Figure 2.
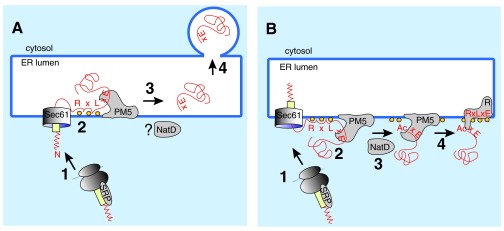
A) The hypothesis. Most proteins targeted for export into the host cell have a signal sequence (yellow) or transmembrane domain, which leads to their SRP-mediated targeting to the protein translocation channel (Sec61) in the ER membrane of the parasite (1). Many but not all of the P. falciparum exported proteins have an N-terminal extension (red zigzag) whose function is unknown. In addition, host cell targeted proteins contain, in a distance of 20–40 amino acids from the signal peptide, a PEXEL sequence (RxLxE), which is also required for binding to phosphoinositol-3-phosphate (PI3P; orange balls) and for cleavage by the ER-membrane associated protease plasmepsin V (PM5). The current hypothesis in the field is that, after signal-peptide mediated translocation into the ER lumen, the PEXEL sequence binds to PI3P in the lumenal face of the ER membrane and is cleaved by PM5 (2). The cleaved protein is released from PM5 (3) and continues through the P. falciparum secretory pathway by vesicular transport (4). Note that this model cannot explain the NatD-mediated N-acetylation of the PM5-cleaved N-terminus, because NatD resides in the cytosplasm. B) The alternative. After SRP-mediated targeting of the protein destined for export to the parasite ER (1) and insertion into the Sec61 channel, the N-terminal extension of the signal peptide (red zigzag) delays signal cleavage, perhaps by preventing reorientation of the signal peptide in the Sec61 channel. This delay in completing translocation allows the RxLxE sequence to bind to PI3P (orange balls) on the cytoplasmic face of the ER membrane, which creates a recognition signal for PM5 and results in proteolytic cleavage (2). Cleavage releases the protein from the translocation machinery and allows N-acetylation by NatD (3). The mature protein is handed over to a PI3P-associated putative transmembrane receptor (R; 4), which may itself be a PEXEL protein.
