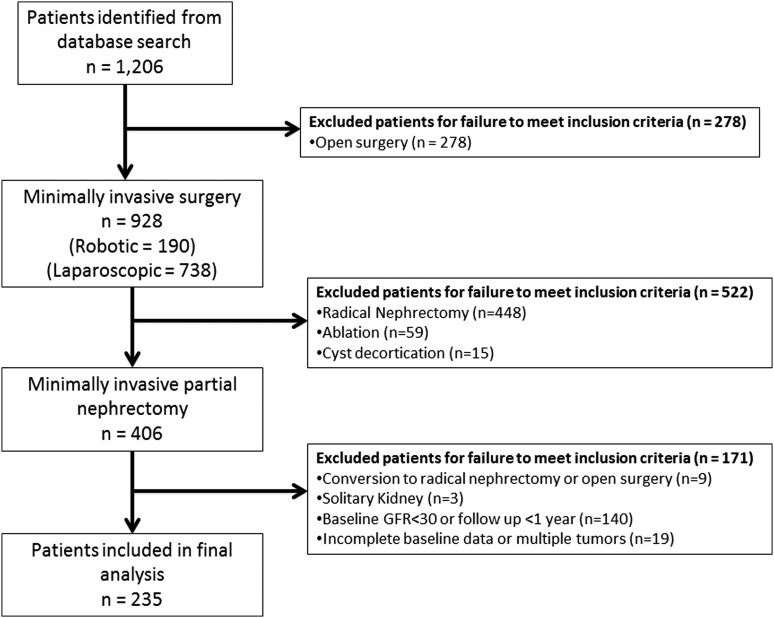
Before initiating this analysis, we obtained approval from our institutional review board in recognition of and compliance with the United States Health Insurance Portability and Accountability Act of 1996 guidelines. Our prospectively maintained institutional renal mass database was queried from August 2002 through March 2013 identifying 1206 patients who underwent kidney surgery. Inclusion criteria were any patient with follow-up of ≥12 months who underwent MIPN for a solitary renal tumor and a normal contralateral kidney. Exclusion criteria included incomplete baseline data, off-clamp techniques, conversion to radical nephrectomy, conversion to open surgery, or baseline CKD stage ≥IV (baseline estimated glomerular filtration rate [eGFR] <30 mL/minute/1.73 m2 as defined by the National Kidney Foundation).12 Eligibility criteria were met by 235 patients (Fig. 1), establishing the foundation for this study.
FIG. 1.
Flowchart outlining selection of cohort.
Data collection
MIPN was performed by one of the two surgeons (A.L.S. and S.E.E.). Follow-up has been maintained every 6 months for 2 years and yearly thereafter with measurement of kidney function at baseline and at each follow-up visit. The abbreviated Modification of Diet in Renal Disease (MDRD) formula was utilized to assess baseline and postoperative eGFR.13,14 Furthermore, patients were categorized according to the National Kidney Foundation classification based on eGFR as CKD stages I to V (I=eGFR >90 mL/minute/1.73 m2, II=eGFR 60 to 89 mL/minute/1.73 m2, III=eGFR 30–59 mL/minute/1.73 m2, IV=eGFR 15 to 29 mL/minute/1.73 m2, and V=eGFR <15 mL/minute/1.73 m2).12 In accordance with WHO BMI classification, patients were categorized as underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to 24.9 kg/m2), preobese (BMI 25 to 29.9 kg/m2), obese class 1 (BMI 30 to 34.9 kg/m2), obese class 2 (BMI 35 to 39.9 kg/m2), or obese class 3 (BMI ≥40 kg/m2).15 To allow for similar numbers of patients in each category, obese class 2 and 3 patients were grouped together for analysis. Various perioperative clinical, operative, and pathologic variables were analyzed, including age, gender, race, Charlson comorbidity score (CCI),16 tumor size, pathologic stage, operative time, ischemia time, estimated blood loss, and length of hospital stay.
Statistical analysis
Statistical analysis was performed using Stata 12 (College Station, TX). Comparison of means was performed across the BMI groupings using ANOVA. Fisher's exact and chi-squared tests were used for comparison of categorical variables. A multivariable logistic regression model was performed for the aforementioned perioperative variables to identify independent predictors of CKD stage ≥3 one year following MIPN. Additionally, a multivariable linear regression model was performed with the same variables to identify independent predictors of absolute reduction in eGFR 1 year following MIPN. Kaplan–Meier survival analysis and stratified log-rank test were utilized to compare cumulative incidence of CKD ≥3 based on BMI category. A two-sided p-value of <0.05 was considered statistically significant.
Results
Of the 235 patients meeting inclusion criteria, overall median follow-up was 29 months (interquartile range [IQR] 19, 45). Among all patients, 42% were preobese with a similar distribution of patients being normal weight (19%), obese class 1 (21%), and obese class ≥2 (18%). Notably, no patients were underweight (Table 1). The sex distribution was different among the four BMI categories, with men comprising 76% of obese class 1 and 48% of obese class ≥2 (p=0.024). BMI was inversely correlated with age (mean age decreased across BMI categories; p<0.001), but there was no difference in CCI across BMI categories (p=0.11). There was no difference in baseline renal function or tumor size between the four BMI groups (Table 2).
Table 1.
Body Mass Index Classification of Cohort
| BMI category (kg/m2) | Classification | Total (%) |
|---|---|---|
| 18.5–24.9 | Normal weight | 45 (19) |
| 25–29.9 | Preobese | 99 (42) |
| 30–34.9 | Obese class 1 | 50 (21) |
| 35–39.9 | Obese class 2 | 20 (9) |
| ≥40 | Obese class 3 | 21 (9) |
| Total | 235 |
BMI=body mass index.
Table 2.
Baseline Preoperative Characteristics of Cohort
| BMI categories | |||||
|---|---|---|---|---|---|
| 18.5–24.9 | 25–29.9 | 30–34.9 | ≥35 | p-Value | |
| Number of patients (%) | 45 (19) | 99 (42) | 50 (21) | 41 (18) | |
| Male, n (%) | 26 (57.8) | 69 (69.7) | 38 (76.0) | 20 (48.8) | 0.024 |
| Age, mean | 61.2 | 60.9 | 55.3 | 52.7 | <0.001 |
| CCI, n (%) | |||||
| 0 | 7 (15.6) | 29 (29.3) | 21 (42.0) | 14 (34.2) | 0.105 |
| 1 | 13 (28.9) | 22 (22.2) | 14 (28.0) | 10 (24.4) | |
| >1 | 25 (55.5) | 48 (48.5) | 15 (30.0) | 17 (41.4) | |
| Baseline creatinine (ng/dL), mean | 1.1 | 1.1 | 1.2 | 0.9 | 0.363 |
| Baseline eGFR (mL/min/BSA), mean | 73.8 | 75.1 | 75.8 | 80.5 | 0.599 |
| Tumor size (cm), mean | 3.1 | 2.9 | 3.1 | 3.3 | 0.554 |
CCI=Charlson comorbidity score; eGFR=estimated glomerular filtration rate.
Of the entire cohort, 143 (61%) underwent laparoscopic PN and 92 (39%) underwent robotic PN with no difference in distribution across BMI groups (Table 3). There was no significant difference in pathologic stage between the four BMI groups: 84.4%, 78.8%, 88.0%, and 75.6% of the tumors were pT1a for normal weight, preobese, obese class 1, and obese class ≥2, respectively (p=0.25). Likewise, OR time, estimated blood loss (EBL), ischemia time, and length of hospital stay were similar across BMI groups (Table 3).
Table 3.
Perioperative Characteristics of the Cohort
| BMI categories | |||||
|---|---|---|---|---|---|
| 18.5–24.9 | 25–29.9 | 30–34.9 | ≥35 | p-Value | |
| Number of patients (%) | 45 (19) | 99 (42) | 50 (21) | 41 (18) | |
| Surgery type, n (%) | |||||
| Laparoscopic | 30 (66.7) | 63 (63.6) | 29 (58.0) | 21 (51.2) | 0.435 |
| Robotic | 15 (33.3) | 36 (36.4) | 21 (42.0) | 20 (48.8) | |
| Path stage, n (%) | |||||
| T1a | 38 (84.4) | 78 (78.8) | 44 (88) | 31 (75.6) | 0.247 |
| T1b | 5 (11.1) | 15 (15.2) | 4 (8.0) | 4 (9.8) | |
| T2a | 2 (4.5) | 0 | 0 | 2 (4.9) | |
| T2b | 0 | 5 (5.0) | 2 (4.0) | 3 (7.3) | |
| T3a | 0 | 0 | 0 | 1 (2.4) | |
| T3b | 0 | 0 | 0 | 0 | |
| Benign | 0 | 1 (1.0) | 0 | 0 | |
| OR time (min), mean | 231.2 | 215.5 | 220.7 | 236.2 | 0.088 |
| EBL (mL), mean | 169.6 | 165.9 | 175.1 | 175.1 | 0.994 |
| Ischemia time (min), mean | 23.9 | 27.8 | 27.9 | 26.2 | 0.198 |
| Hospital stay (days), mean | 1.6 | 1.7 | 1.8 | 1.9 | 0.823 |
EBL=estimated blood loss; OR=odds ratio.
A total of 90 patients (38%) were noted to have CKD stage ≥3 one year after MIPN. Table 4 details the unadjusted and adjusted logistic regression models identifying independent risk factors for the development of CKD stage ≥3 one year after MIPN. Adjusting for gender, race, CCI, tumor size, and ischemia time, obese class 1 (OR 4.68, 95% CI 1.28–17.1, p=0.019), obese class ≥2 (OR 4.27, 95% CI 1.13–16.2, p=0.033), and age (OR 1.06, 95% CI 1.01–1.12, p=0.014) were associated with significantly increased risk of CKD ≥3. In contrast, higher baseline eGFR (OR 0.91, 95% CI 0.89–0.94, p<0.001) was associated with a reduced risk of CKD stage ≥3 at 1 year. Table 5 lists the unadjusted and adjusted linear regression models identifying independent predictors for absolute change in eGFR 1 year after MIPN. Controlling for gender, race, CCI, and ischemia time, increasing BMI was associated with a significant absolute reduction in eGFR at 1 year (0.38 mL/minute/1.73 m2 reduction in GFR per 1 kg/m2 increase in BMI, 95% CI 0.10–0.67, p=0.009). Adjusting for these same variables, increased age, tumor size, and baseline eGFR were also associated with significant absolute reduction in eGFR at 1 year. Kaplan–Meier analysis of time to CKD stage ≥3 stratified by BMI category revealed a decrease in time to CKD stage ≥3 as BMI increased (p=0.04) (Fig. 2).
Table 4.
Logistic Regression Analysis Assessing Odds of Chronic Kidney Disease Stage ≥3 1 Year After Minimally Invasive Partial Nephrectomy
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-Value | Odds ratio | 95% CI | p-Value | |
| Age | 1.07 | 1.04–1.10 | <0.001 | 1.06 | 1.01–1.12 | 0.014 |
| Gender | ||||||
| Male | REF | REF | ||||
| Female | 0.70 | 0.40–1.23 | 0.216 | 0.52 | 0.22–1.22 | 0.134 |
| Race | ||||||
| Caucasian | REF | REF | ||||
| African American | 2.53 | 1.33–4.83 | 0.005 | 1.44 | 0.58–3.60 | 0.431 |
| BMI category | ||||||
| Normal weight | REF | REF | ||||
| Preobese | 1.32 | 0.62–2.80 | 0.467 | 1.70 | 0.57–5.08 | 0.344 |
| Obese class 1 | 1.88 | 0.81–4.37 | 0.139 | 4.68 | 1.28–17.1 | 0.019 |
| Obese class ≥2 | 1.42 | 0.58–3.45 | 0.443 | 4.27 | 1.13–16.2 | 0.033 |
| CCI | ||||||
| CCI 0 | REF | REF | ||||
| CCI 1 | 1.39 | 0.64–3.03 | 0.402 | 0.38 | 0.12–1.24 | 0.109 |
| CCI >1 | 3.49 | 1.79–6.80 | <0.001 | 0.59 | 0.16–2.11 | 0.416 |
| Tumor size (per cm) | 1.20 | 1.01–1.41 | 0.036 | 1.12 | 0.85–1.50 | 0.403 |
| Baseline eGFR (per mL/min/1.73 m2) | 0.91 | 0.89–0.93 | <0.001 | 0.91 | 0.89–0.94 | <0.001 |
| Ischemia time (per min) | 0.99 | 0.97–1.02 | 0.53 | 0.97 | 0.94–1.01 | 0.154 |
REF=referent group.
Table 5.
Linear Regression Analysis Identifying Independent Predictors for Absolute Change in eGFR at 1 Year Following Minimally Invasive Partial Nephrectomy
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p-Value | Coefficient | 95% CI | p-Value | |
| Age | 0.02 | −0.17–0.21 | 0.836 | −0.40 | −0.62 to −0.19 | <0.001 |
| Gender | ||||||
| Male | REF | REF | ||||
| Female | 3.56 | −1.19–8.31 | 0.141 | 2.36 | −1.67–6.39 | 0.250 |
| Race | ||||||
| Caucasian | REF | REF | ||||
| African American | 3.71 | −1.87–9.28 | 0.191 | −0.22 | −5.04–4.61 | 0.929 |
| BMI (continuous variable) | −0.45 | −0.78 to −0.12 | 0.008 | −0.38 | −0.67 to −0.10 | 0.009 |
| CCI | ||||||
| CCI 0 | REF | REF | ||||
| CCI 1 | 0.23 | −5.87–6.33 | 0.941 | 1.80 | −3.63–7.24 | 0.514 |
| CCI >1 | 1.88 | −3.49–7.25 | 0.491 | 0.93 | −4.85–6.72 | 0.751 |
| Tumor size (per cm) | −0.96 | −2.45–0.54 | 0.208 | −1.59 | −2.88 to −0.30 | 0.016 |
| Baseline eGFR (per mL/min/1.73 m2) | −0.39 | −0.47 to −0.31 | <0.001 | −0.46 | −0.55 to −0.38 | <0.001 |
| Ischemia time (per min) | −0.06 | −0.27–0.16 | 0.598 | 0.06 | −0.12–0.24 | 0.520 |
FIG. 2.
Kaplan–Meier curve of cumulative incidence of chronic kidney disease stage ≥3 stratified by body mass index categories following minimally invasive partial nephrectomy.
A subgroup univariable logistic regression analysis was performed to assess the impact of BMI on development of CKD stage ≥3 one year after MIPN stratifying patients by operative approach (laparoscopic or robotic). Increasing BMI category was not independently associated with the development of CKD stage ≥3 when stratified by operative approach.
Discussion
The incidences of renal cell carcinoma and obesity have steadily increased over the past 20 years. Furthermore, renal cell carcinoma, obesity, and CKD are interrelated. For example, increasing BMI is a known risk factor for kidney cancer.3 Additionally, in a large European longitudinal cohort study of 17,375 healthy volunteers, increasing BMI was identified as an independent predictor of new-onset CKD (OR 1.04, 95% CI 1.02–1.06, p<0.001).17 Finally, renal cell carcinoma is a risk factor for CKD: up to a quarter of patients who present with renal tumors eligible for PN have CKD stage ≥3 at baseline and nearly 30% of patients with CKD stage 1–2 at baseline will develop CKD stage ≥3 following PN.7 As renal functional decline is a significant risk factor for death, cardiovascular events, and hospitalization, preservation of renal function in the already at-risk obese population is critical.5 To our knowledge, this is the first study to evaluate long-term renal functional outcomes and additional predictors of CKD stage ≥3 one year following MIPN in obese patients.
PN has traditionally been performed by an open approach and remains the gold standard treatment for localized renal tumors <7 cm in size.18 Over the past two decades, minimally invasive options, including laparoscopic PN and robot-assisted PN, have emerged as safe and effective treatment options. The advantages of MIPN include less blood loss, shorter hospital stay, improved cosmesis, less postoperative pain, and fewer postoperative complications than open PN while maintaining early oncologic and functional outcomes.19,20 However, the ability to manage intra-abdominal visceral and perinephric adipose tissue in obese patients during MIPN can pose a significant challenge for exposure, hilar dissection, tumor resection, and renorrhaphy.
Surrogate measures of operative difficulty include EBL, OR time, and ischemia time. In our study, these measures did not increase with increasing BMI. Naeem et al. compared perioperative outcomes between 48 patients with BMI <30 kg/m2 and 49 patients with BMI ≥30 kg/m2 who underwent robotic PN noting that the obese patients had higher EBL (150 vs 100 mL, p=0.027) and a trend toward a longer OR time (265 vs 243 minutes, p=0.085) and warm ischemia time (26.5 vs 22.5 minutes, p=0.074).10 In a single-institution retrospective series of 250 patients who underwent robotic PN, Isac et al. reported that BMI did not have a significant impact on OR time, warm ischemia time, or postoperative complications. However, patients with BMI ≥40 did have increased EBL compared to the other BMI groups (median 250 mL, p=0.03).9 Finally, in an adjusted linear regression analysis in a single-institution series of 283 patients who underwent robotic PN, Kiziloz et al. found that BMI was significantly correlated with EBL and OR time.8 While the subtle variability in these results combined with the results from our study suggests that the metrics currently available for measuring operative difficulty may not be able to quantify the difference as BMI increases, it does appear that, in general, MIPN in obese patients is more challenging.
BMI may serve as a fairly crude metric for measuring what really matters—intra-abdominal and perinephric adipose tissue. Indeed, IAF has been shown to be associated with adverse surgical outcomes independent of BMI or measurements of outer abdominal fat (OAF).21 A similar approach was utilized in a single-surgeon retrospective series of 195 patients undergoing MIPN, in which IAF, OAF, and BMI were evaluated for associations with perioperative outcomes.11 Both BMI and IAF were significantly associated with any postoperative complication, whereas OAF was not. Ioffe et al. came to a different conclusion after retrospectively measuring perinephric, visceral, and subcutaneous fat in 118 consecutive patients who underwent MIPN.22 They noted no association between perioperative variables and amount of perinephric, visceral, or subcutaneous fat; however, they did identify statistically significant increases in BMI as the corresponding fat content increased, thus suggesting that BMI may be an adequate surrogate for intra-abdominal and perinephric fat content.
Nonetheless, no matter the complexity of surgery, open PN or MIPN, a critical component is preservation of renal function. In a large, retrospective multi-institutional study of 1228 patients with baseline CKD stage ≤3, Clark et al. identified increasing age, female gender, increasing tumor size, clamping of the renal artery and vein, and lower preoperative eGFR as independent predictors of newly acquired CKD stage ≥3 at long-term follow-up (3–18 months) in a multivariable model.7 However, BMI and operative technique did not predict for progression to CKD stage ≥3, although a majority (72%) of the PN were performed open. In our multivariable logistic regression model, age, obese class 1, obese class ≥2, and lower preoperative eGFR were also independent predictors of progression to CKD stage ≥3. With BMI as a continuous variable, each 1 kg/m2 increase in BMI predicted an absolute reduction in eGFR of 0.38 mL/minute/1.73 m2 1 year following MIPN in our multivariable linear regression model while controlling for potential confounders, including CCI, tumor size, and ischemia time. In the linear regression model, increasing age, tumor size, and baseline eGFR also predicted an absolute reduction in eGFR. It is likely that patients with a higher preoperative eGFR will note a greater absolute change in eGFR compared with patients with lower baseline eGFR potentially explaining the phenomenon noted. Previous studies have not noted significant alterations in renal functional outcomes following MIPN in obese patients.8–10,22 However, these studies report short-term results (≤3 months postoperative) focusing on perioperative outcomes and feasibility of MIPN in obese patients, thus neglecting the potential for longer term renal functional consequences.
The results of this study should be interpreted with caution due to inherent limitations. As a retrospective review of a prospectively maintained database, we were unable to accurately measure and account for the amount of renal volume reduction performed at the time of PN, a variable that has been shown to be an important predictor of renal functional decline following PN.23,24 We were also unable to correlate outcomes with renal mass complexity (i.e., nephrometry scores) due to incomplete data collection in our database. Furthermore, although BMI is an imperfect metric, we were unable to measure IAF, perinephric fat, or visceral fat due to inability to re-review preoperative imaging in a large percentage of our cohort. Nonetheless, BMI does seem to correlate with the amount of perinephric, visceral, and subcutaneous fat.22 Finally, measurement of eGFR using the MDRD equation was initially developed for patients up to age 70 with CKD stage ≥3 and may lack the sensitivity to detect significant postoperative changes (it may not be as accurate in patients with GFR >60 or the obese).25 The CKD Epidemiology Collaboration (CKD-EPI) equation has been developed to obviate some of these concerns and has shown increased accuracy especially for patients with eGFR >60 mL/minute/1.73 m2.26 Further study in urologic patients using CKD-EPI should be pursued.
Conclusion
MIPN is feasible in obese patients with similar perioperative outcomes to nonobese patients. BMI, age, and lower baseline eGFR are independent risk factors for worsening long-term kidney function following MIPN, and these patients should be counseled accordingly.
Abbreviations Used
- BMI
body mass index
- CCI
Charlson comorbidity score
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- IAF
intra-abdominal fat
- MDRD
modification of diet in renal disease
- MIPN
minimally invasive partial nephrectomy
- OAF
outer abdominal fate
- PN
partial nephrectomy
- REF
referent group
- WHO
World Health Organization
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Rigby N. Eating and obesity–the new world disorder. Nutrients 2013;5:4206–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578 [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 6.Govindarajan G, Whaley-Connell A, Mugo M, Stump C, Sowers JR. The cardiometabolic syndrome as a cardiovascular risk factor. Am J Med Sci 2005;330:311–318 [DOI] [PubMed] [Google Scholar]
- 7.Clark MA, Shikanov S, Raman JD, et al. Chronic kidney disease before and after partial nephrectomy. J Urol 2011;185:43–48 [DOI] [PubMed] [Google Scholar]
- 8.Kiziloz H, Dorin R, Finnegan KT, Shichman S, Meraney A. The impact of body mass index on perioperative outcomes in robot-assisted laparoscopic partial nephrectomy. J Endourol 2013;27:1000–1007 [DOI] [PubMed] [Google Scholar]
- 9.Isac WE, Autorino R, Hillyer SP, Hernandez AV, Stein RJ, Kaouk JH. The impact of body mass index on surgical outcomes of robotic partial nephrectomy. BJU Int 2012;110:E997–E1002 [DOI] [PubMed] [Google Scholar]
- 10.Naeem N, Petros F, Sukumar S, et al. Robot-assisted partial nephrectomy in obese patients. J Endourol 2011;25:101–105 [DOI] [PubMed] [Google Scholar]
- 11.Gorin MA, Mullins JK, Pierorazio PM, Jayram G, Allaf ME. Increased intra-abdominal fat predicts perioperative complications following minimally invasive partial nephrectomy. Urology 2013;81:1225–1230 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–S266 [PubMed] [Google Scholar]
- 13.Lane BR, Babineau DC, Poggio ED, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol 2008;180:2363–2368 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 15.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251 [DOI] [PubMed] [Google Scholar]
- 17.Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-European population. Nephrol Dial Transplant 2008;23:1265–1273 [DOI] [PubMed] [Google Scholar]
- 18.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: The 2010 update. Eur Urol 2010;58:398–404 [DOI] [PubMed] [Google Scholar]
- 19.Ficarra V, Minervini A, Antonelli A, et al. A multicenter matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int 2014;113:936–941 [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Oh J, Hong SK, Lee SE, Byun SS. Open versus robot-assisted partial nephrectomy: Effect on clinical outcome. J Endourol 2011;25:1181–1185 [DOI] [PubMed] [Google Scholar]
- 21.Morris K, Tuorto S, Gonen M, et al. Simple measurement of intra-abdominal fat for abdominal surgery outcome prediction. Arch Surg 2010;145:1069–1073 [DOI] [PubMed] [Google Scholar]
- 22.Ioffe E, Hakimi AA, Oh SK, et al. Effect of visceral obesity on minimally invasive partial nephrectomy. Urology 2013;82:612–619 [DOI] [PubMed] [Google Scholar]
- 23.Song C, Bang JK, Park HK, Ahn H. Factors influencing renal function reduction after partial nephrectomy. J Urol 2009;181:48–53 [DOI] [PubMed] [Google Scholar]
- 24.Chan AA, Wood CG, Caicedo J, Munsell MF, Matin SF. Predictors of unilateral renal function after open and laparoscopic partial nephrectomy. Urology 2010;75:295–302 [DOI] [PubMed] [Google Scholar]
- 25.Nyman HA, Dowling TC, Hudson JC, Peter WL, Joy MS, Nolin TD. Comparative evaluation of the Cockcroft-Gault equation and the modification of diet in renal disease (MDRD) study equation for drug dosing. Pharmacotherapy 2011;31:1130–1144 [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 2010;56:486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]