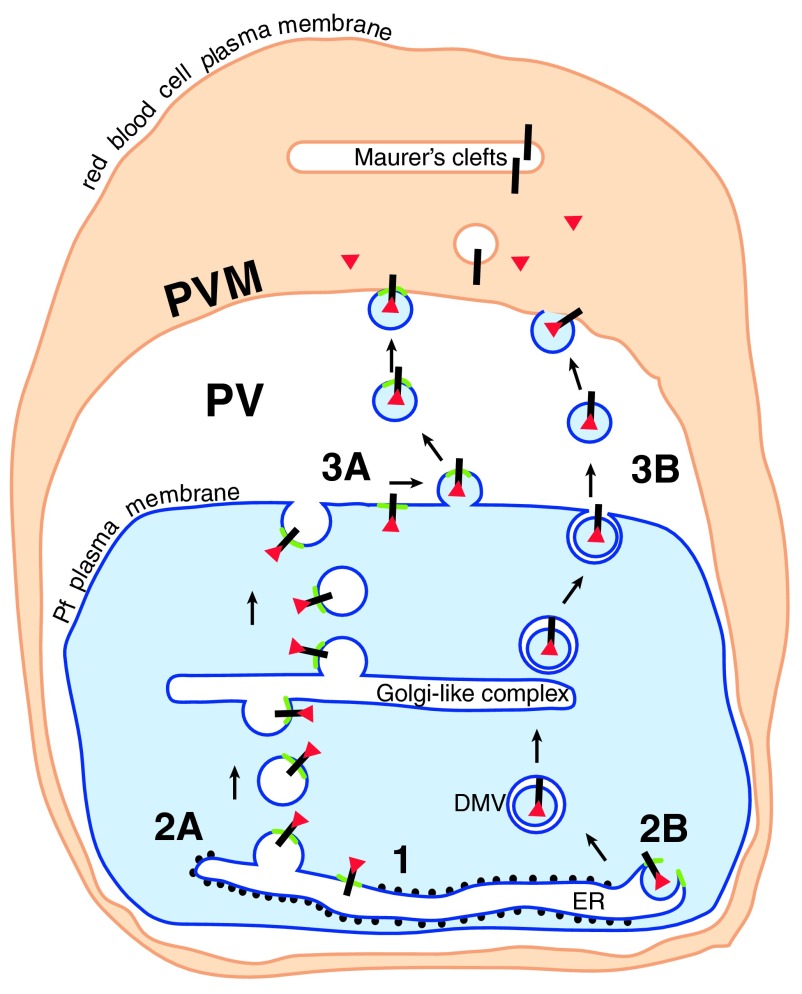
Figure 3. The way out.
Cleaved N-acetylated PEXEL proteins (red triangles) associated with their receptor (black bar) could be transported to the cell surface in a complex (1) in one of two ways. Either the receptor/soluble protein complex remains associated with the PI3P patch (green) in the ER membrane. The patch and associated proteins are transported by vesicle budding (2A) and fusion through the parasite secretory pathway to the cell surface, where a further budding event (3A) liberates vesicles containing the PI3P patch and the PEXEL proteins. These vesicles then fuse with the PVM (4A). Alternatively, similar to the formation of omegasomes during autophagy, the PI3P patch may trigger the budding of processed PEXEL protein-containing vesicles into the ER (2B). PEXEL proteins would then be transported through the secretory pathway in double membrane vesicles (DMV), and released into the PV by fusion of the outer membrane with the parasite plasma membrane (3B). The released vesicle may then fuse with the PVM (4B) (Figure modified from Römisch, 2005) 6.