Abstract
Advances in microscopy allow one to probe the structure of neurons and their interactions with astrocytes in brain slices and in vivo at ever increasing resolution. Moreover, the dynamic interactions between the cells can be examined in live preparation. In this paper we discuss how a variety of imaging approaches: confocal microscopy, electron microscopy, and multiphoton time-lapse microscopy are employed to probe neuron glia interactions in the developing cerebellum.
Keywords: cerebellum, synapse, astrocytes, dendritic spines
INTRODUCTION
Most central nervous system synapses have an intimate relationship with the glial processes that ensheath them (Peters & Kaiserman-Abramof, 1970; Spacek, 1985; Ventura & Harris, 1999; Grosche et al., 2002). Glial cells have been traditionally believed to have solely support functions, such as clearing neurotransmitters and ions from the synaptic cleft. Recently, however, glial cells have also been shown to have an active role in regulating synaptic development, regeneration, and physiological function (Son & Thompson, 1995; Iino et al., 2001; Seil, 2001; Ullian et al., 2001; Volterra et al., 2002; Auld & Robitaille, 2003; Ullian et al., 2004; Haber et al., 2006; Nishida & Okabe, 2007; Henneberger et al., 2010). For example, in the presence of astroglia, retinal ganglion cells have an increased number of mature functional synapses compared to cells grown in the absence of astroglia (Ullian et al., 2001). Furthermore, astrocytes were implicated in regulating the critical periods of synaptic plasticity in the visual system (Muller & Best, 1989; Muller, 1990). In the cerebellum it has been demonstrated that Bergmann glia (BG) appendages consist of repetitive units or “microdomains.” Based on calcium imaging experiments, these microdomains are thought to be capable of autonomous interactions with groups of synapses (Grosche et al., 1999). It has been suggested that these processes are involved in the structural and functional maturation of the synapses between parallel fibers and Purkinje cells (Yamada et al., 2000).
While the close apposition of the glial process to the synapse allows for easy interaction between the cells, the primary function of the ensheathment remains unclear. We hypothesized that the glial ensheathment of dendritic spines and synapses regulates spine dynamics and synaptogenesis. Using a combination of electron, confocal and multiphoton time-lapse microscopy, we first outlined the development of ensheathment of dendritic spines by astrocytes, next we determined the role of ensheathment in regulating dendritic spine motility, and last we determined the role of glial ensheathment in synaptogenesis (Lippman et al., 2008; Lippman Bell et al., 2010).
ENSHEATHMENT OF DENDRITIC SPINE BY GLIAL PROCESSES INCREASES WITH AGE
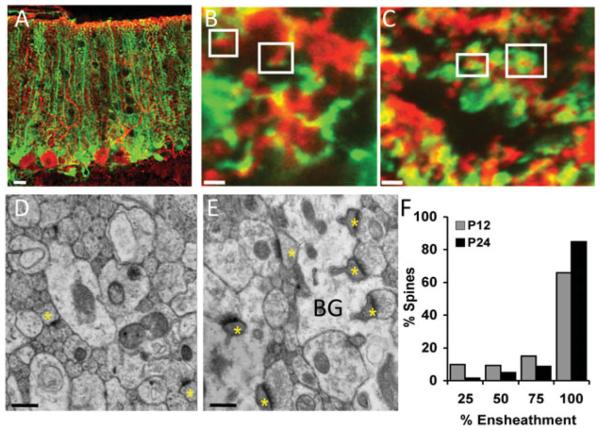
Ensheathment of Purkinje cell dendritic spines, sites of excitatory synaptic input, by BG processes was compared between the second and fourth postnatal weeks. Light level analysis of ensheathment was performed following virally transducing BG cells with green fluorescent protein (GFP) and immunostaining the Purkinje cells with an antibody directed against Calbindin (Fig. 1A). Due to the high tropism of the adenovirus to astrocytes, GFP was almost exclusively expressed in BG when injected into the cerebellar cortex despite the use of a ubiquitous (cytomegalovirus) promoter. High-resolution confocal images demonstrated that while in the younger animals most spines were sparsely ensheathed, in older animals the vast majority of dendritic spines were fully ensheathed by BG processes (Figs. 1B, 1C). This result was confirmed by electron microscopy analysis of cerebellar thin sections from postnatal days 12 and 24 (Figs. 1D, 1E). Measurement of the proportion of the perimeter of the dendritic spine in contact with astrocytic processes demonstrated that BG more completely surround spines in older mice than in younger mice (Fig. 1F). Thus during the first 4 postnatal weeks, a time when synapses form and mature, glial ensheathment of dendritic spines, the postsynaptic structures increases substantially.
Figure 1.
Developmental increase in ensheathment of PC dendritic spines by BG processes. (A) Purkinje cells immunostained with calbindin (red) in acute slice from a GFP-adenovirus injected mouse. Glial ensheathment grows more complete between (B) P13 and (C) P24. Electron micrographs of the cerebellar molecular layer from (D) P12 and (E) P24 mice show that synapses (yellow asterisks) are more fully ensheathed by glial processes (BG) in the older animals. (F) Quantification of glial ensheathment of spines demonstrated increased ensheathment in older animals. (P , 0.0001, chi-square.) Scale bar: (A) 20 mm, (B, C) 1 mm, and (D, E) 500 nm. Modified from Lippman et al. (2008).
ENSHEATHMENT OF DENDRITIC SPINES BY BG DOES NOT REGULATE SPINE MOTILITY
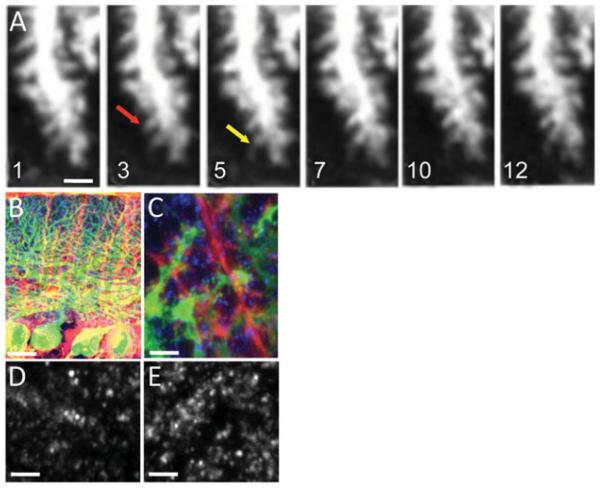
Dendritic spines are highly dynamic structures (Nimchinsky et al., 2002; Lippman & Dunaevsky, 2005). Spine motility is high in young animals but is much reduced with increased age (Dunaevsky et al., 1999; Deng & Dunaevsky, 2005; Lippman & Dunaevsky, 2005). Spine motility has been postulated to facilitate the formation of synaptic contacts between postsynaptic and presynaptic cells during development (Ziv & Smith, 1996). The mechanisms that regulate the reduction in spine motility with age are not well understood. We hypothesized that increased glial ensheathment in older animals might contribute to the reduced spine motility. We tested this hypothesis by experimentally reducing ensheathment of dendritic spines by BG. Reduction in glial ensheathment was achieved by ectopically expressing either the glutamate receptor subunit 2 (GluR2) (Iino et al., 2001) or the dominant negative form of the small GTPase Rac1 (Lippman et al., 2008) using adenovirus constructs. Both approaches resulted in reduced BG process length and spine ensheathment as was determined by light or electron microscopy. Motility of dendritic spines was analyzed in acute cerebellar slices (300 mm) from transgenic mice (Tg(Pcp2-cre)137Gsat) that expressed green fluorescent protein (GFP) in Purkinje cells, following reduction in glial processes length and ensheathment. Multiphoton time-lapse microscopy was performed on a Radiance 2000, Bio-Rad system (Bio-Rad, Hercules, CA, USA) coupled to a Nikon E-600-FN microscope with a Ti-Sapphire laser tuned to 920 nm. Images were collected every 30 s for 15 min with a 60X, N.A. 1 objective at a pixel size of 0.08 X 0.08 mm. These experiments determined that spine motility, measured by the Motility Index described in Dunaevsky et al. (1999), was not significantly different in slices with normal or reduced glial ensheathment (Fig. 2A). This led to the conclusion that although glial processes are increased at the same developmental stage that spine motility wanes, ensheathment of spines by glial processes does not regulate spine motility and other mechanisms must be involved.
Figure 2.
Decreased ensheathment by GluR2 expression in BG does not affect dendritic spine motility but increases synapse number. (A) Multiphoton time-lapse images of Purkinje cell dendritic spines in acute cerebellar slices from a P24 mouse. At this age, very little spine motility is observed. An example of a stable spine is highlighted by a red arrow and an example of a more motile spine is highlighted by a yellow arrow. Time is indicated in minutes. Low (B) and high (C) magnification confocal images of BG (red) expressing td-Tomato-GluR2 in cerebellar sections from transgenic mice expressing GFP in Purkinje cells (green), immunostained with the presynaptic marker VGluT1 (blue). Presynaptic terminals are identified with VGluT1 labeled puncta in cerebella injected with (D) control td-Tomato or with (E) td-Tomato-GluR2 expressing virus. Decreasing the level of glial ensheathment with GluR2 results in increase in the number of VGluT1 puncta. (P = 0.013, t-test.) Scale bar: (A) 2 mm, (B) 40 mm, and (C–E) 6 mm. Modified from Lippman Bell et al. (2010).
GLIAL ENSHEATHMENT REGULATES SYNAPSE FOR MATION IN THE CEREBELLUM
The synapse number increases during the first postnatal weeks, but the mechanisms that end the period of synaptogenesis are not well understood. To determine if glial ensheathment of dendritic spines regulates synaptogenesis in the cerebellum, we have quantified the number of presynaptic terminals and dendritic spines following reduction in glial ensheathment with ectopic expression of GluR2 or dnRac1 in BG. Cerebellar frozen sections (45 mm) in areas that express the viral constructs were immunostained for the presynaptic marker vGLuT1 (Fig. 2) that marks excitatory presynaptic terminals. Three channel confocal imaging was performed to visualize the tdTomato expressing BG, the vGluT1 stained presynaptic terminals and the GFP expressing Purkinje cell dendritic spines. Imaging was performed on a Zeiss LSM 510 confocal microscope with a 63X, 1.4 N.A. objective at pixel size of 0.05 X 0.05 mm. Imaging of each channel was performed sequentially using laser lines of 488, 561, and 633 nm to visualize GFP, tdTomato, and Alexa Fluor 647 conjugated antibody, respectively. We have found that the number of both vGluT1 labeled puncta and the number of dendritic spines was increased in regions with reduced BG ensheathment.
SUMMARY
Here we have described how several different microscopy approaches were used to determine how the structural interaction between neurons and glia changes with development and probe the role of this interaction in synapse formation and spine motility.
We found that ensheathment of Purkinje cells dendritic spines by BG processes increases over the first 4 postnatal weeks. Upon reduction in BG process outgrowth and ensheathment of dendritic spines, dendritic spine motility did not change, but the overall number of synapses increased. These results indicate that full spine coverage by BG in the cerebellum is not necessary for stabilization of spine dynamics, but is important in the regulation of synapse number. These experiments identify a novel role for astrocytes in capping synaptogenesis during development.
REFERENCES
- Auld DS, Robitaille R. Glial cells and neurotransmission: An inclusive view of synaptic function. Neuron. 2003;40(2):389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Deng J, Dunaevsky A. Dynamics of dendritic spines and their afferent terminals: Spines are more motile than presynaptic boutons. Dev Biol. 2005;277:366–377. doi: 10.1016/j.ydbio.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res. 2002;68:138–149. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuronglia interaction: Parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26(35):8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292(5518):926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64(1):47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- Lippman JJ, Lordkipanidze T, Buell ME, Yoon SO, Dunaevsky A. Morphogenesis and regulation of Bergmann glial processes during Purkinje cell dendritic spine ensheathment and synaptogenesis. Glia. 2008;56(13):1463–1477. doi: 10.1002/glia.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Bell JJ, Lordkipanidze T, Cobb N, Dunaevsky A. Bergmann glial ensheathment of dendritic spines regulates synapse number without affecting spine motility. Neuron Glia Biol. 2010;2:1–8. doi: 10.1017/S1740925X10000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CM. Dark-rearing retards the maturation of astrocytes in restricted layers of cat visual cortex. Glia. 1990;3(6):487–494. doi: 10.1002/glia.440030607. [DOI] [PubMed] [Google Scholar]
- Muller CM, Best J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature. 1989;342(6248):427–430. doi: 10.1038/342427a0. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27(2):331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–356. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Interactions between cerebellar Purkinje cells and their associated astrocytes. Histol Histopathol. 2001;16(3):955–968. doi: 10.14670/HH-16.955. [DOI] [PubMed] [Google Scholar]
- Son Y-J, Thompson W. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berlin) 1985;171(2):245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47(3):209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19(16):6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Magistretti P, Haydon P. The Tripartite Synapse: Glia in Synaptic Transmission. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;28:106–120. [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]