Abstract
Background
Mitochondrial dysfunction and defects in oxidative metabolism are a characteristic feature of many chronic illnesses not currently classified as mitochondrial diseases. Examples of such illnesses include bipolar disorder, multiple sclerosis, Parkinson’s disease, schizophrenia, depression, autism, and chronic fatigue syndrome.
Discussion
While the majority of patients with multiple sclerosis appear to have widespread mitochondrial dysfunction and impaired ATP production, the findings in patients diagnosed with Parkinson’s disease, autism, depression, bipolar disorder schizophrenia and chronic fatigue syndrome are less consistent, likely reflecting the fact that these diagnoses do not represent a disease with a unitary pathogenesis and pathophysiology. However, investigations have revealed the presence of chronic oxidative stress to be an almost invariant finding in study cohorts of patients afforded each diagnosis. This state is characterized by elevated reactive oxygen and nitrogen species and/or reduced levels of glutathione, and goes hand in hand with chronic systemic inflammation with elevated levels of pro-inflammatory cytokines.
Summary
This paper details mechanisms by which elevated levels of reactive oxygen and nitrogen species together with elevated pro-inflammatory cytokines could conspire to pave a major road to the development of mitochondrial dysfunction and impaired oxidative metabolism seen in many patients diagnosed with these disorders.
Keywords: Autism, Bipolar disorder, Schizophrenia, Chronic fatigue syndrome, Cytokines, Depression, Immune dysfunction, Inflammatory, Mitochondrial dysfunction, Multiple sclerosis, Nitric oxide, Oxidative stress, Parkinson’s disease, Peroxynitrite, Psychiatry, Neurology
Background
Syndromic or non-syndromic mitochondrial diseases, classified as cytopathies or encephalomyopathies, arise as a result of mutations in mitochondrial or nuclear DNA [1]. However, mitochondrial dysfunction and impaired bioenergetics are implicated in the pathogenesis of many chronic illnesses, mainly neuroimmune or autoimmune in nature, despite these not being currently categorized as primary mitochondrial diseases [1-5]. Mitochondrial dysfunction with concomitant oxidative stress is evidenced in the brains and periphery of many patients with the diagnoses of multiple sclerosis (MS) [6], chronic fatigue syndrome (CFS) [6], Parkinson’s disease (PD) [7], and autism [8].
Mitochondrial dysfunction in such individuals may well result from the presence of oxidative stress, as there is now ample evidence implicating oxidative stress as one of the major contributing factors in the development of mitochondrial dysfunction and compromised bioenergetic performance [9-13]. In fact, the causative role of chronic oxidative stress in the development of mitochondrial damage and localized or systemic bioenergetic failure has now been established beyond reasonable doubt [4,14-16]. Chronic oxidative stress develops in a cellular environment whenever production of reactive nitrogen species (RNS) and reactive oxygen species (ROS) exceeds the clearance ability of the cell’s antioxidant defenses such as the glutathione (GSH) and thioredoxin systems [17-19]. ROS and RNS are natural products of oxidative phosphorylation [18,20]. These reactive species can also be generated by activated inflammatory cells, including macrophages and microglia [21-24]. Oxidative stress and chronic inflammation are inextricably interconnected.
Oxidative stress activates a number of transcription factors, such as NF-kappaB and activated protein 1, leading to the production of pro-inflammatory cytokines (PICs), various chemokine species, and activation and proliferation of lymphocytes. The activation of other immune cells in turn leads to the production of more ROS and RNS, principally in the form of superoxide, nitric oxide (NO), and peroxynitrite [24-27]. The tissue damage characteristic of chronic inflammation is mediated directly by macrophages, neutrophils, and eosinophils via the production of PICs [28]. This intricate bidirectional self-amplifying and self-sustaining relationship between the development of chronic oxidative stress and chronic systemic inflammation is sometimes described as an ‘autotoxic loop’ [25,29].
ROS and RNS can also contribute to the development of chronic oxidative stress and inflammation via the oxidative and nitrosative modification of proteins, lipids, and DNA, resulting in modification of DNA bases and tertiary protein structure, lipid peroxidation of cell membranes, and the production of highly reactive aldehydes and ketones. The net result of these processes is the indirect and direct formation of damage-associated molecular patterns capable of activating pathogen sensing receptors on the surface and in the cytoplasm of immune cells [15,29-32].
The origin of oxidative stress in the brains of people suffering from a range of neuroimmune diseases, such as MS and PD, is still a matter of debate. There is now however strong evidence supporting the hypothesis that the oxidative stress in the brains of such patients stems from the transduction of inflammatory signals to the brain following the establishment of chronic inflammation and oxidative stress in the periphery. There is ample evidence demonstrating that systemic inflammation can lead to the development of chronic neuroinflammation [33-35]. Communication of inflammatory signals to the brain is mediated by PICs via a number of routes, including innervation of the vagus nerve, carrier-enabled transport across the blood brain barrier (BBB), activation of endothelial cells within the BBB and perivascular macrophages, and finally via transport through circumventricular organs devoid of a functional BBB [36,37]. The transduced inflammatory signals may lead to the development of chronic neuroinflammation via the activation of microglia if of sufficient intensity and/or duration or lead to the development of ‘primed’ microglia [34,36,38]. Microglial priming involves the up-regulation of a range of surface receptors such as MHC class II, CD11b, and CD11c integrins, co-stimulatory molecule CD86, and Toll-like receptor TLR4 [38].
Following the up-regulation of these receptors, such microglia become exquisitely sensitive to subsequent inflammatory stimuli, leading to an exaggerated production of neurotoxic molecules that may exacerbate the pre-existing pathology and may even accelerate the progression of existing neuroinflammatory or neurodegenerative diseases [39-41]. Activated microglia exert their neurotoxic effects by releasing PICs, such as tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, and interferon (IFN), and free radicals including superoxide NO and peroxynitrite as well as inflammatory molecules such as prostaglandin E2. Moreover, TNF, IL-1, and IFN can act as secondary sources of RNS and other inflammatory molecules acting as potent inducers of inducible NO synthase (iNOS) and via their capacity to upregulate Cox-2 with the resultant production of prostaglandin E2 [36,38,42]. While the relationship between the establishment of chronic systemic inflammation and the development of chronic neuroinflammation is highlighted by numerous authors, the increase in levels of systemic inflammation following the development of neuroinflammation is perhaps under-appreciated. This may occur via a number of different mechanisms, including cytokine leakage from the central nervous system (CNS) into the circulation, increased cytokine synthesis in the periphery, primarily in the liver, and the escape of antigenic molecules likely activating Toll-like receptors on peripheral immune cells flowing ingestion by antigen presentation cells [38,39,43-45]. While these mechanisms could account for the presence of activated cell-mediated immunity in patients with MS, CFS, PD, bipolar disorer, depression, schizophrenia and autism as discussed below, they would not appear to explain the changes in lymphocyte populations and T cell differentiation patterns seen in illnesses like MS and PD. Whatever the cause, chronic inflammation in the body and/or the brain is characterized by the presence of elevated ROS and RNS together with increased levels of PICs.
The aim of this paper is to outline how excessive levels of PICs, notably TNF-α, ROS, and RNS, can lead to mitochondrial dysfunction and compromised bioenergetics in PD, MS, CFS, depression, bipolar disorder, schizophrenia and autism, by inhibiting the electron transport chain (ETC), the tricarboxylic acid cycle, and fatty acid oxidation, adversely affecting the activity and structure of structural and regulatory proteins and the integrity of essential functional lipid membranes. In short, this paper aims to demonstrate that chronically elevated levels of these pro-inflammatory entities are common denominators in paving the many roads to mitochondrial dysfunction.
Discussion
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in MS
Evidence of immune dysfunction in MS
Chronic activation of the humoral and innate arms of the immune system in both the periphery and the CNS is a characteristic finding in MS patients. Commonly reported abnormalities include elevated levels of activated T helper (Th)2, Th17, and Th1 T cells, abnormal function of regulatory T cells, and activated naive B cells with impaired tolerance together with changes in the overall B cell subpopulation distribution pattern [6,29,46]. The relation between an activated peripheral immune system and the development of neuro-inflammation is thrown into stark relief in relapsing remitting MS by the proven efficacy of treatment with the monoclonal antibodies rituximab [47] and natalizumab [48], which target peripheral immune cells and ameliorate disease activity in the CNS [49]. Increased TNF-α levels in the periphery often precede active disease, and levels of this cytokine predict disability levels as measured by the expanded disability status scale (EDSS) [50-52]. Significant increases in plasma levels of IL-2, IL-1β, IL-4, and IL-13 have also been reported [53]. Given the strong positive relationship between TNF-α levels and degree of physical disability, it is of theoretical relevance that the levels of TNF-α and other PICs correlate significantly with the severity of fatigue which affects the vast majority of people with this illness [6,54-56]. The existence of a chronically activated peripheral immune system goes some way to explain the development and/or the maintenance of chronic systemic inflammation seen in sufferers of this disease, which we will now turn to illustrate. However, before doing so, this would seem to be an opportune juncture to emphasize the accumulating and persuasive evidence that a diagnosis of MS represents a spectrum of illnesses where different disease processes converge to produce a similar pathology [57,58]. For a fuller consideration of the evidence that has led many workers to this conclusion, the reader is referred to the work of Ortiz et al. [29].
Evidence of chronic oxidative stress in MS
There is now ample evidence highlighting the pivotal role of oxidative stress in the pathogenesis of MS [59-61]. Several authors have reported the existence of oxidative damage in the brain cerebrospinal fluid (CSF) and blood of MS sufferers [29,62]. Elevated levels of protein carbonyls have been detected in post-mortem brains of patients suffering from this disease [63]. Significantly elevated levels of other surrogate markers of oxidative stress have also been detected in the CSF and plasma of MS patients [60,63,64]. Studies investigating markers of oxidative and nitrosative stress in CSF have demonstrated increased levels of ethane and pentane, which are acknowledged markers of lipid peroxidation [65], malondialdehyde [66], hydroxynonenal [29], and isoprostanes [67]. Nitrotyrosine, a surrogate marker for peroxynitrite formation, is often found in active demyelinated lesions [68]. Unsurprisingly, iNOS levels are also elevated in lesions [69] and in CSF of patients with this illness [66]. High levels of NO, peroxynitrite, and superoxide have also been observed in spinal fluid extracted from patients with MS [66]. Interestingly, CSF levels of NO metabolites correlate positively with relapses [70]. Furthermore, Tasset et al. [9,71] have reported significant peripheral levels of oxidative stress in patients with relapsing remitting MS. Further evidence of the causative role of oxidative stress in the pathophysiology of MS is provided by a recent longitudinal study demonstrating that levels of oxidative stress increased dramatically during relapses but its presence was barely detectable in patients during remission [72]. It is also worthy to note that research teams have discovered that levels of oxidative stress in the blood and CSF correlate significantly and positively with levels of disability as measured by the EDSS [11,73]. The latter study also reported that levels of oxidative stress correlated significantly and positively with the extent of gadolinium-enhanced lesions [73].
Evidence of mitochondrial dysfunction in MS
Although the weight of evidence demonstrates that, while the development of pathology in the early stages of MS is largely driven by inflammation [74], mitochondrial dysfunction appears to have a crucial role in the progression of this disease [75,76]. Mitochondrial abnormalities in MS include altered structure and distribution coupled with a wide array of molecular and biochemical abnormalities [18,75,77-79]. Oxidative damage to mitochondrial DNA and impaired Complex I activity is a characteristic finding in active MS lesions [80]. Complex I and Complex III activity is also reduced in normal tissue within the motor cortex [81]. Complex IV activity is also decreased in lesions as well as in normal-appearing white and grey matter [82,83]. Studies utilizing NMR spectroscopy have demonstrated evidence of globally impaired bioenergetics and increased production of lactate in the CSF [84,85]. Lazzarino et al. [86] provided tantalizing evidence in a longitudinal investigation suggesting a global impairment of adenosine triphosphate (ATP) synthesis in MS when they reported that progressive central ATP depletion over a 3-year period correlated significantly and positively with increased physical disability as measured by changes in EDSS.
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in autism
Immune abnormalities in autism
A diagnosis of autism similarly in all probability represents a group of illnesses with heterogeneous etiology [87,88]. Epigenetics, rather than genetics, seemingly plays a dominant role in driving the development and persistence of these illnesses [89-91]. Several studies have investigated the presence of immune abnormalities in children afforded a diagnosis of autism herein described as children with autism (CWA) and in the parents of such children. Overall, the results demonstrate that CWA and their immediate family members, especially mothers, display markers of autoimmunity, abnormal cellular immunity, and aberrant expression of cytokines and other soluble mediators [92-95]. Abnormal expression of PICs and anti-inflammatory cytokines and their signaling effector molecules is commonly detected in CWA. These findings have been noted in the brain [96-99], gastrointestinal tract [100,101], and peripheral blood [102,103]. CWA commonly have increased plasma IL-1Β and abnormal cellular IL-1Β responses following mitotic stimulation of peripheral mononuclear blood leucocytes [103,104]. Abnormal levels of IL-6 in peripheral blood [103,105] and the brain [92,96,106] is another common finding. The observations relating to elevated levels of PICs extend to TNF-α [107,108] and INF-γ, which are once again elevated in the brain [92] and the peripheral circulation, interestingly, correlated with the levels of other inflammatory mediators such as NO [109,110]. The vast range of immune abnormalities displayed by many CWA is beyond the scope of this paper. An interested reader is referred to previous work for a more in depth treatment of the issue [94,103]. What remains unclear is if this evidence of immune dysregulation reflects a narrow sense of immunity in terms of cellular defense against exogenous pathogens or reflects dysregulation of signaling moieties with a wider range of intracellular signaling roles.
Evidence of oxidative stress in autism
The presence of chronic oxidative stress is commonly reported in CWA [111-114]. Interestingly, this abnormal state is sometimes reported in parents [115]. Several authors have also reported genetic abnormalities in GSH pathways in CWA [116-119], and some of these abnormalities correlate positively with the severity of symptoms [2,120]. Several researchers have detected lower concentrations of reduced GSH increased concentrations of oxidized GSH and a decreased GSH/glutathione disulfide ratio [117,121,122]. The mitochondrial reduced GSH reserve appears decreased in at least some children afforded this diagnosis compared to healthy controls. Additionally, in many studies, decreased GSH levels and several other markers of increased oxidative stress correlate positively with disease severity [123,124]. Other authors have reported a positive correlation between the severity of gastrointestinal dysfunction and surrogate markers of oxidative stress [125]. It is worthy of note that the aforementioned studies measured markers of oxidative stress in the periphery, but there is also a robust body of evidence demonstrating the existence oxidative stress in post-mortem brain samples from CWA compared to healthy controls [126-132].
Mitochondrial dysfunction and impaired bioenergetics in autism
Numerous workers have also reported the presence of mitochondrial dysfunction in CWA [133-139]. In many instances, biomarkers of mitochondrial dysfunction appear associated with disease severity [140,141]. It must be stressed, however, that not all children afforded a diagnosis of autism display evidence of mitochondrial dysfunction, as might be expected if this diagnosis actually represents several different diseases. Systematic reviews place the percentage of CWA displaying evidence of mitochondrial dysfunction as between 30 and 50% [114,142]. Historically, the bulk of published literature examining bioenergetic impairments have focused on blood and urine measures. However, an increasing number of researchers in recent years have reported evidence of impaired mitochondrial function in the brains of CWA compared to healthy controls [126,129,132,143-146]. Studies involving 31P-magnetic resonance spectroscopy have reported decreased production of ATP elevated levels of lactate, reduced levels of carnitine [140,147-149], and other measures of mitochondrial dysfunction [150,151].
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in Parkinson’s disease (PD)
Immune abnormalities in PD
There is evidence that a diagnosis of PD also represents a range of illnesses of heterogeneous etiology [152,153]. A wide array of peripheral immune abnormalities have been detected in patients with PD. The reduction in lymphocyte numbers in general and CD19 B, together with CD3 and CD4 subsets, is especially commonplace. Of the remaining subsets, an increased frequency of T cells secreting Th1 cytokines and a reduced frequency of Th2 cytokine-secreting T lymphocytes is also a common finding. For details of these immune abnormalities and the evidence supporting their existence, the reader is referred to an excellent review by Mosely et al. [45]. The cause of these immune abnormalities in the periphery is far from clear. One suggestion proposes that various elements of the adaptive and innate immune system could become activated as a result of the escape of CNS proteins into the periphery, which could function as damage-associated molecular patterns [30]. It is worthy of note that corrupted species of proteins specific to this disease, including the phosphorylated form of α-synuclein, are found in peripheral tissues in PD patients [154]. Other authors have suggested prolonged pathogen infection or chronic exposure to environmental toxins as the root cause of immune dysregulation and chronic inflammation seen in people with this illness [38]. Further, albeit indirect, evidence of abnormalities in immune and inflammatory pathways in patients with PD stems from the existence of elevated levels of Cox-2 and members of the NF-kappaB family in the substantia nigra, and elevated levels of IL-15, IL-10, and RANTES in the CNS and peripheral circulation in people afforded this diagnosis [155,156]. A number of authors have reported abnormally elevated serum levels of TNF-α and TNF receptor 1 in patients with PD [43,157,158]. Elevated levels of IL-6 have also been detected in the plasma of PD patients, which correlate positively with an increased disease risk [159]. Elevated levels of IL-1β has also been detected in the CSF and the strata of patients with PD, with the latter findings being post-mortem [160-162]. Abnormally, high levels of TNF-α and INF-γ are also commonly observed in the CSF and post-mortem tissue of people suffering from this illness [161,163]. It is also particularly noteworthy that peripheral immune responses have the capacity to trigger exacerbation of PD symptoms, probably on the basis of neuroinflammation [163-165].
Evidence of oxidative stress in PD
Oxidative stress is considered to be the common underlying mechanism driving cellular dysfunction and ultimate demise in genetic and idiopathic cases of PD. The wealth of evidence supporting this viewpoint includes increased levels of oxidized lipids [166], proteins and DNA [167], and decreased levels of reduced GSH in the substantia nigra of PD patients [168-171]. Other abnormalities indicative of oxidative stress observed in the substantia nigra and other regions of the brain include carbonyl modifications of soluble proteins [172,173], oxidized DNA [167,174], and increased levels of malondialdehyde and 4-hydroxy-2-nonenal, and reduced levels of polyunsatrurated fatty acids [175,176]. Nitration and nitrosylation of several proteins, including of alpha-synuclein and parkin, have also been repeatedly documented [177-179]. Many studies have also reported strong evidence of chronic oxidative stress in PD blood and CSF strongly suggesting that PD is a generalized disease [167,180-185].
Mitochondrial dysfunction and bioenergetic abnormalities in PD
Early evidence demonstrating a link between mitochondrial dysfunction and the pathogenesis of PD involved a number of reports illustrating Complex I impairment in the post-mortem substantia nigra pars compacta of patients [186,187]. This Complex I deficiency is also evident in the frontal cortex of PD [188], and remarkably in peripheral tissues such as skeletal muscle [189] and platelets [190], strongly suggesting the presence of global impairment in mitochondrial Complex I activity in this disease. It is also worthy of note that oxidative damage to Complex I and subsequent complex miss-assembly is a common feature of isolated mitochondria in the brains of PD sufferers [191].
Decreased function of Complex III is also commonly detected in the platelets and lymphocytes of PD patients [190,192]. A strong link between impairments in the assembly of mitochondrial Complex III and an increase in free radical damage in the mitochondria isolated from PD patients has also been reported [193]. It is possible that the increase in free radical damage stems from an increased production in ROS and RNS. This increase in free radical release may be due to the increased leakage of electrons from Complex III (as explained below). Alternatively, the inhibition of Complex III assembly causes a severe reduction in the levels of functional Complex I in mitochondria [194], which could lead to an increase in free radical production through Complex I deficiency. The use of magnetic resonance spectroscopy has revealed evidence of in vivo widespread mitochondrial dysfunction in virtually every region of the brain in PD patients, demonstrating that bioenergetic abnormalities and a shift to anaerobic metabolism are not confined to the substantia nigra [195-197]. It is worth stressing, however, that studies investigating mitochondrial dysfunction in PD highlight that its pathophysiological heterogeneity as mitochondrial function is normal in many patients afforded this diagnosis [198].
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in chronic fatigue syndrome (CFS)
Immune abnormalities in patients with CFS
Metzger et al. [199] reported evidence of abnormal Th17 T cell activity in driving the symptoms of people within their trial cohort. It is of interest that Th17 cells have a critical role in mucosal defense, with particular functions in gut and respiratory defenses. Other studies examining receptors expressed on the surface of T cells extracted from people with CFS have also provided evidence of impaired T cell activation with a possible Th17 differentiation pattern [200,201]. Other studies report the presence of activated but anergic T cells (Review [6]). Recent evidence has challenged the view that people with CFS display immune abnormalities consistent with a Th2 pattern of T cell differentiation. While some patients present with a Th2 profile and a preponderance of anti-inflammatory cytokine production, others present with a Th1 or possibly Th17 profile, with the synthesis of PICs being dominant [202-204]. Elevated levels of TNF-α and IL-1β are, in fact, particularly commonplace observations in patients recruited into studies using the internationally agreed diagnostic guidelines [202,205-211]. However, some patients also present with elevated levels of Foxp3-expressing regulatory T cells likely in an attempt to counter the proliferation of activated T cells [212,206]. While there is ample evidence that many patients afforded a diagnosis of CFS display profound immunological abnormalities characteristic of a chronically activated but dysregulated peripheral immune system, it must be stressed that some patients with such a diagnosis do not (review [213]). Such disparate often conflicting findings, between and within cohorts, are typical of studies investigating the existence of diverse neuropathology (review [1]). These and other lines of evidence strongly argue that a diagnosis of CFS does not represent a unitary illness with a single pathogenesis and pathophysiology but rather represents a spectrum of illnesses where different pathophysiological processes converge to produce a very similar phenotype [214-217]. This is a core issue across neurobiology, where diagnoses, in the absence of coherent knowledge of pathophysiology, are made on the basis of symptomatology. Nowhere in the rest of medicine does phenomenology parallel pathophysiology, nor should we expect it to do so in neuropsychiatric disorders. The situation is thus made more complex as a diagnosis of CFS is also afforded to people who present with weariness of uncertain or overtly psychological origin either with or without additional non-specific and intermittent symptoms [218-220]. Furthermore, patients afforded a diagnosis of CFS using one of these localized or department-specific protocols are often recruited into studies using predetermined scores on varius non-specific fatigue scales and symptom inventories [218,221]. It must be emphasized that there is therefore no evidence of a consistent pattern immune or neurological abnormalities and, indeed, no evidence of mitochondrial dysfunction in patients afforded a diagnosis of CFS using any of these alternative approaches [213,222-224].
Evidence of chronic oxidative stress in patients with a diagnosis of CFS
Elevated oxidative stress is an almost invariant finding in studies investigating this phenomenon in patients afforded a diagnosis of CFS, with many studies reporting a significant positive correlation between markers of oxidative stress and symptom severity. Several authors have reported that oxidative and nitrosative stress measures demonstrate a significant and positive correlation with symptom severity [225-232]. Miwa and Fujita [233] reported that a fall in the oxidative stress levels of patients corresponded with their transition into remission. Several authors have reported systemic increases in markers of nitrosative and oxidative stress including malondialdehyde, isoprostane, 8-OH-deoxyguanosine, 2,3-diphosphoglyceric acid, thiobutyric acid, and protein carbonyls [225-230,233-235]. iNOS and NO production is significantly increased in many patients relative to levels in normal controls [225,236]. Oxidative imbalance is reported in skeletal muscle, and its severity has been reported to correlate positively with objective measures of muscle fatigability reported by affected patients [237]. Finally, a recent NMR spectroscopy study reported significantly decreased cortical GSH levels in the brains of patients diagnosed according to the Fukuda guidelines [232]. As this review has emphasized, oxidative stress and chronic inflammation, metaphorically like pyrexia, are ubiquitous findings in diverse and seemingly unrelated disorders.
Evidence of mitochondrial dysfunction in patients afforded a diagnosis of CFS
A number of studies investigating bioenergetic performance in patients diagnosed with CFSs have reported evidence of mitochondrial dysfunction, including a loss of mitochondrial membrane integrity and oxidative damage to translocatory proteins in a class of peripheral mononuclear blood cells [238-240]. These findings support earlier work reporting abnormal mitochondrial morphology in muscle biopsy tissue and defects in aerobic metabolism not characteristic of muscle disuse [241-244]. Several authors utilizing 31P NMR spectroscopy to investigate the bioenergetic performance of striated muscles in CFS sufferers have reported profound defects in oxidative phosphorylation, as evidenced by direct or surrogate markers of ATP re-synthesis and low basal levels of ATP production [245-250]. Another line of evidence, once again produced via the use of NMR spectroscopy, demonstrates the existence of abnormal lactate responses to exercise in some patients with CFS [251-253]. Notably, the observed changes in the heart rate of patients coupled with an examination of muscle fiber morphology could not be attributed to deconditioning [252,253]. The pathophysiological heterogeneity within the trial cohorts, however, was striking, with approximately 50% of patients displaying these abnormalities while the other cohort members displayed no metabolic abnormalities in muscle function [251-253]. This gives further support to the view that a diagnosis of CFS, even using internationally agreed criteria as in these studies, does not represent a single illness. This is even more graphically illustrated in a study by Barnes et al. [254], where only 20% of patients were found to have muscle defects in oxidative metabolism. In a recent review, Filler et al. [223] concluded that there was ample evidence of mitochondrial dysfunction and impaired bioenergetic performance in patients afforded a diagnosis of CFS, but once again it was confined to patients diagnosed according to internationally agreed criteria and not apparent in all patients. Vermeulen et al. [255] conducted two exercise tests using cycle ergonometry, on CFS patients on consecutive days, and found that patients attained their anaerobic threshold at a markedly lower oxygen consumption than their putatively healthy counterparts in the first test. Importantly, the anaerobic threshold attained by patients occurred at a much lower oxygen consumption in the subsequent test. These findings were also evidenced in the patients maximal exercise capacity relative to healthy controls, which was also attained at a much lower oxygen capacity than the control group and correlated with differences in ATP production [255]. In a follow-up study, Vermeulen and Vermeulen [256] examined exercise performance in a cohort of CFS patients and reported a loss in the linear relationship between heart rate and cardiac output and the dissipation of oxygen concentration gradient between venous and arterial blood characteristic of mitochondrial dysfunction. Finally, the use of NMR spectroscopy also revealed that some patients display significantly increased ventricular lactate levels, indicative of widespread mitochondrial dysfunction [232,257]. Readers interested in a detailed explanation of the characteristic changes in exercise physiology characteristic of mitochondrial dysfunction are referred to previous studies [1,258]. Again, as with oxidative stress, these increases in lactate are found in seemingly divergent disorders that are not overtly mitochondrial in nature, including schizophrenia [259], and reflect a shift to anaerobic or possibly aerobic glycolysis as a mode of ATP generation.
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in bipolar disorder
Evidence of immune dysfunction in bipolar disorder
Many lines of evidence converge to suggest a role of immune dysregulation in bipolar disorder. Bipolar disorder is commonly associated with autoimmune disorders including MS, thyrotoxicosis, ulcerative colitis, psoriasis, and rheumatoid arthritis [260]. To date, a number of studies have consistently shown that there are elevated C-reactive protein levels in bipolar disorder, both in acute mania and remission [261,262]. Similarly, TNF-α and IL-6 have shown consistent patterns of elevation in the disorder. Interestingly, there is a suggestion of stage-specific changes in these markers, with elevated levels of PICs in early and late stages, but loss of elevated IL-10, an anti-inflammatory cytokine, in late stage illness [263,264]. A recent meta-analysis showed that there are higher concentrations of TNF-α, soluble IL-2 receptor, and soluble TNF receptor type 1 in bipolar patients than in controls [265]. The study did not find significant differences between bipolar disorder patients and healthy control subjects for IL-1, IL-2, IL-5, IL-6, IL-8, IL-10, IL-12, IL-1β, IL-1 receptor antagonist, IFN-γ, transforming growth factor-β1 (TGF-β1), and TNF receptor type 2 [265]. A range of anti-inflammatory agents, including aspirin, minocycline, N-acetylcysteine, curcumin, anti-TNF-α agents, celecoxib, and omega-3 fatty acids are being investigated as an adjunct to treatment as usual for use in mood disorders, and the extant, albeit preliminary, evidence shows promise [266]. Finally, many of the known risk factors for the development of mood disorders drive systemic inflammation, including physical inactivity, stress, poor diet, obesity, smoking, atopy, altered gut permeability, dental caries, vitamin D deficiency, and dysregulated sleep [35].
Evidence of chronic oxidative stress in patients with a diagnosis of bipolar disorder
There is consistent evidence from peripheral marker studies that the brain’s primary antioxidants, GSH, catalase (CAT), superoxide dismutase (SOD), and GSH peroxidase are altered in those with bipolar disorder [267]. In addition, there is post-mortem data that GSH is depleted in those individuals who have bipolar disorder, as well as in people with schizophrenia [268]. In parallel, there is now meta-analysis level data showing increased markers of oxidative stress. The most consistent findings are increased lipid peroxidation, DNA/RNA damage, and raised NO in bipolar disorder compared to controls, with high effect sizes for lipid peroxidation [269]. Oxidative damage to proteins (protein carbonylation) is also consistently shown [270]. Clinical data suggests there are correlations between illness severity and the extent of oxidative stress, such that those with greater illness duration, and a larger number of prior episodes show decreased antioxidant defenses [264,271]. Atypical antipsychotic drugs, as a class, possess redox-active properties although the extent to which they mediate their pharmacological benefits is uncertain [272,273]. Lithium and valproate also have extensive effects on oxidative markers [274,275]. Remission in bipolar depression was mirrored by increases in oxidative defenses and reductions in oxidative stress measures [276]. Lastly, many of the known environmental precipitants and risk factors for depression appear to be transduced via redox signaling [277].
Mitochondrial dysfunction and bioenergetic abnormalities in bipolar disorder
Of all the disorders mentioned in this review, bipolar disorder has the highest face validity as a primary disorder or mitochondrial bioenergetics, being a biphasic disorder of symptomatically increased and decreased energy and activity. Mania is known to be associated with increased brain energy generation, while depression is associated with decreased energy generation [278]. Patients with bipolar disorder have a higher prevalence of primary mitochondrial disorders than the general population, particularly mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes [279]. Abnormalities in brain and lymphocyte mitochondrial distribution and morphology using electron microscopy have been observed in bipolar disorder [280]. Proton magnetic resonance imaging studies show an increased brain glutamate/glutamine ratio in bipolar disorder [281]. This increased excitatory glutamate creates a high energy demand. Additionally, elevated lactate levels and decreased intracellular pH suggest a shift to glycolysis and imply dysregulation of mitochondrial bioenergetics [282,283]. Furthermore, there is evidence of changes in expression of genes encoding for mitochondrial complexes, particularly Complex I and IV [284,285]. Mitochondrial dysfunction is thus a target of novel therapeutic endeavors in this disorder [286].
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in major depression (MDD)
Evidence of immune dysfunction in MDD
MDD is characterized by evidence of activated cell-mediated immunity with many patients demonstrating (Th1) style response with elevated levels of IFN-γ [287,288]. Several meta-analyses and numerous recent studies have demonstrated elevated levels of IL-1β, TNF-α, and IL-6 together with increased levels of neopterin and soluble IL-2 receptors, which globally indicate increased cell-mediated immunity and macrophage activity [289-293]. However, there is evidence of biologically distinct MDD subtypes where Th2 cytokines predominate [294]. Investigating therapeutic responses to the antidepressant duloxetine, the existence of patients with a Th2-biased cytokine profile whose positive response to treatment was indicated by a Th1 shift in their cytokine profile was reported; this contrasted with other patients whose baseline cytokine profile was characteristic of a Th1 profile which shifted towards a Th2 cytokine pattern in response to treatment [294]. This supports very similar findings in an earlier study by the same authors [295] and is in line with the work of other researchers where the positive effects of treatment were evidenced by increased levels of TNF-α and decreased levels of IL-4 [296]. Other findings include evidence of increased numbers of circulating Th17 T cells, diminished numbers of regulatory T cells (Tregs), and a significantly increased Th17/Treg ratio [297].
Evidence of oxidative stress in patients with a diagnosis of MDD
Nitrosative and oxidative stress are now considered to play a major role in the pathophysiology of MDD [298-301]. It seems likely that this state arises as a result of elevated production of ROS and RNS and compromised cellular antioxidant defenses. Galecki et al. [302] reported elevated levels of SOD and CAT activity and a global deficit of antioxidant defenses [302]. Deficiencies have also been reported in other antioxidant compounds such as vitamins C and E [303-305]. There is also evidence of widespread lipid peroxidation in crucial areas of the brain such as the prefrontal cortex whose levels in female patients correlate with the severity of symptoms [306,307]. The existence of lipid peroxidation is further evidenced by high levels of serum malondialdehyde and oxidative damage to lipids in peripheral tissues [302,308]. Elevated levels of urine and plasma isoprostane and 8-oxo-2’-deoxyguanosine have also been reported, which bears testimony to excessive levels of ROS and RNS produced outside the brain [303-305]. It is significant that the concentration of oxidative stress markers in the periphery correlates positively with the chronicity and severity of the illness irrespective of patient gender [299,307-309]. Given such a relationship, it is not surprising that antioxidant compounds are being trialed as potential antidepressant treatments [310,311]. The level of oxidative damage to lipids and DNA is sufficient to form neoepitopes and provoke antibody responses [312,313]. The presence of oxidative damage to mtDNA bears further testimony to the severity of oxidative stress in sufferers of this illness [314,315].
Evidence of bioenergetic impairments and mitochondrial dysfunction in patients with MDD
As previously discussed, there is copious evidence implicating the activation of immuno-inflammatory pathways and chronic oxidative and nitrosative stress in the genesis, persistence, and severity of MDD [316-320]. However, there is a growing awareness that the archetypal symptoms of MDD, such as neurocognitive impairment, sleep disturbances lethargy, fatigue, and loss of motivation, may also be driven by mitochondrial dysfunction primarily in the domain of the ETC [321-323]. This is perhaps expected as the bidirectional association between elevated PICs, chronic oxidative and nitrosative stress, and mitochondrial dysfunction has been clearly established and will be discussed in detail below [1,36]. There is also accumulating evidence that mitochondrial dysfunction plays a role in the etiology of the illness.
Several neuroimaging studies utilizing PET or SPECT technology have detected impaired bioenergetic metabolism in numerous regions of MDD patient brains, notably in the basal ganglia and prefrontal cortices [6,321]. Other authors have reported widespread abnormalities in blood flow, energy and glucose metabolism, as well as a reliance on glycolysis as a source of ATP production [324-327,321]. A number of studies have demonstrated the existence of mitochondrial dysfunction in the peripheral tissues of MDD patients, which is of interest, as it would be expected in light of growing data implicating systemic inflammation in the genesis of the illness [317]. For example, Gardner et al. [328] demonstrated that striated muscle mitochondria of patients with MDD, who concomitantly presented with physical symptoms, synthesized a significantly lower amount of ATP and showed impairments in respiratory chain enzyme activity, particularly at Complex III and IV [328]. Hroudová et al. [329] were the first research team to demonstrate mitochondrial dysfunction in the peripheral mononuclear blood cells of MDD patients, and this finding has been confirmed in an even more recent study by Karabatsiakis et al. [330]. These authors assessed mitochondrial respiration in intact peripheral blood mononuclear cells via the use of high-resolution respirometry using a healthy volunteer control group [330]. They demonstrated that MDD patients displayed grossly impaired mitochondrial functioning along several dimensions. Importantly, mitochondrial respiration correlated significantly and negatively with the severity of many depressive symptoms, notably loss of energy, fatigue, and difficulties concentrating strongly, suggesting a causative role for ATP shortage in the genesis of such symptoms [330].
Immune dysfunction, oxidative stress, and mitochondrial dysfunction in schizophrenia
Evidence of immune dysfunction in schizophrenia
A range of immunological abnormalities have been detected by several authors in patients with schizophrenia [331,332]. Most researchers have focused on levels of plasma cytokines following mitogenic stimulation of peripheral blood mononuclear cells, and have broadly revealed the existence of a Th2-biased immune system, although the detailed picture is somewhat mixed [333-336]. However, more recent data also indicates the presence of elevated levels of TNF-α, IL-1β, and IL-6 in treatment-naive patients, which appear to drive the genesis and maintenance of neuroinflammation in at least some patients [337-340]. Moreover, accumulating data also indicates the existence of elevated numbers of effector and memory Th17 cells which are responsible for the development of neuropathology and autoimmunity in other illnesses [337]. The probable contribution of Th17 T cells to the pathophysiology of schizophrenia was highlighted in a recent study by Ding et al. [338]. These authors reported the presence of activated Th17 T cells in drug-naive first episode schizophrenia patients and also noted a significant positive relationship between the proportion of activated Th17 cells and the levels of IL-17, TNF-α, IL-6, and INF-γ with the negative symptom on the positive and negative syndrome scale [338]. Perhaps even more importantly, the proportion of Th17 cells decreased in patients displaying a positive response to risperidone which correlated positively with the change in score [338].
Evidence of oxidative stress in schizophrenia
Chronic systemic inflammation and oxidative stress is an invariant feature of schizophrenia [337,341,342]. It is now recognized that levels of inflammation and oxidative stress correlate with the level of cognitive impairment in patients with first episode schizophrenia [343]. It would also appear that levels of oxidative stress correlate with severity of positive symptoms [344]. It probably unsurprising to learn that oxidative and nitrosative stress is causatively implicated in the pathogenesis and pathophysiology of the illness [345-347]. Numerous authors have reported the presence of oxidative damage to proteins, lipids, and DNA [346,348]. There is evidence of ROS and RNS overproduction and reduced levels of antioxidants [349,350]. Numerous research teams have detected the presence of oxidative stress in the prefrontal cortex and CSF in vivo [351]. Post-mortem studies have revealed the presence of this phenomenon in the anterior cingulate cortex [352].
The presence of oxidative stress is not confined to the brains of those with schizophrenia, but is also found in plasma and peripheral tissues of patients [353-355]. Specific abnormalities include elevated levels of malondialdehyde and NO coupled with significantly reduced levels of GSH relative to healthy controls [344,356,357]. It is worthy of note that the presence of thiobarbituric acid reactive substances and protein carbonyls are seen both in the early and late stages of the disease [358]. It is also worthy of note that the presence and levels of molecules signifying the presence of oxidative and nitrosative stress correlate with the enzymatic activity of Complex I [359]. It is fair to state, however, that some controversy remains regarding the activities and levels of enzymatic antioxidant activity evaluated by SOD, CAT, and GSH peroxidase, with normal and abnormal levels and activities being reported by different research teams [360-362]. Reflecting the classification of Davis [363], a recent meta-analysis suggested that total antioxidant status, red cell catalase, and plasma nitrite are potential state or acuity markers of the disorder, while others, including red cell SOD, are trait markers [364].
Evidence of bioenergetic impairments and mitochondrial dysfunction in schizophrenia
Several authors demonstrated a range of ultrastructural abnormalities in the mitochondria of patients suffering from schizophrenia, which may play a major role in its pathogenesis [365,366]. Many of these studies have relied on the examination of post-mortem brain tissue by electron microscopy and abnormalities reported have included denuded numbers, reduced density, and significant defects in cross-sectional profiles [367,368]. Enlarged mitochondria with disrupted cristae have been detected in astrocytes of patients with longstanding illness, and may be the source of the progressive astrocyte dysfunction seen in the illness [369]. Interestingly, ultrastructural abnormalities in these organelles are not confined to the brain, as impaired mitochondrial numbers and density are also evident in peripheral blood mononuclear cells [370,371]. Abnormalities in Complex I activity are also a frequently reported finding [372,373]. The pattern of these abnormalities in the brain is somewhat inconsistent, but dependent on the location and function of the tissue sampled [374-377]. A recent study conducted by Akarasu et al. [378] detected Complex I hyperactivity in peripheral blood from schizophrenia patients, which the authors proposed as a diagnostic marker for the illness. Increased activity of Complex I in peripheral tissues was reported in an earlier study [375]. The evidence demonstrating impaired activity of mitochondrial complex enzymes induced by conformational changes in the frontal or prefrontal cortex appears to be more consistent, however, with the decreased activity of Complex I, III, and IV [377,379-381].
Impaired bioenergetics and an increase in glycolytic ATP production secondary to mitochondrial dysfunction has been detected in the post-mortem brains of schizophrenia patients via the use of NMR spectroscopy [382-384]. The same technique has also revealed elevated levels of lactate and a decrease in levels of pyruvate dehydrogenase, indicative of mitochondrial dysfunction and a shift to energy production via glycolysis in the CSF of patients with this illness [385,386]. Several studies have reported a positive association between a range of defects in the mitochondrial genome and the development of schizophrenia [387,388]. There is also a growing body of evidence suggesting an increased maternal transmission of schizophrenia, which may indirectly indicate a potential role for mitochondrial inheritance in the etiology of the illness [389,390]. Numerous authors have demonstrated an association between certain mitochondrial haplotypes and disease risk, and the associations also extend to age of onset[390]. In a similar vein, functional single nucleotide polymorphisms in mitochondrial genes encoding for Complex I, ATP synthase, and Cox subunits also confer an increased risk of developing the illness [391-393]. Table 1 provides a summary of the various mitochondrial and bioenergetic abnormalities recorded in the illnesses discussed above.
Table 1.
Range of mitochondrial abnormalities seen in MS, CFS, PD, AUT, MDD, Schiz and BPD
| Mitochondrial abnormality | MS | CFS | PD | AUT | MDD | Schiz | BPD |
|---|---|---|---|---|---|---|---|
| Ultrastructural abnormalities in mitochondria | + | ++ | + | + | + | +++ | + |
| Evidence to shift in energy production via glycolysis | + | ++ | ++ | ++ | ++ | ++ | |
| Mitochondrial dysfunction in peripheral immune cells | + | ++ | ++ | ++ | ++ | ++ | + |
| Mitochondria DNA damage | + | ++ | |||||
| Damage to the electron transport chain | ++ | +++ | + | ++ | + | ||
| High lactate in brain or cerebrospinal fluid | + | + | + | + | + | ++ | + |
| Bioenergetic impairment Skeletal muscle | +++ | + | |||||
| Decreased mitochondrial membrane potential | + | + |
MS, Multiple sclerosis; CFS, Chronic fatigue syndrome; PD, Parkinson’s disease; AUT, Autism; MDD, Major depression; Schiz, Schizophrenia; BPD, Bipolar disorder.
The role of NO peroxynitrite and proinflammatory cytokines in the genesis of mitochondrial dysfunction and impaired oxidative metabolism
Nitric oxide (NO)-mediated impairment of energy production
NO impacts on mitochondrial performance and levels of ATP production in a number of ways, notably, by playing a major role in governing oxygen delivery [394,395] and, crucially, by inhibiting the performance of the ETC. NO inhibits the activity of Complex IV via a number of mechanisms, not least by acting as a competitive antagonist for the binding of oxygen to the enzyme’s active site [396-398]. NO also inhibits electron transfer between cytochrome b and c, namely the electron transfer at Complex III [399,400], which directly leads to increased production of ROS [401]. Finally, NO inhibits NADH dehydrogenase activity and electron transfer at Complex I [399,402,403]. Although the reaction with Complex III is somewhat ponderous [404], the reaction between Complex I and Complex IV, otherwise known as cytochrome c oxidase (COX), is extremely rapid and generally reversible. It is worth noting at this juncture that inhibition of these components of the ETC can be a source of pathology other than by direct inhibition of ATP production. Both reactions produce a number of derivatives responsible for generating the nitrosative stress of mitochondrial origin observed in a number of different illnesses, mainly neuroimmune or autoimmune [397,401,405-407]. NO reacts with the iron and copper ions of the heme-CuB and sulfhydryl groups located at the active site of COX [408-411], while inhibition of Complex I stems from the S-nitrosylation of Cys39 prominent on the ND3 subunit [410,412,413].
The inhibition of Complex I and COX is normally reversible but is less so following prolonged excessive production of NO [402,413,414]. While the reaction of NO with both ETC complexes is extremely rapid compared to the reaction with Complex III, inhibition of Complex IV following exposure to NO occurs within seconds or milliseconds [415], while inhibition of Complex I may occur within minutes [413]. The onset of NO inhibition on Complex I is slow (minutes) [413], whereas on COX is very fast (milliseconds to seconds) [415,396]. As previously discussed, when excessively elevated levels of NO persists in the cellular environment, enabling prolonged nitrosylation, the inhibition of COX and Complex I may become virtually irreversible [416-419], leading to a substantial inhibition of the ETC, impaired oxidative phosphorylation, and a decreased synthesis of ATP [1,36,396,420]. This situation leads to the induction of glycolysis even in an environment of abundant oxygen in an attempt to compensate for loss of ATP [421,422]. This is very similar, in principle, to the Warburg effect [423], and the weight of evidence suggests that the efficiency of this compensatory mechanism varies with cell type and location [424,425]. Prolonged nitrosylation of COX, however, likely overcomes this compensatory mechanism leading to a situation of chronic ATP depletion [396]. NO also has a positive effect on net ATP production by playing a crucial role in mitochondrial biogenesis within skeletal muscle [384]. This positive contribution is considered to be mediated by its capacity to upregulate the transcription of peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1α) [426-428]. In an inflammatory environment, however, this stimulatory effect is countered by the presence of elevated levels of TNF-α, which reduces the expression of PGC-α, a key regulator of energy metabolism, via the upregulation of NF-kappaB and p38 MAPK kinases [132,429,430]. Moreover, inhibition of the ETC predisposes to the excessive production of superoxide anions, which react with NO to form the highly dangerous peroxynitrite [1,36,402]. This reactive species has the capacity to compromise virtually every element and system involved in the generation and regulation of energy production as we will now illustrate.
Peroxynitrite-mediated impairment of energy production
Peroxynitrite has a much longer half-life than its molecular ancestors and is much more reactive [431,432]. Thiol oxidation and nitration of tyrosine residues are the major mechanism by which peroxynitrite induces conformational change in proteins [433,434]. Peroxynitrite also causes oxidative damage to mitochondrial structural proteins and enzymes and peroxidative damage to lipids within membranes leading to profound changes in function and membrane integrity [1,435,436]. Peroxynitrite inhibits mitochondrial respiration by inactivation of ETC I and III [437,438]. Inactivation of mitochondrial electron transport enzymes increases mitochondrial production of superoxide and hydrogen peroxide generated by mitochondria [437], creating adaptive and synergistic damage [439].
Peroxynitrite can make an indirect contribution towards mitochondrial dysfunction by inhibiting SOD [440] and glutaredoxin [441], and by oxidizing reduced GSH and other thiols [442-444]. GSH depletion, in turn, exacerbates peroxynitrite-induced pathology [445]. This intimate bidirectional relationship with oxidative and nitrosative stress is reviewed in Morris and Maes [1] and Morris et al. [36]. The oxidation of critical cysteine groups by this highly reactive species inactivates a plethora of enzymes playing indispensable roles in bioenergetic processes, including glyceraldehyde-3-phosphate dehydrogenase [446,447], NADH dehydrogenase [403], creatine kinase [448], succinate dehydrogenase [449], cytochrome c reductase [450], and ATP synthase [451,452]. These enzymes are also inactivated by nitration of tyrosine damage to their iron sulfur centres [451,452] and, thus, are highly prone to inactivation by chronically elevated levels of peroxynitrite. The redox activity of cytochrome C is severely impaired by nitration and, hence, this cytochrome is also very readily disabled in an environment of chronically elevated peroxynitrite. Nitration of cytochrome c significantly elevates its peroxidatic activity, leading to increased synthesis of hydrogen peroxide accelerating further oxidative corruption of mitochondrial proteins [453,435]. Peroxynitrite also disrupts the ferrous-sulfur active site of the tricarboxylic acid cycle enzyme aconitase, leading to its inhibition and impairing ATP production [454,455]. The enzyme nicotinamide nucleotide transhydrogenase, which catalyzes the reduction of NAD, is another crucial mitochondrial enzyme readily inactivated by peroxynitrite-mediated nitration and oxidation [456]. The subsequent depletion of NADPH impairs the ability of mitochondria to further regenerate reduced GSH, exacerbating the pre-existing oxidative stress within the organelle [457,458]. Chronically elevated levels of peroxynitrite lead to mitochondrial membrane depolarization [459,460], which is probably mediated by thiol oxidation of cysteine residues of proteins within the permeability transition pore complex [461,457].
Peroxynitrite can inhibit cellular energy production via yet another mechanism, the activation of poly [ADP-ribose] polymerase 1 [462,463], the chronic activation of which leads to impoverished levels of NAD+, an essential cofactor enabling the performance of the tricarboxylic acid cycle, glycolytic pathway, and the ETC [462,464-466]. Depletion of NAD+ thus results in severely diminished cellular ATP stores, resulting in profound cellular dysfunction [467,468]. Peroxinitrite can also grossly impair function of p53 by inducing conformational change in the transcription factor’s tertiary structure [469-471]. This altered structure impairs or even eliminates the capacity of the p53 protein to bind to DNA and thus exert its normal functions [472,469]. p53 plays a crucial role in coordinating increases in cellular metabolic activity to match increasing energy demands [473-475]. Loss of p53 facilitates the switch to anaerobic glycolysis as a source of ATP [476,477], resulting in dramatically reduced oxygen uptake and mitochondrial respiration [478] and a markedly diminished capacity for exercise [479]. Elevated levels of peroxynitrite can also impact the activity of proteins with a regulatory role in mitochondrial function, such as parkin and DJ-1, by inducing conformation changes leading to their loss of function or affecting post-translational signaling mechanisms rendering their protective actions ineffective [480]. Schematic representations of the deleterious effects of chronically elevated levels of ROS and RNS on mitochondrial function and energy production are presented in Figures 1 and 2 below.
Figure 1.
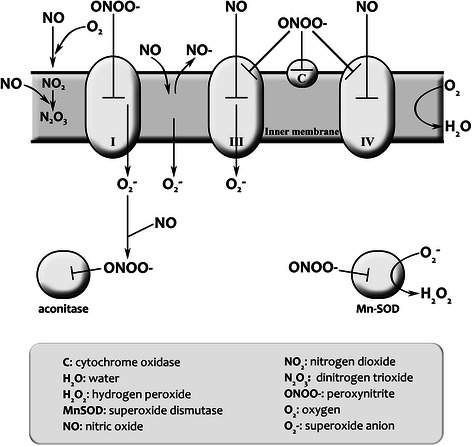
Schematic representation of the inhibitory effects of NO and ONOO- on the ETC, enzymes of the tricarboxylic cycle and antioxidant enzymes. NO and peroxynitrite inhibit the mitochondrial respiration via different mechanisms: NO itself causes selective, rapid, potent, but readily reversible inhibition of cytochrome oxidase and increased production of RNS within the intermembrane space. On the other hand, excessive levels of peroxynitrite and other RNS leads to slow, weak non-selective, but essentially irreversible inhibition of a wide range of mitochondrial components. Peroxynitrite inhibits Complex I, Complex II, cytochrome oxidase ATP synthase, MnSOD, aconitase, creatine kinase, and a plethora of other proteins playing an essential role in energy production. In addition, peroxynitrite is a potent oxidant capable of inducing peroxidation of mitochondrial membrane lipid components, hence increasing membrane permeability and disrupting the potential difference between the inner and outer membrane and inducing mitochondrial membrane transition. Inhibition of ATP production and electron chain dysfunction leads to the production of ever increasing production of ROS and RNS leading to a vicious circle culminating in eventual bioenergetic failure and often cellular necrosis or apoptosis.
Figure 2.
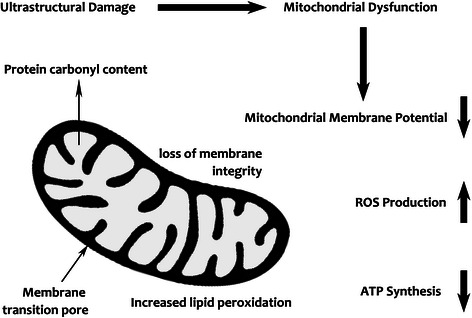
Mitochondrial ultrastructural damage and impaired capacity for energy generation in an environment of chronic nitro-oxidative stress. Excessive levels of peroxynitrite cause oxidative and peroxidative damage to lipids and proteins, leading to profound ultrastructural damage, including disrupted cristae, loss of outer membrane integrity, mitochondrial permeability transition, and uncoupling of ETC activity from oxidative phosphorylation.
Pro-inflammatory cytokine (PIC)-mediated impairment of energy production
The adverse effects of elevated levels of TNF-α on mitochondrial biogenesis have already been discussed; however, excessive levels of TNF-α and other PICs, typifying a state of chronic systemic inflammation, can additionally disable oxidative phosphorylation [1]. This is evidenced by elevated levels of lactate levels and impaired mitochondrial function characteristic of chronic inflammatory states [481-483]. It is worthy of note, however, that this PIC-mediated suppression of ETC function is ultimately mediated by NO via a number of different mechanisms [484,485]. PICs can also inhibit mitochondrial respiration directly. TNF-α, one of the major PICs, can block electron transfer at Complex I [486,487], Complex III [488,489], and COX [490-492], leading to a significant reduction in the rate of respiration and the activities of the enzymes in the ETC [492,493]. TNF-α increases mitochondrial membrane permeability leading to membrane depolarization, increased intracellular calcium and a marked decrease in mitochondrial membrane potential [494,495], and increased generation of ROS [496,497]. TNF-α and IL-1β collude to inhibit the ETC and suppress pyruvate dehydrogenase activity [498,499]. IL-1β and TNF-α, acting in concert, have also been shown to increase aerobic glycolysis and inhibit oxidative phosphorylation [499]. Prolonged excessive levels of TNF-α also induces the development of aerobic glycolysis and appears to be yet another mechanism for inducing Warburg-like metabolism, whereby cells predominantly generate energy by glycolytic, non-oxidative breakdown of glucose, in an environment of excessive oxidative stress [490,491].
Summary
This paper has detailed some of the evidence demonstrating the existence of immune dysfunction, oxidative stress, and mitochondrial dysfunction in many patients diagnosed with MS, PD, autism, bipolar disorder, depression, schizophrenia, and CFS. It is proposed that these apparently non-specific findings may contribute to the pathophysiology in each illness. Excessive levels of peroxynitrite, NO, and PICs clearly have the capacity to inhibit the activity of the ETC at several points, alone or synergistically, leading to the depletion of ATP production and promoting a switch to anaerobic glycolysis. Peroxynitrite and TNF-α can also depolarize the mitochondrial membrane via a number of different mechanisms once again having a deleterious effect on the generation of ATP. Peroxynitrite in particular can damage lipids and proteins, altering their conformation and function, causing structural damage to integral mitochondrial proteins and lipid membranes and to proteins regulating the function of the organelle. The capacity of peroxynitrite to inactivate a range of enzymes with an essential function in the generation of energy and the regulation of energy generation, such as p53, can provide other pathways to impaired oxidative metabolism. However, peroxynitrite is not alone in its ability to impair the activity of essential transcription factors as evidenced by the capacity of TNF-α to inhibit the production of PGC-1α and indirectly impair activity-stimulated mitochondrial biogenesis. It must be emphasized, however, that the presence of these inflammatory entities in an environment of oxidative stress is highly unlikely to be the sole cause of the mitochondrial dysfunction and impaired energy production seen in people with these illnesses. Genetic and epigenetic factors are also surely involved. Consequently, it is impossible to calculate the extent of the contribution that these entities make to the phenomenon of bioenergetic impairment seen in these apparently disparate illnesses, but it is likely that they play at least a part.
Acknowledgements
MB is supported by a NHMRC Senior Principal Research Fellowship 1059660.
Abbreviations
- ATP
Adenosine triphosphate
- BBB
Blood brain barrier
- CAT
Catalase
- CFS
Chronic fatigue syndrome
- CNS
Central nervous system
- COX
Cytochrome c oxidase
- CSF
Cerebrospinal fluid
- CWA
Children with autism
- EDSS
Expanded disability status scale
- ETC
Electron transport chain
- GSH
Glutathione
- IFN-γ
Interferon-γ
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- MDD
Major depression
- MS
Multiple sclerosis
- NO
Nitric oxide
- PD
Parkinson disease
- PGC-1α
Proliferator-activated receptor gamma coactivator-1
- PIC
Pro-inflammatory cytokine
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TGF-β1
Transforming growth factor-β1
- Th
T helper
- TNF-α
Tumor necrosis factor-alpha
- Treg
T regulatory
Footnotes
Competing interests
No specific funding was obtained for this review. MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier and Woolworths, has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth, and served as a consultant to Astra Zeneca, Bioadvantex, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck Merck and Servier.
Authors’ contributions
GM and MB participated in the design of this review and contributed equally to this paper. Both authors read and approved the final version.
Contributor Information
Gerwyn Morris, Email: activatedmicroglia@gmail.com.
Michael Berk, Email: mikebe@barwonhealth.org.au.
References
- 1.Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29:19–36. doi: 10.1007/s11011-013-9435-x. [DOI] [PubMed] [Google Scholar]
- 2.Guo T, Chen H, Liu B, Ji W, Yang C. Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet Test Mol Biomarkers. 2012;16:968–73. doi: 10.1089/gtmb.2012.0091. [DOI] [PubMed] [Google Scholar]
- 3.Pagano G, Castello G, Pallardó FV. Sjøgren’s syndrome-associated oxidative stress and mitochondrial dysfunction: prospects for chemoprevention trials. Free Radic Res. 2013;47:71–3. doi: 10.3109/10715762.2012.748904. [DOI] [PubMed] [Google Scholar]
- 4.López-Erauskin J, Galino J, Bianchi P, Fourcade S, Andreu AL, Ferrer I, et al. Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain. 2012;135:3584–98. doi: 10.1093/brain/aws292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perl A, Hanczko R, Doherty E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. Methods Mol Biol. 2012;900:61–89. doi: 10.1007/978-1-60761-720-4_4. [DOI] [PubMed] [Google Scholar]
- 6.Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013;11:205. doi: 10.1186/1741-7015-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciccone S, Maiani E, Bellusci G, Diederich M, Gonfloni S. Parkinson’s disease: a complex interplay of mitochondrial DNA alterations and oxidative stress. Int J Mol Sci. 2013;14:2388–409. doi: 10.3390/ijms14022388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150. doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasset I, Agüera E, Sánchez-López F, Feijóo M, Giraldo AI, Cruz AH, et al. Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin Biochem. 2012;45:440–4. doi: 10.1016/j.clinbiochem.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–9. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira SR, Kallaur AP, Simão AN, Morimoto HK, Lopes J, Panis C, et al. Oxidative stress in multiple sclerosis patients in clinical remission: association with the expanded disability status scale. J Neurol Sci. 2012;321:49–53. doi: 10.1016/j.jns.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Kalman B, Laitinen K, Komoly S. The involvement of mitochondria in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;188:1–12. doi: 10.1016/j.jneuroim.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Pinniger GJ, Bakker AJ, Moss TJ, Noble PB, Berry CA, et al. Lipopolysaccharide-induced weakness in the preterm diaphragm is associated with mitochondrial electron transport chain dysfunction and oxidative stress. PLoS One. 2013;8:e73457. doi: 10.1371/journal.pone.0073457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Pavliv O, et al. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One. 2014;9:e85436. doi: 10.1371/journal.pone.0085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galley HF. Bench-to-bedside review: targeting antioxidants to mitochondria in sepsis. Crit Care. 2010;14:230. doi: 10.1186/cc9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 17.Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014;50:1059–84. doi: 10.1007/s12035-014-8705-x. [DOI] [PubMed] [Google Scholar]
- 18.Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–99. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–8. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 20.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–40. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci. 2012;69:2409–27. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–61. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 23.Honorat JA, Kinoshita M, Okuno T, Takata K, Koda T, Tada S, et al. Xanthine oxidase mediates axonal and myelin loss in a murine model of multiple sclerosis. PLoS One. 2013;8:e71329. doi: 10.1371/journal.pone.0071329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prolo C, Alvarez MN, Radi R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. Biofactors. 2014;40:215–25. doi: 10.1002/biof.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaziri ND. Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis. 2008;2:1–10. [PubMed] [Google Scholar]
- 27.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286:6627–40. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, Ramírez-Anguiano AC, Flores-Alvarado LJ, Ramírez-Ramírez V, et al. Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol. 2013;2013:708659. doi: 10.1155/2013/708659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas K, Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang O. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 2013;22:11–7. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witherick J, Wilkins A, Scolding N, Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010;2011:164608. doi: 10.4061/2011/164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–24. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 35.Steel C, Breving K, Tavakoli S, Kim W, Sanford L, Ciavarra R. Role of peripheral immune response in microglia activation and regulation of brain chemokine and proinflammatory cytokine responses induced during VSV encephalitis. Journal Of Neuroimmunology. 2014;267(1-2):50–60. doi:10.1016/j.jneuroim.2013.12.002. [DOI] [PMC free article] [PubMed]
- 36.Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) Curr Neuropharmacol. 2014;12:168–85. doi: 10.2174/1570159X11666131120224653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris G, Maes M. A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis. 2013;28:523–40. doi: 10.1007/s11011-012-9324-8. [DOI] [PubMed] [Google Scholar]
- 38.Su X, Federoff HJ. Immune responses in Parkinson’s disease: interplay between central and peripheral immune systems. Biomed Res Int. 2014;2014:275178. doi: 10.1155/2014/275178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari CC, Tarelli R. Parkinson’s disease and systemic inflammation. Parkinsons Dis. 2011;2011:436813. doi: 10.4061/2011/436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunnon K, Teeling JL, Tutt AL, Cragg MS, Glennie MJ, Perry VH. Systemic inflammation modulates Fc receptor expression on microglia during chronic neurodegeneration. J Immunol. 2011;186:7215–24. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- 41.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–86. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 42.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 2010;16:2766–78. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 43.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Reale M, Greig NH, Kamal MA. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev Med Chem. 2009;9:1229–41. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009381. doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura M, Matsuoka T, Chihara N, Miyake S, Sato W, Araki M, et al. Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult Scler. 2014;20:1371–80. doi: 10.1177/1352458514523496. [DOI] [PubMed] [Google Scholar]
- 47.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 48.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 49.Romme Christensen J, Börnsen L, Hesse D, Krakauer M, Sørensen PS, Søndergaard HB, et al. Cellular sources of dysregulated cytokines in relapsing-remitting multiple sclerosis. J Neuroinflammation. 2012;9:215. doi: 10.1186/1742-2094-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations. Acta Neurol Scand. 1988;78:318–23. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 51.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-G. [DOI] [PubMed] [Google Scholar]
- 52.Navikas V, Link H. Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996;45:322–33. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Martins TB, Rose JW, Jaskowski TD, Wilson AR, Husebye D, Seraj HS, et al. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. Am J Clin Pathol. 2011;136:696–704. doi: 10.1309/AJCP7UBK8IBVMVNR. [DOI] [PubMed] [Google Scholar]
- 54.Gold SM, Krüger S, Ziegler KJ, Krieger T, Schulz KH, Otte C, et al. Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. J Neurol Neurosurg Psychiatry. 2011;82:814–8. doi: 10.1136/jnnp.2010.230029. [DOI] [PubMed] [Google Scholar]
- 55.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77:34–9. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler. 2004;10:165–9. doi: 10.1191/1352458504ms991oa. [DOI] [PubMed] [Google Scholar]
- 57.Disanto G, Berlanga AJ, Handel AE, Para AE, Burrell AM, Fries A, et al. Heterogeneity in multiple sclerosis: scratching the surface of a complex disease. Autoimmune Dis. 2010;2011:932351. doi: 10.4061/2011/932351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucchinetti CF, Brück W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–74. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gironi M, Borgiani B, Mariani E, Cursano C, Mendozzi L, Cavarretta R, et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res. 2014;2014:961863. doi: 10.1155/2014/961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller E, Walczak A, Saluk J, Ponczek MB, Majsterek I. Oxidative modification of patient’s plasma proteins and its role in pathogenesis of multiple sclerosis. Clin Biochem. 2012;45:26–30. doi: 10.1016/j.clinbiochem.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 61.Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 62.Stavropoulou C, Zachaki S, Alexoudi A, Chatzi I, Georgakakos VN, Terzoudi GI, et al. The C609T inborn polymorphism in NAD(P)H:quinone oxidoreductase 1 is associated with susceptibility to multiple sclerosis and affects the risk of development of the primary progressive form of the disease. Free Radic Biol Med. 2011;51:713–8. doi: 10.1016/j.freeradbiomed.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 63.Bizzozero OA, DeJesus G, Callahan K, Pastuszyn A. Elevated protein carbonylation in the brain white matter and gray matter of patients with multiple sclerosis. J Neurosci Res. 2005;81:687–95. doi: 10.1002/jnr.20587. [DOI] [PubMed] [Google Scholar]
- 64.Greco A, Minghetti L, Sette G, Fieschi C, Levi G. Cerebrospinal fluid isoprostane shows oxidative stress in patients with multiple sclerosis. Neurology. 1999;53:1876–9. doi: 10.1212/WNL.53.8.1876. [DOI] [PubMed] [Google Scholar]
- 65.Toshniwal PK, Zarling EJ. Evidence for increased lipid peroxidation in multiple sclerosis. Neurochem Res. 1992;17:205–7. doi: 10.1007/BF00966801. [DOI] [PubMed] [Google Scholar]
- 66.Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, et al. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–7. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- 67.Mattsson N, Haghighi S, Andersen O, Yao Y, Rosengren L, Blennow K, et al. Elevated cerebrospinal fluid F2-isoprostane levels indicating oxidative stress in healthy siblings of multiple sclerosis patients. Neurosci Lett. 2007;414:233–6. doi: 10.1016/j.neulet.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 68.Jack C, Antel J, Brück W, Kuhlmann T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia. 2007;55:926–34. doi: 10.1002/glia.20514. [DOI] [PubMed] [Google Scholar]
- 69.Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:12041–5. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giovannoni G, Heales SJ, Land JM, Thompson EJ. The potential role of nitric oxide in multiple sclerosis. Mult Scler. 1998;4:212–6. doi: 10.1177/135245859800400323. [DOI] [PubMed] [Google Scholar]
- 71.Tasset I, Bahamonde C, Agüera E, Conde C, Cruz AH, Pérez-Herrera A, et al. Effect of natalizumab on oxidative damage biomarkers in relapsing-remitting multiple sclerosis. Pharmacol Rep. 2013;65:624–31. doi: 10.1016/S1734-1140(13)71039-9. [DOI] [PubMed] [Google Scholar]
- 72.Fiorini A, Koudriavtseva T, Bucaj E, Coccia R, Foppoli C, Giorgi A, et al. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: the spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS One. 2013;8:e65184. doi: 10.1371/journal.pone.0065184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rejdak K, Petzold A, Stelmasiak Z, Giovannoni G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult Scler. 2008;14:59–66. doi: 10.1177/1352458507082061. [DOI] [PubMed] [Google Scholar]
- 74.Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell GR, Mahad DJ. Clonal expansion of mitochondrial DNA deletions and the progression of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:589–97. doi: 10.2174/187152712801661194. [DOI] [PubMed] [Google Scholar]
- 76.Centonze D, Muzio L, Rossi S, Furlan R, Bernardi G, Martino G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010;17:1083–91. doi: 10.1038/cdd.2009.179. [DOI] [PubMed] [Google Scholar]
- 77.Kidd PM. Neurodegeneration from mitochondrial insufficiency: nutrients, stem cells, growth factors, and prospects for brain rebuilding using integrative management. Altern Med Rev. 2005;10:268–93. [PubMed] [Google Scholar]
- 78.Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, van Het Hof B, et al. Reduced expression of PGC-1α partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013;125:231–43. doi: 10.1007/s00401-012-1052-y. [DOI] [PubMed] [Google Scholar]
- 79.Blokhin A, Vyshkina T, Komoly S, Kalman B. Variations in mitochondrial DNA copy numbers in MS brains. J Mol Neurosci. 2008;35:283–7. doi: 10.1007/s12031-008-9115-1. [DOI] [PubMed] [Google Scholar]
- 80.Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/S0022-510X(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 81.Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–89. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 82.Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol. 2008;34:577–89. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, et al. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–74. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinke S, Broadhurst D, Sykes B, Baker G, Catz I, Warren K, et al. Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult Scler. 2014;20:1396–400. doi: 10.1177/1352458513516528. [DOI] [PubMed] [Google Scholar]
- 85.Lutz NW, Viola A, Malikova I, Confort-Gouny S, Ranjeva JP, Pelletier J, et al. High-resolution 1 H NMR spectroscopy reveals differences in CSF metabolic profiles for MS patients with inflammatory vs. non-inflammatory plaques. Proc Intl Soc Mag Reson Med. 2006;14:1986. [Google Scholar]
- 86.Lazzarino G, Amorini AM, Eikelenboom MJ, Killestein J, Belli A, Di Pietro V, et al. Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult Scler. 2010;16:549–54. doi: 10.1177/1352458510364196. [DOI] [PubMed] [Google Scholar]
- 87.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 89.Wong CC, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, et al. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19:495–503. doi: 10.1038/mp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:255–74. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–6. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–76. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 94.Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–23. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–92. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 97.Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, et al. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122:e438–45. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ziats MN, Rennert OM. Expression profiling of autism candidate genes during human brain development implicates central immune signaling pathways. PLoS One. 2011;6:e24691. doi: 10.1371/journal.pone.0024691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeFelice ML, Ruchelli ED, Markowitz JE, Strogatz M, Reddy KP, Kadivar K, et al. Intestinal cytokines in children with pervasive developmental disorders. Am J Gastroenterol. 2003;98:1777–82. doi: 10.1111/j.1572-0241.2003.07593.x. [DOI] [PubMed] [Google Scholar]
- 101.Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24:664–73. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- 102.Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 103.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–5. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–5. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 106.Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–5. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Chez M, Low R, Parise C, Donnel T. Safety and observations in a pilot study of lenalidomide for treatment in autism. Autism Res Treat. 2012;2012:291601. doi: 10.1155/2012/291601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. J Autism Dev Disord. 2004;34:583–6. doi: 10.1007/s10803-004-2552-y. [DOI] [PubMed] [Google Scholar]
- 110.Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–5. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 111.Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42:1032–40. doi: 10.1016/j.clinbiochem.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 112.Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012;42:367–77. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rose S, Melnyk S, Trusty TA, Pavliv O, Seidel L, Li J, et al. Intracellular and extracellular redox status and free radical generation in primary immune cells from children with autism. Autism Res Treat. 2012;2012:986519. doi: 10.1155/2012/986519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.James SJ, Melnyk S, Jernigan S, Hubanks A, Rose S, Gaylor DW. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J Autism Dev Disord. 2008;38:1966–75. doi: 10.1007/s10803-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boris M, Goldblatt A, Galanko J, James SJ. Association of MTHFR gene variants with autism. J Am Phys Surg. 2004;9:106–8. [Google Scholar]
- 117.James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–56. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bowers K, Li Q, Bressler J, Avramopoulos D, Newschaffer C, Fallin MD. Glutathione pathway gene variation and risk of autism spectrum disorders. J Neurodev Disord. 2011;3:132–43. doi: 10.1007/s11689-011-9077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52:2128–41. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 120.Goin-Kochel RP, Porter AE, Peters SU, Shinawi M, Sahoo T, Beaudet AL. The MTHFR 677C– > T polymorphism and behaviors in children with autism: exploratory genotype-phenotype correlations. Autism Res. 2009;2:98–108. doi: 10.1002/aur.70. [DOI] [PubMed] [Google Scholar]
- 121.James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–7. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 122.James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009;89:425–30. doi: 10.3945/ajcn.2008.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghezzo A, Visconti P, Abruzzo PM, Bolotta A, Ferreri C, Gobbi G, et al. Oxidative stress and erythrocyte membrane alterations in children with autism: correlation with clinical features. PLoS One. 2013;8:e66418. doi: 10.1371/journal.pone.0066418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. The severity of autism is associated with toxic metal body burden and red blood cell glutathione levels. J Toxicol. 2009;2009:532640. doi: 10.1155/2009/532640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gorrindo P, Lane CJ, Lee EB, McLaughlin B, Levitt P. Enrichment of elevated plasma F2t-isoprostane levels in individuals with autism who are stratified by presence of gastrointestinal dysfunction. PLoS One. 2013;8:e68444. doi: 10.1371/journal.pone.0068444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem. 2011;117:209–20. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–8. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- 128.Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. 2012;37:1681–9. doi: 10.1007/s11064-012-0775-4. [DOI] [PubMed] [Google Scholar]
- 129.Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gu F, Chauhan V, Kaur K, Brown WT, LaFauci G, Wegiel J, et al. Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl Psychiatry. 2013;3:e299. doi: 10.1038/tp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu F, Chauhan V, Chauhan A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: alterations in the activities and protein expression of glutathione-related enzymes. Free Radic Biol Med. 2013;65:488–96. doi: 10.1016/j.freeradbiomed.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 132.Tang G, Gutierrez Rios P, Kuo SH, Akman HO, Rosoklija G, Tanji K, et al. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis. 2013;54:349–61. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–96. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guevara-Campos J, González-Guevara L, Briones P, López-Gallardo E, Bulán N, Ruiz-Pesini E, et al. Autism associated to a deficiency of complexes III and IV of the mitochondrial respiratory chain. Invest Clin. 2010;51:423–31. [PubMed] [Google Scholar]
- 135.Shoffner J, Hyams L, Langley GN, Cossette S, Mylacraine L, Dale J, et al. Fever plus mitochondrial disease could be risk factors for autistic regression. J Child Neurol. 2010;25:429–34. doi: 10.1177/0883073809342128. [DOI] [PubMed] [Google Scholar]
- 136.Zhang B, Angelidou A, Alysandratos KD, Vasiadi M, Francis K, Asadi S, et al. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J Neuroinflammation. 2010;7:80. doi: 10.1186/1742-2094-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dhillon S, Hellings JA, Butler MG. Genetics and mitochondrial abnormalities in autism spectrum disorders: a review. Curr Genomics. 2011;12:322–32. doi: 10.2174/138920211796429745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frye RE, Delatorre R, Taylor H, Slattery J, Melnyk S, Chowdhury N, et al. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry. 2013;3:e273. doi: 10.1038/tp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frye RE, Rossignol DA. Mitochondrial physiology and autism spectrum disorder. OA Autism. 2013;1:5. doi: 10.13172/2052-7810-1-1-433. [DOI] [Google Scholar]
- 140.Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol Psychiatry. 1993;33:762–73. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- 141.Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2:179–88. [Google Scholar]
- 142.Frye RE. Biomarkers of abnormal energy metabolism in children with autism spectrum disorder. NAJ Med Sci. 2012;5:141–7. [Google Scholar]
- 143.Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- 144.Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, et al. Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism. 2012;3:12. doi: 10.1186/2040-2392-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anitha A, Nakamura K, Thanseem I, Matsuzaki H, Miyachi T, Tsujii M, et al. Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol. 2013;23:294–302. doi: 10.1111/bpa.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ginsberg MR, Rubin RA, Falcone T, Ting AH, Natowicz MR. Brain transcriptional and epigenetic associations with autism. PLoS One. 2012;7:e44736. doi: 10.1371/journal.pone.0044736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chugani DC, Sundram BS, Behen M, Lee ML, Moore GJ. Evidence of altered energy metabolism in autistic children. Prog Europsychopharmacol Biol Psych. 1999;23:635–41. doi: 10.1016/S0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 148.Coleman M, Blass JP. Autism and lactic acidosis. J Autism Dev Disord. 1985;15:1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- 149.Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–23. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- 150.Filipek PA, Juranek J, Smith M, Mays LZ, Ramos ER, Bocian M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53:801–4. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 151.Fillano JJ, Goldenthal MJ, Rhodes CH, Marín-García J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002;17:435–9. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- 152.Holiga Š, Mueller K, Möller HE, Sieger T, Schroeter ML, Vymazal J, et al. Motor matters: tackling heterogeneity of Parkinson’s disease in functional MRI studies. PLoS One. 2013;8:e56133. doi: 10.1371/journal.pone.0056133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Erro R, Vitale C, Amboni M, Picillo M, Moccia M, Longo K, et al. The heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. PLoS One. 2013;8:e70244. doi: 10.1371/journal.pone.0070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Arizona Parkinson’s Disease Consortium. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, et al. Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol Scand. 2009;119:332–7. doi: 10.1111/j.1600-0404.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- 156.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, et al. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–25. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 157.Scalzo P, Kümmer A, Cardoso F, Teixeira AL. Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s disease. J Neuroimmunol. 2009;216:122–5. doi: 10.1016/j.jneuroim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 158.Dufek M, Hamanová M, Lokaj J, Goldemund D, Rektorová I, Michálková Z, et al. Serum inflammatory biomarkers in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:318–20. doi: 10.1016/j.parkreldis.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 159.Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol. 2008;167:90–5. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 160.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–50. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 161.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–6. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 162.Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000;2000:143–51. [PubMed] [Google Scholar]
- 163.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 164.Hasegawa Y, Inagaki T, Sawada M, Suzumura A. Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macrophages in Parkinson’s disease. Acta Neurol Scand. 2000;101:159–64. doi: 10.1034/j.1600-0404.2000.101003159.x. [DOI] [PubMed] [Google Scholar]
- 165.Arai H, Furuya T, Mizuno Y, Mochizuki H. Inflammation and infection in Parkinson’s disease. Histol Histopathol. 2006;21:673–8. doi: 10.14670/HH-21.673. [DOI] [PubMed] [Google Scholar]
- 166.Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Jr, et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2:249–53. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 167.Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K. Oxidative damage in nucleic acids and Parkinson’s disease. J Neurosci Res. 2007;85:919–34. doi: 10.1002/jnr.21191. [DOI] [PubMed] [Google Scholar]
- 168.Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother. 2008;62:236–49. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 169.Perry TL, Godin DV, Hansen S. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–10. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 170.Perry TL, Yong VW. Idiopathic Parkinson’s disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–74. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 171.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett. 1992;142:128–30. doi: 10.1016/0304-3940(92)90355-B. [DOI] [PubMed] [Google Scholar]
- 172.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326–9. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 173.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–75. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 174.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 175.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52:381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 177.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–9. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 178.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:1328–31. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 179.Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–4. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic Biol Med. 1998;25:169–74. doi: 10.1016/S0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 181.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002;9:244–8. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 182.Abe T, Isobe C, Murata T, Sato C, Tohgi H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci Lett. 2003;336:105–8. doi: 10.1016/S0304-3940(02)01259-4. [DOI] [PubMed] [Google Scholar]
- 183.Buhmann C, Arlt S, Kontush A, Möller-Bertram T, Sperber S, Oechsner M, et al. Plasma and CSF markers of oxidative stress are increased in Parkinson’s disease and influenced by antiparkinsonian medication. Neurobiol Dis. 2004;15:160–70. doi: 10.1016/j.nbd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 184.Sohmiya M, Tanaka M, Tak NW, Yanagisawa M, Tanino Y, Suzuki Y, et al. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J Neurol Sci. 2004;223:161–6. doi: 10.1016/j.jns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 185.Prigione A, Begni B, Galbussera A, Beretta S, Brighina L, Garofalo R, et al. Oxidative stress in peripheral blood mononuclear cells from patients with Parkinson’s disease: negative correlation with levodopa dosage. Neurobiol Dis. 2006;23:36–43. doi: 10.1016/j.nbd.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 186.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–7. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 187.Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163:1450–5. doi: 10.1016/0006-291X(89)91141-8. [DOI] [PubMed] [Google Scholar]
- 188.Parker WD, Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–8. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bindoff LA, Birch-Machin MA, Cartlidge NE, Parker WD, Jr, Turnbull DM. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. J Neurol Sci. 1991;104:203–8. doi: 10.1016/0022-510X(91)90311-T. [DOI] [PubMed] [Google Scholar]
- 190.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, et al. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol. 1995;37:714–22. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 191.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–64. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shinde S, Pasupathy K. Respiratory-chain enzyme activities in isolated mitochondria of lymphocytes from patients with Parkinson’s disease: preliminary study. Neurol India. 2006;54:390–3. doi: 10.4103/0028-3886.28112. [DOI] [PubMed] [Google Scholar]
- 193.Rana M, de Coo I, Diaz F, Smeets H, Moraes CT. An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann Neurol. 2000;48:774–81. doi: 10.1002/1531-8249(200011)48:5<774::AID-ANA11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 194.Acín-Pérez R, Bayona-Bafaluy MP, Fernández-Silva P, Moreno-Loshuertos R, Pérez-Martos A, Bruno C, et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell. 2004;13:805–15. doi: 10.1016/S1097-2765(04)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rango M, Bonifati C, Bresolin N. Parkinson’s disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2006;26:283–90. doi: 10.1038/sj.jcbfm.9600192. [DOI] [PubMed] [Google Scholar]
- 196.Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbé C, Cunningham VJ, et al. Cortical dysfunction in non-demented Parkinson’s disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain. 2000;123:340–52. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- 197.Bowen BC, Block RE, Sanchez-Ramos J, Pattany PM, Lampman DA, Murdoch JB, et al. Proton MR spectroscopy of the brain in 14 patients with Parkinson disease. AJNR Am J Neuroradiol. 1995;16:61–8. [PMC free article] [PubMed] [Google Scholar]
- 198.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–9. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 199.Metzger K, Frémont M, Roelant C, De Meirleir K. Lower frequency of IL-17 F sequence variant (His161Arg) in chronic fatigue syndrome patients. Biochem Biophys Res Commun. 2008;376:231–3. doi: 10.1016/j.bbrc.2008.08.135. [DOI] [PubMed] [Google Scholar]
- 200.Mihaylova I, DeRuyter M, Rummens JL, Bosmans E, Maes M. Decreased expression of CD69 in chronic fatigue syndrome in relation to inflammatory markers: evidence for a severe disorder in the early activation of T lymphocytes and natural killer cells. Neuro Endocrinol Lett. 2007;28:477–83. [PubMed] [Google Scholar]
- 201.Maes M, Mihaylova I, Leunis JC. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol Lett. 2005;26:745–51. [PubMed] [Google Scholar]
- 202.Maes M, Twisk FN, Johnson C. Myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), and chronic fatigue (CF) are distinguished accurately: results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry Res. 2012;200:754–60. doi: 10.1016/j.psychres.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 203.Maher KJ, Klimas NG, Fletcher MA. Chronic fatigue syndrome is associated with diminished intracellular perforin. Clin Exp Immunol. 2005;142:505–11. doi: 10.1111/j.1365-2249.2005.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209–17. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 206.Brenu EW, van Driel ML, Staines DR, Ashton KJ, Hardcastle SL, Keane J, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2012;10:88. doi: 10.1186/1479-5876-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brenu EW, van Driel ML, Staines DR, Ashton KJ, Ramos SB, Keane J, et al. Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2011;9:81. doi: 10.1186/1479-5876-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lombardi VC, Hagen KS, Hunter KW, Diamond JW, Smith-Gagen J, Yang W, et al. Xenotropic murine leukemia virus-related virus-associated chronic fatigue syndrome reveals a distinct inflammatory signature. In Vivo. 2011;25:307–14. [PubMed] [Google Scholar]
- 209.Moss RB, Mercandetti A, Vojdani A. TNF-alpha and chronic fatigue syndrome. J Clin Immunol. 1999;19:314–6. doi: 10.1023/A:1020595709352. [DOI] [PubMed] [Google Scholar]
- 210.Borish L, Schmaling K, DiClementi JD, Streib J, Negri J, Jones JF. Chronic fatigue syndrome: identification of distinct subgroups on the basis of allergy and psychologic variables. J Allergy Clin Immunol. 1998;102:222–30. doi: 10.1016/S0091-6749(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 211.Patarca R, Klimas N, Lugtendorf S, Antoni M, Fletcher M. Dysregulated expression of tumor necrosis factor in chronic fatigue syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin Infect Dis. 1994;18:147–53. doi: 10.1093/clinids/18.Supplement_1.S147. [DOI] [PubMed] [Google Scholar]
- 212.Brenu E, Johnston S, Hardcastle S, Huth T, Fuller K, Ramos S, et al. Immune abnormalities in patients meeting new diagnostic criteria for chronic fatigue syndrome/myalgic encephalomyelitis. J Mol Biomark Diagn. 2013;4:2. [Google Scholar]
- 213.Natelson BH, Haghighi MH, Ponzio NM. Evidence for the presence of immune dysfunction in chronic fatigue syndrome. Clin Diagn Lab Immunol. 2002;9:747–52. doi: 10.1128/CDLI.9.4.747-752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Natelson BH. Brain dysfunction as one cause of CFS symptoms including difficulty with attention and concentration. Front Physiol. 2013;4:109. doi: 10.3389/fphys.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26:24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 216.Kennedy G, Norris G, Spence V, McLaren M, Belch JJ. Is chronic fatigue syndrome associated with platelet activation? Blood Coagul Fibrinolysis. 2006;17:89–92. doi: 10.1097/01.mbc.0000214705.80997.73. [DOI] [PubMed] [Google Scholar]
- 217.Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Scheeres K, Wensing M, Knoop H, Bleijenberg G. Implementing cognitive behavioral therapy for chronic fatigue syndrome in a mental health center: a benchmarking evaluation. J Consult Clin Psychol. 2008;76:163–71. doi: 10.1037/0022-006X.76.1.163. [DOI] [PubMed] [Google Scholar]
- 219.Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, et al. International Chronic Fatigue Syndrome Study Group. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. 2003;3:25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sharpe MC, Archard LC, Banatvala JE, Borysiewicz LK, Clare AW, David A, et al. A report–chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991;84:118–21. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 222.Perrin R, Embleton K, Pentreath VW, Jackson A. Longitudinal MRI shows no cerebral abnormality in chronic fatigue syndrome. Br J Radiol. 2010;83:419–23. doi: 10.1259/bjr/85621779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Filler K, Lyon D, Bennett J, McCain N, Elswisk R, Lukkahatai N, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clinical. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Smits B, van den Heuvel L, Knoop H, Küsters B, Janssen A, Borm G, et al. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion. 2011;11:735–8. doi: 10.1016/j.mito.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 225.Maes M, Mihaylova I, Leunis JC. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett. 2006;27:615–21. [PubMed] [Google Scholar]
- 226.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol Lett. 2009;30:715–22. [PubMed] [Google Scholar]
- 227.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30:470–6. [PubMed] [Google Scholar]
- 228.Maes M, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides as a marker of oxidative stress in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med Sci Monit. 2011;17:SC11–5. doi: 10.12659/MSM.881699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335:151–4. doi: 10.1016/S0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 230.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 2005;39:584–9. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 231.Richards RS, Roberts TK, McGregor NR, Dunstan RH, Butt HL. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000;5:35–41. doi: 10.1179/rer.2000.5.1.35. [DOI] [PubMed] [Google Scholar]
- 232.Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–87. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Miwa K, Fujita M. Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25:319–23. doi: 10.1007/s00380-009-1206-6. [DOI] [PubMed] [Google Scholar]
- 234.Manuel y Keenoy B, Moorkens G, Vertommen J, Noe M, Nève J, De Leeuw I. Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. J Am Coll Nutr. 2000;19:374–82. doi: 10.1080/07315724.2000.10718934. [DOI] [PubMed] [Google Scholar]
- 235.Manuel y Keenoy B, Moorkens G, Vertommen J, De Leeuw I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001;68:2037–49. doi: 10.1016/S0024-3205(01)01001-3. [DOI] [PubMed] [Google Scholar]
- 236.Maes M, Mihaylova I, Kubera M, Bosmans E. Not in the mind but in the cell: increased production of cyclo-oxygenase-2 and inducible NO synthase in chronic fatigue syndrome. Neuro Endocrinol Lett. 2007;28:463–9. [PubMed] [Google Scholar]
- 237.Fulle S, Pietrangelo T, Mancinelli R, Saggini R, Fanò G. Specific correlations between muscle oxidative stress and chronic fatigue syndrome: a working hypothesis. J Muscle Res Cell Motil. 2007;28:355–62. doi: 10.1007/s10974-008-9128-y. [DOI] [PubMed] [Google Scholar]
- 238.Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- 239.Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Int J Clin Exp Med. 2012;5:208–20. [PMC free article] [PubMed] [Google Scholar]
- 240.Myhill S, Booth NE, McLaren-Howard J. Targeting mitochondrial dysfunction in the treatment of myalgic ncephalomyelitis/chronic fatigue syndrome (ME/CFS) – a clinical audit. Int J Clin Exp Med. 2013;6:1–15. [PMC free article] [PubMed] [Google Scholar]
- 241.Behan W, Holt I, Kay D, Moonie P. In vitro study of muscle aerobic metabolism in chronic fatigue syndrome. JCFS. 1999;5:3–16. [Google Scholar]
- 242.Behan WMH, More IAR, Downie I, Gow JW. Mitochondrial studies in the chronic fatigue syndrome. EOS Riv Immunol Immunofarmacol. 1995;15:36–9. [Google Scholar]
- 243.Behan WMH, Gow JW, Simpson K, More IAR, Downie I, Holt IJ, et al. Mitochondrial findings in the chronic fatigue syndrome. J Pathol. 1994;173:SI7. [Google Scholar]
- 244.Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83:61–5. doi: 10.1007/BF00294431. [DOI] [PubMed] [Google Scholar]
- 245.Jones DE, Hollingsworth KG, Taylor R, Blamire AM, Newton JL. Abnormalities in pH handling by peripheral muscle and potential regulation by the autonomic nervous system in chronic fatigue syndrome. J Intern Med. 2010;267:394–401. doi: 10.1111/j.1365-2796.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- 246.Hollingsworth KG, Jones DE, Taylor R, Blamire AM, Newton JL. Impaired cardiovascular response to standing in chronic fatigue syndrome. Eur J Clin Invest. 2010;40:608–15. doi: 10.1111/j.1365-2362.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 247.McCully KK, Natelson BH. Impaired oxygen delivery to muscle in chronic fatigue syndrome. Clin Sci (Lond). 1999;97:603–8. doi: 10.1042/cs0970603. [DOI] [PubMed] [Google Scholar]
- 248.McCully KK, Natelson BH, Iotti S, Sisto S, Leigh JS., Jr Reduced oxidative muscle metabolism in chronic fatigue syndrome. Muscle Nerve. 1996;19:621–5. doi: 10.1002/(SICI)1097-4598(199605)19:5<621::AID-MUS10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 249.Wong R, Lopaschuk G, Zhu G, Walker D, Catellier D, Burton D, et al. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest. 1992;102:1716–22. doi: 10.1378/chest.102.6.1716. [DOI] [PubMed] [Google Scholar]
- 250.Arnold DL, Bore PJ, Radda GK, Styles P, Taylor DJ. Excessive intracellular acidosis of skeletal muscle on exercise in a patient with a post-viral exhaustion/fatigue syndrome. A 31P nuclear magnetic resonance study. Lancet. 1984;1:1367–9. doi: 10.1016/S0140-6736(84)91871-3. [DOI] [PubMed] [Google Scholar]
- 251.Lane RJ, Soteriou BA, Zhang H, Archard LC. Enterovirus related metabolic myopathy: a postviral fatigue syndrome. J Neurol Neurosurg Psychiatry. 2003;74:1382–6. doi: 10.1136/jnnp.74.10.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lane RJ, Barrett MC, Woodrow D, Moss J, Fletcher R, Archard LC. Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1998;64:362–7. doi: 10.1136/jnnp.64.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lane RJ, Barrett MC, Taylor DJ, Kemp GJ, Lodi R. Heterogeneity in chronic fatigue syndrome: evidence from magnetic resonance spectroscopy of muscle. Neuromuscul Disord. 1998;8:204–9. doi: 10.1016/S0960-8966(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 254.Barnes PR, Taylor DJ, Kemp GJ, Radda GK. Skeletal muscle bioenergetics in the chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1993;56:679–83. doi: 10.1136/jnnp.56.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vermeulen RC, Kurk RM, Visser FC, Sluiter W, Scholte HR. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010;8:93. doi: 10.1186/1479-5876-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vermeulen RC, Vermeulen van Eck IW. Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J Transl Med. 2014;12:20. doi: 10.1186/1479-5876-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mathew SJ, Mao X, Keegan KA, Levine SM, Smith EL, Heier LA, et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR Biomed. 2009;22:251–8. doi: 10.1002/nbm.1315. [DOI] [PubMed] [Google Scholar]
- 258.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–19. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 259.Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms, and implications. Neurosci Biobehav Rev. 2015;48C:10–21. doi: 10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 260.Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638–46. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. 2002;17:175–9. doi: 10.1002/hup.390. [DOI] [PubMed] [Google Scholar]
- 262.Tsai SY, Chen KP, Yang YY, Chen CC, Lee JC, Singh VK, et al. Activation of indices of cell-mediated immunity in bipolar mania. Biol Psychiatry. 1999;45:989–94. doi: 10.1016/S0006-3223(98)00159-0. [DOI] [PubMed] [Google Scholar]
- 263.Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Gonçalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34:263–71. [PMC free article] [PubMed] [Google Scholar]
- 264.Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol. 2009;12:447–58. doi: 10.1017/S1461145708009310. [DOI] [PubMed] [Google Scholar]
- 265.Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–33. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 266.Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53C:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 267.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 268.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–30. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 269.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–8. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 270.Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–44. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 271.Yumru M, Savas HA, Kalenderoglu A, Bulut M, Celik H, Erel O. Oxidative imbalance in bipolar disorder subtypes: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1070–4. doi: 10.1016/j.pnpbp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 272.Park SW, Lee CH, Lee JG, Kim LW, Shin BS, Lee BJ, et al. Protective effects of atypical antipsychotic drugs against MPP(+)-induced oxidative stress in PC12 cells. Neurosci Res. 2011;69:283–90. doi: 10.1016/j.neures.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 273.Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479:317–20. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 274.Jornada LK, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, et al. Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. J Psychiatr Res. 2011;45:162–8. doi: 10.1016/j.jpsychires.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 275.Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience. 2007;144:1447–53. doi: 10.1016/j.neuroscience.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 276.Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107:89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 277.Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, et al. Oxidative & nitrosative stress in depression: Why so much stress? Neurosci Biobehav Rev. 2014;45C:46–62. doi: 10.1016/j.neubiorev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 278.Baxter LR, Jr, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry. 1985;42:441–7. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- 279.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–90. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 280.Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177:575–85. doi: 10.2353/ajpath.2010.081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Michael N, Erfurth A, Ohrmann P, Gössling M, Arolt V, Heindel W, et al. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl). 2003;168:344–6. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- 282.Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci. 2004;58:82–8. doi: 10.1111/j.1440-1819.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- 283.Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry. 2009;65:489–94. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 285.Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- 286.Dean OM, Turner A, Malhi GS, Ng C, Cotton SM, et al. Design and rationale of a 16-week adjunctive randomized placebo-controlled trial of mitochondrial agents for the treatment of bipolar depression. Rev Bras Psiquiatr. 2014;0:0 [Epub ahead of print]. [DOI] [PubMed]
- 287.Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology. 1990–1991;24:115–20. [DOI] [PubMed]
- 288.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–75. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 289.Maes M, Mihaylova I, Kubera M, Ringel K. Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:169–75. doi: 10.1016/j.pnpbp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 290.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–9. doi: 10.1016/S0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 291.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 292.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 293.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 294.Fornaro M, Rocchi G, Escelsior A, Contini P, Martino M. Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological backgrounds. J Affect Disord. 2013;145:300–7. doi: 10.1016/j.jad.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 295.Fornaro M, Bandini F, Ogliastro C, Cordano C, Martino M, Cestari L, et al. Electroretinographic assessment in major depressed patients receiving duloxetine: Might differences between responders and non-responders indicate a differential biological background? J Affect Disord. 2011;135:154–9. doi: 10.1016/j.jad.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 296.Song C, Halbreich U, Han C, Leonard B, Luo H. Imbalance between Pro- and Anti-inflammatory Cytokines, and between Th1 and Th2 Cytokines in Depressed Patients: The Effect of Electroacupuncture or Fluoxetine Treatment. Pharmacopsychiatry. 2009;42:182–8. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- 297.Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 299.Anderson G, Maes M. Oxidative/Nitrosative Stress and Immuno-inflammatory Pathways in Depression: Treatment Implications. Current Pharmaceutical Design. 2014;20(23):3812–3847. doi:10.2174/13816128113196660738. [DOI] [PubMed]
- 300.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 301.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–75. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gałecki P, Szemraj J, Bieńkiewicz M, Florkowski A, Gałecki E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep. 2009;61:436–47. doi: 10.1016/S1734-1140(09)70084-2. [DOI] [PubMed] [Google Scholar]
- 303.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 304.Czarny P, Kwiatkowski D, Kacperska D, Kawczyńska D, Talarowska M, Orzechowska A, et al. Elevated Level of DNA Damage and Impaired Repair of Oxidative DNA Damage in Patients with Recurrent Depressive Disorder. Medical Science Monitor. 2015;21:412–418. doi:10.12659/msm.892317. [DOI] [PMC free article] [PubMed]
- 305.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–6. doi: 10.1016/S0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 306.Milaneschi Y, Cesari M, Simonsick E, Vogelzangs N, Kanaya A, Yaffe K, et al. Lipid peroxidation and depressed mood in community-dwelling older men and women. Plos ONE. 2013;8:e65406. doi: 10.1371/journal.pone.0065406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tsuboi H, Shimoi K, Kinae N, Oguni I, Hori R, Kobayashi F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J Psychosom Res. 2004;56:53–8. doi: 10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- 308.Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35:1356–62. doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 309.Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, et al. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15:249–52. doi: 10.1016/j.euroneuro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 310.Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, et al. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75:628–36. doi: 10.4088/JCP.13m08454. [DOI] [PubMed] [Google Scholar]
- 311.Dean OM, Maes M, Ashton M, Berk L, Kanchanatawan B, Sughondhabirom A, et al. Protocol and rationale-the efficacy of minocycline as an adjunctive treatment for major depressive disorder: a double blind, randomised. Placebo Controlled Trial Clin Psychopharmacol Neurosci. 2014;12:180–8. doi: 10.9758/cpn.2014.12.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Maes M, Kubera M, Mihaylova I, Geffard M, Galecki P, Leunis JC, et al. Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: implications for the pathways to chronic depression and neuroprogression. J Affect Disord. 2013;149:23–9. doi: 10.1016/j.jad.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 313.Nunes S, Vargas H, Prado E, Barbosa D, de Melo L, Moylan S, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev. 2013;37:1336–45. doi: 10.1016/j.neubiorev.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 314.Tobe E. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat. 2013;9:567–73. doi: 10.2147/NDT.S44282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Anglin R, Rosebush P, Mazurek M, Tarnopolsky M, Noseworthy M. The psychiatric manifestations of mitochondrial cytopathies: A clinical and MR spectroscopy investigation. Mitochondrion. 2011;11:639–40. doi: 10.1016/j.mito.2011.03.018. [DOI] [Google Scholar]
- 316.Maes M, Galecki P, Chang Y, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:676–92. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 317.Berk M, Williams L, Jacka F, O’Neil A, Pasco J, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 319.Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jpn J Cancer Res. 2001;92:367–76. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Irie M, Miyata M, Kasia H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–60. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 321.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 322.Fattal O, Budur K, Vaughan AJ, Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 323.Klinedinst N, Regenold W. A mitochondrial bioenergetic basis of depression. Journal Of Bioenergetics And Biomembranes. 2014;47(1-2):155–171. doi:10.1007/s10863-014-9584-6. [DOI] [PubMed]
- 324.Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- 325.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 326.Moretti A, Gorini A, Villa R. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–85. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- 327.Rigucci S, Serafini G, Pompili M, Kotzalidis G, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11:165–80. doi: 10.3109/15622970903131571. [DOI] [PubMed] [Google Scholar]
- 328.Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, Hagenfeldt L, et al. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/S0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 329.Hroudová J, Fišar Z, Kitzlerová E, Zvěřová M, Raboch J. Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion. 2013;6:795–800. doi: 10.1016/j.mito.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 330.Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich E, et al. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Translational Psychiatry. 2014;4:e397. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rothermundt M, Arolt V, Weitzsch C, Eckhoff D, Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–93. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- 332.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. doi: 10.1016/S0920-9964(00)00007-4. [DOI] [PubMed] [Google Scholar]
- 333.Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naïve and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–9. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- 334.Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–34. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 335.Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res. 2002;57:247–58. doi: 10.1016/S0920-9964(01)00296-1. [DOI] [PubMed] [Google Scholar]
- 336.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 337.Debnath M, Berk M. Th17 pathway-mediated immunopathogenesis of schizophrenia: mechanisms and implications. Schizophr Bull. 2014;40:1412–21. doi: 10.1093/schbul/sbu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Na K, Jung H, Kim Y. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog NeuroPsychopharmacol Biol Psychiatry. 2014;48:277–86. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 339.Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, et al. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology (Berl). 2013;231:319–25. doi: 10.1007/s00213-013-3382-4. [DOI] [PubMed] [Google Scholar]
- 340.Song X, Fan X, Song X, Zhang J, Zhang W, Li X, et al. Elevated levels of adiponectin and other cytokines in drug naive, first episode schizophrenia patients with normal weight. Schizophr Res. 2013;150:269–73. doi: 10.1016/j.schres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 341.Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:78–82. doi: 10.1016/j.pnpbp.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 342.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–9. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Micó JA, Fernandez M, Echevarría E, et al. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res. 2012;137:66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 344.Pavlović D, Tamburić V, Stojanović I, Kocić G, Jevtović T, Đorđević V. Oxidative stress as marker of positive symptoms in schizophrenia. Facta Universitatis. 2002;9:157–61. [Google Scholar]
- 345.Wood SJ, Yücel M, Pantelis C, Berk M. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38:396. [PubMed] [Google Scholar]
- 346.Bitanihirwe BKY, Woo TUW. Oxidative stress in schizophrenia: an inte-grated approach. Neurosci Biobehav Rev. 2011;35:878–93. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.O’Donnell P, Do KQ, Arango C. Oxidative/nitrosative stress in psychiatricdisorders: are we there yet? Schizophr Bull. 2014;40:960–2. doi: 10.1093/schbul/sbu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuroprogression inschizophrenia: pathways underpinning clinical staging and therapeutic corol-laries. Aust NZ J Psychiatry. 2014;48:512–29. doi: 10.1177/0004867414533012. [DOI] [PubMed] [Google Scholar]
- 349.Singh OP, Chakraborty I, Datta S. A comparative study of oxidative stressand interrelationship of important antioxidants in haloperidol and olanzap-ine treated patients suffering from schizophrenia. Indian J Psychiatry. 2008;50:1–8. doi: 10.4103/0019-5545.39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Raffa M, Mechri A, Othman L, Ben Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treatedschizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–83. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 351.Do K, Trabesinger A, Kirsten-Krüger M, Lauer C, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 352.Wang J, Shao L, Sun X, Young L. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:523–9. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 353.Prabakaran S, Wengenroth M, Lockstone H, Lilley K, Leweke F, Bahn S. 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. J Proteome Res. 2007;6:141–9. doi: 10.1021/pr060308a. [DOI] [PubMed] [Google Scholar]
- 354.Yao J, Reddy R, McElhinny L, van Kammen D. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr Res. 1998;32:1–8. doi: 10.1016/S0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 355.Reddy R. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res. 2003;62:205–12. doi: 10.1016/S0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 356.Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21:34–8. doi: 10.1007/BF02912908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Akyol O, Herken H, Uz E, Fadillioğlu E, Unal S, Söğüt S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:995–1005. doi: 10.1016/S0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 358.Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, et al. Similarities inserum oxidative stress markers and inflammatory cytokines in patients withovertschizophrenia at early and late stages of chronicity? J Psychiatr Res. 2012;46:819–24. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 359.Gubert C, Stertz L, Pfaffenseller B, Panizzutti BS, Rezin GT, Massuda R, et al. Mitochondrial activity and oxidative stress markers in peripheral bloodmononuclear cells of patients with bipolar disorder, schizophrenia, and healthy subjects. J Psychiatr Res. 2013;47:1396–402. doi: 10.1016/j.jpsychires.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 360.Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–9. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 361.Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets. 2005;16:386–91. doi: 10.1080/09537100500128872. [DOI] [PubMed] [Google Scholar]
- 362.Kunz M, Gama C, Andreazza A, Salvador M, Ceresér K, Gomes FA, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–81. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 363.Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol Psychiatry. 2014;20:152–3. doi: 10.1038/mp.2014.139. [DOI] [PubMed] [Google Scholar]
- 364.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–9. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Park C, Park SK. Molecular links between mitochondrial dysfunctions andschizophrenia. Mol Cells. 2012;33:105–10. doi: 10.1007/s10059-012-2284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible link-age to dopamine. J Neurochem. 2002;83:1241–51. doi: 10.1046/j.1471-4159.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- 367.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/S0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 368.Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a postmortem. Synapse. 1999;31:67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 369.Kolomeets NS, Uranova N. Ultrastructural abnormalities of astrocytes inthe hippocampus in schizophrenia and duration of illness: a postortem morphometric study. World J Biol Psychiatry. 2010;11:282–92. doi: 10.3109/15622970902806124. [DOI] [PubMed] [Google Scholar]
- 370.Inuwa IM, Peet M, Williams MA. QSAR modeling and transmission electronmicroscopy stereology of altered mitochondrial ultrastructure of white bloodcells in patients diagnosed as schizophrenic and treated with antipsychoticdrugs. Biotech Histochem. 2005;80:133–7. doi: 10.1080/10520290500303349. [DOI] [PubMed] [Google Scholar]
- 371.Uranova N, Bonartsev P, Brusov O, Morozova M, Rachmanova V, Orlovskaya D. The ultrastructure of lymphocytes in schizophrenia. World J Biol Psychiatry. 2007;8:30–7. doi: 10.1080/15622970600960207. [DOI] [PubMed] [Google Scholar]
- 372.Rosenfeld M, Brenner-Lavie H, Ari SGB, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex Idependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–8. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 373.Dror N, Klein E, Karry R, Sheinkman A, Kirsh Z, Mazor M, et al. State-dependent alterations in mitochondrial complex I activity in platelets: a potential peripheral marker for schizophrenia? Mol Psychiatry. 2002;7:995–1001. doi: 10.1038/sj.mp.4001116. [DOI] [PubMed] [Google Scholar]
- 374.Ben-Shachar D, Karry R. Sp1 expression is disrupted in schizophrenia; a possible mechanism for the abnormal expression of mitochondrial complex I genes, NDUFV1 and NDUFV2. PLoS One. 2007;2:e817. doi: 10.1371/journal.pone.0000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ben-Shachar D, Zuk R, Gazawi H, Reshef A, Sheinkman A, Klein E. Increased mitochondrial complex I activity in platelets of schizophrenic patients. Int J Neuropsychopharmacol. 1999;2:245–53. doi: 10.1017/S1461145799001649. [DOI] [PubMed] [Google Scholar]
- 376.Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3:e3676. doi: 10.1371/journal.pone.0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Karry R, Klein E, Ben SD. Mitochondrial complex I subunits expres-sion is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55:676–84. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 378.Akarsu S. Schizophrenia and mitochondrial dysfunction. Psikiyatride Guncel Yaklasimlar - Current Approaches In Psychiatry. 2014;6:340–54. doi: 10.5455/cap.20140121114707. [DOI] [Google Scholar]
- 379.Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neuroscience & Biobehavioral Reviews. 2015;48:10–21. doi:10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed]
- 380.Maurer I, Zierz S, Möller H. Evidence for a mitochondrial oxidative phos-phorylation defect in brains from patients with schizophrenia. Schizophr Res. 2001;48:125–36. doi: 10.1016/S0920-9964(00)00075-X. [DOI] [PubMed] [Google Scholar]
- 381.Manatt M, Chandra S. The effects of mitochondrial dysfunction in schizophrenia. J Med Genet Genomics. 2011;3:84–94. [Google Scholar]
- 382.Blass P. Glucose/mitochondria in neurological conditions. Int Rev Neurobiol. 2002;51:325–76. doi: 10.1016/S0074-7742(02)51010-2. [DOI] [PubMed] [Google Scholar]
- 383.Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW, et al. Proteome analysis of schizophrenia patients Wer-nicke’s area reveals an energy metabolism dysregulation. BMC Psychiatry. 2009;30:17. doi: 10.1186/1471-244X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Martins-de-Souza D, Maccarrone G, Wobrock T, Zerr I, Gormanns P, Reckow S, et al. Proteome analysis of the thalamusand cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkerscandidates for schizophrenia. J Psychiatr Res. 2010;44:1176–89. doi: 10.1016/j.jpsychires.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 385.Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JTJ, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromisedbrain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97. doi: 10.1038/sj.mp.4001532. [DOI] [PubMed] [Google Scholar]
- 386.Volz H, Riehemann S, Maurer I, Smesny S, Sommer M, Rzanny R, et al. Reduced phosphodiesters and high-energy phosphates in the frontal lobe ofschizophrenic patients: a31P chemical shift spectroscopic-imaging study. Biol Psychiatry. 2000;41:954–61. doi: 10.1016/S0006-3223(00)00235-3. [DOI] [PubMed] [Google Scholar]
- 387.Verge B, Alonso Y, Miralles C, Valero J, Vilella E, Boles RG, et al. New evidence for the involvement of mitochondrial inheritance in schizophre-nia: results from a cross-sectional study evaluating the risk of illness in relativesof schizophrenia patients? J Clin Psychiatry. 2012;73:684–90. doi: 10.4088/JCP.10m06718. [DOI] [PubMed] [Google Scholar]
- 388.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience & Biobehavioral Reviews. 2012;36(2):764–785. doi:10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed]
- 389.Li X, Sundquist J, Sundquist K. Age-specific familial risks of psychotic dis-orders and schizophrenia: a nation-wide epidemiological study from Sweden. Schizophr Res. 2007;97:43–50. doi: 10.1016/j.schres.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Flyckt L, Edman G, Venizelos N, Borg K. Aberrant tyrosine transport acrossthe fibroblast membrane in patients with schizophrenia—indications of maternal inheritance? J Psychiatr Res. 2011;45:519–25. doi: 10.1016/j.jpsychires.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 391.Marchbanks RM, Ryan M, Day INM, Owen M, McGuffin P, Whatley SA. A mitochondrial DNA sequence variant associated with schizophrenia andoxidative stress. Schizophr Res. 2003;65:33–8. doi: 10.1016/S0920-9964(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 392.Ueno H, Nishigaki Y, Kong QP, Fuku N, Kojima S, Iwata N, et al. Analysis of mitochondrial DNA variants in Japanese patients with schizophrenia. Mitochondrion. 2009;9:385–93. doi: 10.1016/j.mito.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 393.Ichikawa T, Arai M, Miyashita M, Arai M, Obata N, Nohara I, et al. Schizophrenia: maternal inher-itance and heteroplasmy of mtDNA mutations? Mol Genet Metab. 2012;105:103–9. doi: 10.1016/j.ymgme.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 394.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–62. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 395.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–37. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 396.Sarti P, Forte E, Giuffrè A, Mastronicola D, Magnifico MC, Arese M. The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: reactions, effectors and pathophysiology. Int J Cell Biol. 2012;2012:571067. doi: 10.1155/2012/571067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–31. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 398.Xu W, Charles IG, Moncada S. Nitric oxide: orchestrating hypoxia regulation through mitochondrial respiration and the endoplasmic reticulum stress response. Cell Res. 2005;15:63–5. doi: 10.1038/sj.cr.7290267. [DOI] [PubMed] [Google Scholar]
- 399.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 400.Boveris A, Costa LE, Poderoso JJ, Carreras MC, Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann N Y Acad Sci. 2000;899:121–35. doi: 10.1111/j.1749-6632.2000.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 401.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–20. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 402.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–90. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 403.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658:44–9. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 404.Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 405.Blandini F, Braunewell KH, Manahan-Vaughan D, Orzi F, Sarti P. Neurodegeneration and energy metabolism: from chemistry to clinics. Cell Death Differ. 2004;11:479–84. doi: 10.1038/sj.cdd.4401323. [DOI] [PubMed] [Google Scholar]
- 406.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, et al. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 407.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 408.Sarti P, Giuffrè A, Barone MC, Forte E, Mastronicola D, Brunori M. Nitric oxide and cytochrome oxidase: reaction mechanisms from the enzyme to the cell. Free Radic Biol Med. 2003;34:509–20. doi: 10.1016/S0891-5849(02)01326-6. [DOI] [PubMed] [Google Scholar]
- 409.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–9. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 410.Tengan CH, Rodrigues GS, Godinho RO. Nitric oxide in skeletal muscle: role on mitochondrial biogenesis and function. Int J Mol Sci. 2012;13:17160–84. doi: 10.3390/ijms131217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhang J, Jin B, Li L, Block ER, Patel JM. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol. 2005;288:C840–9. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]
- 412.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem. 2007;282:37448–53. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 413.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–6. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Cooper CE, Davies NA, Psychoulis M, Canevari L, Bates TE, Dobbie MS, et al. Nitric oxide and peroxynitrite cause irreversible increases in the K(m) for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim Biophys Acta. 2003;1607:27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 415.Giuffrè A, Sarti P, D’Itri E, Buse G, Soulimane T, Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem. 1996;271:33404–8. doi: 10.1074/jbc.271.52.33404. [DOI] [PubMed] [Google Scholar]
- 416.Mason MG, Nicholls P, Wilson MT, Cooper CE. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci U S A. 2006;103:708–13. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Antunes F, Boveris A, Cadenas E. On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc Natl Acad Sci U S A. 2004;101:16774–9. doi: 10.1073/pnas.0405368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Antunes F, Boveris A, Cadenas E. On the biologic role of the reaction of NO with oxidized cytochrome c oxidase. Antioxid Redox Signal. 2007;9:1569–79. doi: 10.1089/ars.2007.1677. [DOI] [PubMed] [Google Scholar]
- 419.Cooper CE. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci. 2002;27:33–9. doi: 10.1016/S0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- 420.Brookes PS, Bolaños JP, Heales SJ. The assumption that nitric oxide inhibits mitochondrial ATP synthesis is correct. FEBS Lett. 1999;446:261–3. doi: 10.1016/S0014-5793(99)00217-3. [DOI] [PubMed] [Google Scholar]
- 421.Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–9. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 422.Almeida A, Almeida J, Bolaños JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A. 2001;98:15294–9. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 424.Arese M, Magnifico MC, Mastronicola D, Altieri F, Grillo C, Blanck TJ, et al. Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life. 2012;64:251–8. doi: 10.1002/iub.603. [DOI] [PubMed] [Google Scholar]
- 425.Masci A, Mastronicola D, Arese M, Piane M, De Amicis A, Blanck TJ, et al. Control of cell respiration by nitric oxide in Ataxia Telangiectasia lymphoblastoid cells. Biochim Biophys Acta. 2008;1777:66–73. doi: 10.1016/j.bbabio.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 426.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, et al. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588:3551–66. doi: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.McConell GK, Ng GP, Phillips M, Ruan Z, Macaulay SL, Wadley GD. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol (1985) 2010;108:589–95. doi: 10.1152/japplphysiol.00377.2009. [DOI] [PubMed] [Google Scholar]
- 428.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101:16507–12. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, et al. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010;24:5052–62. doi: 10.1096/fj.09-150714. [DOI] [PubMed] [Google Scholar]
- 430.Palomer X, Alvarez-Guardia D, Rodríguez-Calvo R, Coll T, Laguna JC, Davidson MM, et al. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res. 2009;81:703–12. doi: 10.1093/cvr/cvn327. [DOI] [PubMed] [Google Scholar]
- 431.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 432.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci U S A. 1998;95:3566–71. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 434.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–8. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 435.Jang B, Han S. Biochemical properties of cytochrome c nitrated by peroxynitrite. Biochimie. 2006;88:53–8. doi: 10.1016/j.biochi.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 436.Batthyány C, Souza JM, Durán R, Cassina A, Cerveñansky C, Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–46. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 437.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 438.Riobó NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, et al. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J. 2001;359:139–45. doi: 10.1042/bj3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Boczkowski J, Lisdero CL, Lanone S, Carreras MC, Aubier M, Poderoso JJ. Peroxynitrite-mediated mitochondrial dysfunction. Biol Signals Recept. 2001;10:66–80. doi: 10.1159/000046876. [DOI] [PubMed] [Google Scholar]
- 440.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–7. doi: 10.1016/0003-9861(92)90431-U. [DOI] [PubMed] [Google Scholar]
- 441.Aykaç-Toker G, Bulgurcuoğlu S, Koçak-Toker N. Effect of peroxynitrite on glutaredoxin. Hum Exp Toxicol. 2001;20:373–6. doi: 10.1191/096032701680350578. [DOI] [PubMed] [Google Scholar]
- 442.Trujillo M, Folkes L, Bartesaghi S, Kalyanaraman B, Wardman P, Radi R. Peroxynitrite-derived carbonate and nitrogen dioxide radicals readily react with lipoic and dihydrolipoic acid. Free Radic Biol Med. 2005;39:279–88. doi: 10.1016/j.freeradbiomed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 443.Cuzzocrea S, Zingarelli B, O’Connor M, Salzman AL, Szabó C. Effect of L-buthionine-(S, R)-sulphoximine, an inhibitor of gamma-glutamylcysteine synthetase on peroxynitrite- and endotoxic shock-induced vascular failure. Br J Pharmacol. 1998;123:525–37. doi: 10.1038/sj.bjp.0701612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Arteel GE, Briviba K, Sies H. Protection against peroxynitrite. FEBS Lett. 1999;445:226–30. doi: 10.1016/S0014-5793(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 445.Cuzzocrea S, Costantino G, Mazzon E, Caputi AP. Protective effect of N-acetylcysteine on multiple organ failure induced by zymosan in the rat. Crit Care Med. 1999;27:1524–32. doi: 10.1097/00003246-199908000-00021. [DOI] [PubMed] [Google Scholar]
- 446.Buchczyk DP, Grune T, Sies H, Klotz LO. Modifications of glyceraldehyde-3-phosphate dehydrogenase induced by increasing concentrations of peroxynitrite: early recognition by 20S proteasome. Biol Chem. 2003;384:237–41. doi: 10.1515/BC.2003.026. [DOI] [PubMed] [Google Scholar]
- 447.Souza JM, Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch Biochem Biophys. 1998;360:187–94. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 448.Konorev EA, Hogg N, Kalyanaraman B. Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett. 1998;427:171–4. doi: 10.1016/S0014-5793(98)00413-X. [DOI] [PubMed] [Google Scholar]
- 449.Bolaños JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–72. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 450.Pearce LL, Kanai AJ, Epperly MW, Peterson J. Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide. 2005;13:254–63. doi: 10.1016/j.niox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 451.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383:401–9. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 452.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–64. doi: 10.1016/S0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 453.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, et al. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–15. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 454.Han D, Canali R, Garcia J, Aguilera R, Gallaher TK, Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry. 2005;44:11986–96. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 455.Castro L, Rodriguez M, Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994;269:29409–15. [PubMed] [Google Scholar]
- 456.Forsmark-Andrée P, Persson B, Radi R, Dallner G, Ernster L. Oxidative modification of nicotinamide nucleotide transhydrogenase in submitochondrial particles: effect of endogenous ubiquinol. Arch Biochem Biophys. 1996;336:113–20. doi: 10.1006/abbi.1996.0538. [DOI] [PubMed] [Google Scholar]
- 457.Vieira HL, Belzacq AS, Haouzi D, Bernassola F, Cohen I, Jacotot E, et al. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–16. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 458.Scarlett JL, Packer MA, Porteous CM, Murphy MP. Alterations to glutathione and nicotinamide nucleotides during the mitochondrial permeability transition induced by peroxynitrite. Biochem Pharmacol. 1996;52:1047–55. doi: 10.1016/0006-2952(96)99426-5. [DOI] [PubMed] [Google Scholar]
- 459.Paixão J, Dinis TC, Almeida LM. Protective role of malvidin-3-glucoside on peroxynitrite-induced damage in endothelial cells by counteracting reactive species formation and apoptotic mitochondrial pathway. Oxid Med Cell Longev. 2012;2012:428538. doi: 10.1155/2012/428538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Sharpe MA, Cooper CE. Interaction of peroxynitrite with mitochondrial cytochrome oxidase. Catalytic production of nitric oxide and irreversible inhibition of enzyme activity. J Biol Chem. 1998;273:30961–72. doi: 10.1074/jbc.273.47.30961. [DOI] [PubMed] [Google Scholar]
- 461.Le Bras M, Clément MV, Pervaiz S, Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol. 2005;20:205–19. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- 462.Szabó C, Zingarelli B, O’Connor M, Salzman AL. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996;93:1753–8. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Liaudet L, Szabó G, Szabó C. Oxidative stress and regional ischemia-reperfusion injury: the peroxynitrite-poly(ADP-ribose) polymerase connection. Coron Artery Dis. 2003;14:115–22. doi: 10.1097/00019501-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 465.Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–12. doi: 10.1016/S0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 466.Liaudet L. Poly(adenosine 5’-diphosphate) ribose polymerase activation as a cause of metabolic dysfunction in critical illness. Curr Opin Clin Nutr Metab Care. 2002;5:175–84. doi: 10.1097/00075197-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 467.Liaudet L, Oddo M. Role of poly(adenosine diphosphate-ribose) polymerase 1 in septic peritonitis. Curr Opin Crit Care. 2003;9:152–8. doi: 10.1097/00075198-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 468.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:4469–73. [PubMed] [Google Scholar]
- 470.Buizza L, Cenini G, Lanni C, Ferrari-Toninelli G, Prandelli C, Govoni S, et al. Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS One. 2012;7:e29789. doi: 10.1371/journal.pone.0029789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Buizza L, Prandelli C, Bonini SA, Delbarba A, Cenini G, Lanni C, et al. Conformational altered p53 affects neuronal function: relevance for the response to toxic insult and growth-associated protein 43 expression. Cell Death Dis. 2013;4:e484. doi: 10.1038/cddis.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Cobbs CS, Whisenhunt TR, Wesemann DR, Harkins LE, Van Meir EG, Samanta M. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63:8670–3. [PubMed] [Google Scholar]
- 473.Olovnikov IA, Kravchenko JE, Chumakov PM. Homeostatic functions of the p53 tumor suppressor: regulation of energy metabolism and antioxidant defense. Semin Cancer Biol. 2009;19:32–41. doi: 10.1016/j.semcancer.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ma W, Sung HJ, Park JY, Matoba S, Hwang PM. A pivotal role for p53: balancing aerobic respiration and glycolysis. J Bioenerg Biomembr. 2007;39:243–6. doi: 10.1007/s10863-007-9083-0. [DOI] [PubMed] [Google Scholar]
- 475.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–91. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 476.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–9. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Johnson RF, Witzel II, Perkins ND. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-κB. Cancer Res. 2011;71:5588–97. doi: 10.1158/0008-5472.CAN-10-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Assaily W, Benchimol S. Differential utilization of two ATP-generating pathways is regulated by p53. Cancer Cell. 2006;10:4–6. doi: 10.1016/j.ccr.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 479.Park JY, Wang PY, Matsumoto T, Sung HJ, Ma W, Choi JW, et al. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ Res. 2009;105:705–12. doi: 10.1161/CIRCRESAHA.109.205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 481.Fink M. Cytopathic hypoxia in sepsis. Acta Anaesthesiol Scand Suppl. 1997;110:87–95. doi: 10.1111/j.1399-6576.1997.tb05514.x. [DOI] [PubMed] [Google Scholar]
- 482.L’Her E, Sebert P. A global approach to energy metabolism in an experimental model of sepsis. Am J Respir Crit Care Med. 2001;164:1444–7. doi: 10.1164/ajrccm.164.8.2102098. [DOI] [PubMed] [Google Scholar]
- 483.Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219–37. doi: 10.1016/S0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- 484.Xie YW, Wolin MS. Role of nitric oxide and its interaction with superoxide in the suppression of cardiac muscle mitochondrial respiration. Involvement in response to hypoxia/reoxygenation. Circulation. 1996;94:2580–6. doi: 10.1161/01.CIR.94.10.2580. [DOI] [PubMed] [Google Scholar]
- 485.Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169–75. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 486.Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med. 2009;46:462–70. doi: 10.1016/j.freeradbiomed.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Goossens V, Stangé G, Moens K, Pipeleers D, Grooten J. Regulation of tumor necrosis factor-induced, mitochondria- and reactive oxygen species-dependent cell death by the electron flux through the electron transport chain complex I. Antioxid Redox Signal. 1999;1:285–95. doi: 10.1089/ars.1999.1.3-285. [DOI] [PubMed] [Google Scholar]
- 488.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–20. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 489.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–23. [PubMed] [Google Scholar]
- 490.Vaughan RA, Garcia-Smith R, Trujillo KA, Bisoffi M. Tumor necrosis factor alpha increases aerobic glycolysis and reduces oxidative metabolism in prostate epithelial cells. Prostate. 2013;73:1538–46. doi: 10.1002/pros.22703. [DOI] [PubMed] [Google Scholar]
- 491.Vaughan RA, Garcia-Smith R, Dorsey J, Griffith JK, Bisoffi M, Trujillo KA. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer. 2013;133:2504–10. doi: 10.1002/ijc.28264. [DOI] [PubMed] [Google Scholar]
- 492.Samavati L, Lee I, Mathes I, Lottspeich F, Hüttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–44. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Jia L, Kelsey SM, Grahn MF, Jiang XR, Newland AC. Increased activity and sensitivity of mitochondrial respiratory enzymes to tumor necrosis factor alpha-mediated inhibition is associated with increased cytotoxicity in drug-resistant leukemic cell lines. Blood. 1996;87:2401–10. [PubMed] [Google Scholar]
- 494.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000;20:5680–9. doi: 10.1128/MCB.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Li C, Liu Q, Li N, Chen W, Wang L, Wang Y, et al. EAPF/Phafin-2, a novel endoplasmic reticulum-associated protein, facilitates TNF-alpha-triggered cellular apoptosis through endoplasmic reticulum-mitochondrial apoptotic pathway. J Mol Med (Berl). 2008;86:471–84. doi: 10.1007/s00109-007-0298-7. [DOI] [PubMed] [Google Scholar]
- 496.Uslu R, Bonavida B. Involvement of the mitochondrion respiratory chain in the synergy achieved by treatment of human ovarian carcinoma cell lines with both tumor necrosis factor-alpha and cis-diamminedichloroplatinum. Cancer. 1996;77:725–32. doi: 10.1002/(SICI)1097-0142(19960215)77:4<725::AID-CNCR19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 497.Hennet T, Richter C, Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–92. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Zell R, Geck P, Werdan K, Boekstegers P. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: evidence for primary impairment of mitochondrial function. Mol Cell Biochem. 1997;177:61–7. doi: 10.1023/A:1006896832582. [DOI] [PubMed] [Google Scholar]
- 499.Tredget EE, Yu YM, Zhong S, Burini R, Okusawa S, Gelfand JA, et al. Role of interleukin 1 and tumor necrosis factor on energy metabolism in rabbits. Am J Physiol. 1988;255:E760–8. doi: 10.1152/ajpendo.1988.255.6.E760. [DOI] [PubMed] [Google Scholar]


