Abstract
Objective
To evaluate associations between work-related stress, stressful life events, and perceived stress and semen quality.
Design
Cross-sectional analysis.
Setting
Northern California.
Patient(s)
193 men from the Child Health and Development Studies evaluated between 2005–2008.
Intervention(s)
None.
Main Outcome Measure(s)
Measures of stress including job strain, perceived stress, and stressful life events; outcome measures of sperm concentration, percentage of motile sperm, and percentage of morphologically normal sperm.
Result(s)
We found an inverse association between perceived stress score and sperm concentration (estimated coefficient b = –0.09 × 103/mL; 95% confidence interval [CI] = –0.18, –0.01), motility (b = –0.39; 95% CI = –0.79, 0.01), and morphology (b = –0.14; 95% CI, –0.25, –0.04) in covariate-adjusted linear regression analyses. Men who experienced two or more stressful life events in the past year compared with no stressful events had a lower percentage of motile sperm (b= –8.22; 95% CI, –14.31, –2.13) and a lower percentage of morphologically normal sperm (b = –1.66; 95% CI, –3.35, 0.03) but a similar sperm concentration. Job strain was not associated with semen parameters.
Conclusion(s)
In this first study to examine all three domains of stress, perceived stress and stressful life events but not work-related stress were associated with semen quality.
Keywords: Semen quality, stress, work
Semen quality is a key marker of male reproductive health. Identifying the psychosocial risk factors such as stress for poor semen quality is important for improving fecundity and fertility, and may also have implications for life course and intergenerational health (1). However, the research examining the association between psychosocial stress and semen quality has been inconsistent. In studies examining life stress, one prospective study found no association (2), another study found positive results for only one particular stressful life event (death of a family member) (3), and others were inconsistent in regard to the semen parameter with which an association was found (3–6). One study of work stress found an inverse association with semen quality (7), but two others did not (2, 3).
Several reasons may explain the inconsistent associations. First, many studies are based on populations of couples from prenatal clinics or infertility clinics, which are select populations who may not reflect the levels of stress or semen quality in a more general population. Second, no previous study has included both subjective and objective validated measures of stress as well as measures in both work and life domains. Subjective measures of stress focus on an individual's perception of his ability to cope with the demands of events, whereas objective measures of stress assess experiences thought to be universally associated with a stress response (8); both may be necessary to characterize stress in relation to male reproductive health.
Our objective was to examine sperm concentration, motility, and morphology in association with work stress and both subjective and objective measures of life stress in a sample of 193 healthy men aged 38 to 49 years from the general population in northern California. We hypothesized that lower job control, higher job demands, higher perceived stress, and a higher number of stressful life events would be associated with poorer semen quality. Additionally, we explored a potential neuroendocrine pathway between stress and semen measures by examining covariate-adjusted associations between stress measures and reproductive hormones—total testosterone and follicle-stimulating hormone (FSH)—and between reproductive hormones and semen measures.
MATERIALS AND METHODS
Study Population
This cross-sectional analysis used data collected from men aged 38 to 49 years as part of the Study of the Environment and Reproduction (SER) follow-up to the Child Health and Development Studies (CHDS). The CHDS is a pregnancy cohort study conducted among members of Kaiser Foundation Health Plan in the Oakland, California, area. Over 15,000 women participated between 1959 and 1966, resulting in about 20,000 pregnancies. One of a series of follow-up studies launched in 2005 to explore how fetal and childhood growth and development affect adult health, SER was designed to investigate the effects of prenatal organochlorine exposure on men's reproductive health (9, 10). To become participants, the couples were required to have birth length and weight data, maternal interview data, sufficient second-trimester and postpartum serum available for serologic measures, and to live within approximately 100 miles of the Kaiser Oakland Clinic. Out of 3,531 eligible patients, 654 were traced, 338 of whom (52%) qualified and agreed to participate. Of those, 196 (58%) provided semen samples for analysis. This report is based on the 193 who provided a semen sample and for whom we were able to calculate subject and objective stress scores. The institutional review boards at Columbia University Medical Center, Kaiser Permanente, and the Public Health Institute approved this study.
Data Collection
Data were collected between December 2005 and April 2008. The men traveled to the clinic for 1-hour in-person interviews in which they provided extensive demographic, lifestyle, and occupational information. There were no exclusion criteria for participation, but detailed health and reproductive histories were taken to be considered for later exclusion if necessary. Subsequent to the interview, men were asked to provide semen samples followed by blood samples.
Stress Measures
As part of the in-person interview, participants completed three stress scales to measure work stress and both subjective and objective life stress. Among employed men, work stress over the past 3 months was assessed using 16 items from the Job Content Questionnaire (JCQ) (11). The time frame of 3 months was chosen to correlate with the period of spermato-genesis, approximately 72 days (1). The JCQ measures job characteristics among two dimensions—job demands and job control—and can be characterized as an assessment of the psychological and social structure of the work situation. Respondents are asked their agreement on a Likert scale for job demand items such as “I have time to get my work done,” and job control items such as “At my job, I am given a lot of freedom to decide how I do my work.” We calculated quantitative demands and control dimensions based on recoding for directionality and summing, with a higher score indicating greater job demands or greater job control. We then combined these to create a dichotomous job strain variable by categorizing those subjects above the median job demand score (median score: 32, range: 12–48) and below the median job control score (median score: 74, range: 26–96) as “high job strain.” An additional question was asked regarding the security of the men's current job. We collapsed the 4-point Likert scale response to create a dichotomous security variable (1 = strongly agree/agree; 0 = strongly disagree/disagree).
To measure subjective stress, participants were asked to complete a validated abbreviated version (10 out 14 items) of the Perceived Stress Scale (PSS) (12). The PSS measures how often events are perceived as stressful by asking about feelings during the last month. For example, “During the last month, how often have you felt that things were going your way?” Items in the PSS were scored on a 5-point frequency scale (0 = never, 4 = very often). After recoding for consistent directionality, we summed response numbers to yield a quantitative total PSS score, with a higher score indicating greater perceived stress.
Participants also completed a validated shortened version of the Life Events Inventory (LEI) (13), including the top 10 stressors identified by men in an occupational sample (14), to provide an objective measure of stressful events experienced over the past year. The number of events was summed, and a 3-level ordinal categorical LEI score was used for analysis: 0, 1, and ≥2 negative life events based on the distribution of the variable and to be consistent with previous literature (6).
Semen Quality Measures
Men were asked to provide two semen samples approximately 2 weeks apart, remaining abstinent for 2 to 5 days before each donation. Samples were given at the Kaiser clinic and only counted if all of the ejaculate was captured in the provided container; if not, the man was asked to return for an additional visit. Men were asked the date and time of their last ejaculation, and the date and time of the sample collection were also recorded. Analysis was conducted by three andrology technicians trained at the University of California–Davis (UCD), according to the same protocol used by the National Institute of Child Health and Human Development–funded National Cooperative Reproductive Medicine Network (Fertile Male Study) (15). Briefly, within 1 hour of sample collection at the Kaiser clinic, technicians measured the ejaculate's volume by weight and determined the sperm concentration and percentage of motile sperm. Clinic technicians also prepared seminal smears that were shipped to the andrology laboratory at UCD, where they were stained and then analyzed to determine the percentage of normal morphology. Sperm were classified as having normal or abnormal morphology according to strict criteria (16). Between-technician reliability was excellent (ICC >0.9 for all three outcome measures). Further details of the semen analysis procedure have been published elsewhere (10). A total of 355 semen samples were provided by 196 men. For the men who contributed two samples, the mean concentration, motility, and morphology measures were used for the analysis. To approximate a normal distribution, we square-root transformed sperm concentration; motility and morphology measures were approximately normal and therefore were left untransformed.
Covariates
Information on behavioral, sociodemographic, and medical risk factors for infertility were assessed during the in-person interview. Covariates were identified as potential confounders based on a review of the literature and a causal diagram, including time since last ejaculation, age (continuous), race (white, black, other), education (high school, some college, bachelor's degree, graduate level), body mass index (BMI) in quartiles, smoking status (current vs. ever/ never), ever diagnosed with sexually transmitted infection (STI), frequent biking (≥5 hours per week), and hazardous exposures through job or hobbies. Hazardous exposures were assessed with a checklist regarding exposures in the past 3 months and included items hypothesized to be associated with semen quality such as heat and paint. Exposures were summed, and separate scores were created for occupational and for recreational exposure; after examination of the distribution, scores were dichotomized into any versus none. Coffee and alcohol drinking were assessed but not considered as potential confounders as it has been previously reported that they are not associated with semen quality in this study sample (10).
Statistical Analysis
In bivariate analyses, we conducted analysis of variance (ANOVA) to compare the mean of job demands, job control, and perceived stress score according to levels of categorical covariates. A chi-square test was used to detect association between categorical predictor and outcome variables.
Using the subsample of men employed during the past 3 months, we undertook a series of linear regression analyses to estimate the associations between each of the four job stress parameters and the three continuous outcome measures: sperm concentration, percent motility, and percent normal morphology. For each outcome measure, we created three models: an unadjusted model with only the main effects, a second model with the main effects adjusted for potential confounders, and a third additionally adjusting for life stress measures. In addition to the dichotomous measure of job strain, we evaluated job strain using two other methods: [1] entering a term into the model for job demands divided by job control, and [2] entering into the model a cross-product term for job demands by job control (17).
Using the full sample of men, we repeated unadjusted and covariate-adjusted models to estimate associations between the two life stress scales (LEI and PSS) and the three outcome measures. We did not include the two measures of life stress as predictors in the same model because they are two different measures of the same underlying construct; they also were highly correlated. We then produced scatter plots for PSS score and the three outcome measures along with predicted values from the covariate-adjusted regression models. We also examined a series of covariate-adjusted models to test for additive interaction between each job stress and life stress variable using cross-product terms. Finally, because of the concurrent timing of our study with the economic recession in California, we were interested in unemployment alone as a stressor, so we conducted a secondary analysis in which we compared semen measures between the men who were currently employed at the time of the survey with those who were unemployed.
We also examined the associations using an alternative statistical approach: instead of using the mean of the two semen measures, we applied linear models with repeated measures that used generalized estimating equations to take into account within-person correlation in the semen measures when estimating model parameters and testing the hypotheses. All data were analyzed using SAS 9.2 statistical software (SAS Institute).
RESULTS
Descriptive Analyses
The mean value for sperm concentration was 72.5 × 106/mL (range: 0–429.9), for the percentage of motile sperm was 39.4% (range: 0–76.5), and for the percentage of morphologically normal sperm was 7.6% (range: 0–20.0). In descriptive analyses, higher job demands were associated with college education, older age, and fewer exposures to hazardous substances at work (Table 1). Lower job control was associated with lower education level, lower income, and no hazardous exposures from hobbies. Job strain was not associated with any study characteristic. A higher number of stressful life events was associated with lower education. It also was associated with income but in a nonlinear pattern such that those in the lowest and third quartile of income had a greater number of events than in the other two categories. Current smokers also experienced a greater number of stressful life events. Men with a lower income had a higher perceived stress score; men who currently smoked and had ever had a sexually transmitted infection also had higher perceived stress scores.
Table 1.
Life and work stress exposures by study covariates, California, 2005–2008.
| Job demands | Job control | Job strain (%) | Job security (%) | Life Events Inventory (%) | Perceived Stress Scale | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Mean (SD) | Mean (SD) | High | High | n | 0 adverse events | 1 adverse event | ≥2 adverse events | Mean (SD) |
| Education | 155 | 188 | ||||||||
| High school | 22 | 32.5 (4.5)a | 69.2 (15.7)a | 22.7 | 86.4 | 35 | 28.6 | 22.9 | 48.6a | 13.3 (6.9) |
| Some college | 59 | 33.7 (6.5) | 72.8 (11.5) | 28.8 | 89.8 | 72 | 45.8 | 31.9 | 22.2 | 12.5 (6.6) |
| Bachelor's degree | 54 | 33.0 (6.6) | 75.7 (12.9) | 18.5 | 87.0 | 59 | 59.3 | 23.7 | 17.0 | 12.2 (4.8) |
| Graduate level | 20 | 29.2 (6.0) | 84.5 (9.4) | 10.0 | 80.0 | 22 | 77.3 | 9.1 | 13.6 | 10.4 (4.8) |
| Income | 151 | 183 | ||||||||
| ≤ $50,000 | 33 | 31.3 (5.9) | 68.5 (12.1)b | 30.3 | 81.8 | 56 | 33.9 | 28.6 | 37.5a | 14.4 (7.1)a |
| $50,000–99,000 | 59 | 33.6 (5.6) | 72.1 (13.8) | 23.7 | 86.4 | 66 | 57.6 | 25.8 | 16.7 | 11.6 (5.7) |
| $100,000–149,000 | 29 | 31.4 (7.4) | 81.2 (8.2) | 13.8 | 96.6 | 31 | 48.4 | 19.4 | 32.3 | 10.6 (4.5) |
| ≥ $150,000 | 30 | 33.2 (7.1) | 81.1 (11.6) | 13.3 | 86.7 | 30 | 70.0 | 20.0 | 10.0 | 11.6 (4.5) |
| Age (range: 39–47 y) | 155 | 193 | ||||||||
| ≤43 y | 55 | 34.3 (6.0)a | 72.9 (13.0) | 27.3 | 87.3 | 68 | 55.9 | 26.5 | 17.7 | 12.7 (5.7) |
| >43 y | 100 | 31.8 (6.4) | 75.8 (13.1) | 19.0 | 87.0 | 125 | 46.4 | 25.6 | 28.0 | 12.2 (6.1) |
| Race | 155 | 193 | ||||||||
| White | 90 | 32.3 (7.1) | 75.9 (13.4) | 18.9 | 85.6 | 108 | 50.0 | 27.8 | 22.2 | 12.2 (5.8) |
| Black | 31 | 33.5 (5.7) | 73.0 (12.3) | 29.0 | 90.3 | 48 | 41.7 | 25.0 | 33.3 | 13.4 (5.9) |
| Other | 34 | 33.0 (4.5) | 73.6 (12.8) | 23.5 | 88.2 | 37 | 59.5 | 21.6 | 18.9 | 11.6 (6.6) |
| BMI (kg/cm2) | 154 | 191 | ||||||||
| <25 | 35 | 31.2 (6.6) | 75.0 (15.7) | 20.0 | 82.9 | 44 | 47.7 | 25.0 | 27.3 | 11.5 (5.6) |
| 25–29.9 | 68 | 33.0 (5.8) | 75.5 (12.7) | 19.1 | 88.2 | 79 | 53.2 | 26.6 | 20.3 | 11.9 (5.9) |
| 30–34.9 | 31 | 33.4 (6.6) | 71.0 (11.7) | 32.3 | 87.1 | 44 | 38.6 | 22.7 | 38.6 | 14.3 (6.9) |
| ≥ 35 | 20 | 34.2 (6.5) | 78.2 (10.6) | 20.0 | 90.0 | 24 | 62.5 | 29.2 | 8.3 | 12.5 (4.8) |
| Smoking | 155 | 188 | ||||||||
| Never/ever | 137 | 32.7 (6.6) | 75.2 (13.1) | 21.2 | 86.1 | 155 | 55.5 | 22.6 | 21.9a | 11.9 (5.7)a |
| Current | 18 | 32.7 (3.3) | 71.7 (12.6) | 27.8 | 94.4 | 33 | 27.3 | 36.4 | 36.4 | 14.3 (6.6) |
| STI | 154 | 187 | ||||||||
| None | 113 | 32.7 (5.9) | 75.3 (13.0) | 19.5 | 86.7 | 135 | 54.1 | 23.0 | 23.0 | 11.7 (5.8)a |
| Any | 41 | 32.7 (7.5) | 73.7 (13.3) | 29.3 | 87.8 | 52 | 42.3 | 30.8 | 26.9 | 13.9 (6.3) |
| Biking | 153 | 190 | ||||||||
| < 5 h/wk | 150 | 32.8 (6.0)c | 74.6 (13.1) | 22.0 | 86.7 | 186 | 51.1 | 24.7 | 24.2 | 12.4 (6.0) |
| ≥ 5 h/wk | 3 | 26.3 (17.6) | 83.3 (9.0) | 0.0 | 100.0 | 4 | 25.0 | 50.0 | 25.0 | 10.3 (6.3) |
| Hazardous exposuresd | ||||||||||
| From job | 155 | 193 | ||||||||
| None | 88 | 31.8 (7.0)a | 74.8 (14.1) | 20.5 | 87.5 | 120 | 48.3 | 23.3 | 28.3 | 12.7 (6.2) |
| Any | 67 | 33.9 (5.1) | 74.8 (11.7) | 23.9 | 86.6 | 73 | 52.1 | 30.1 | 17.8 | 11.8 (5.4) |
| From hobbies | 155 | 193 | ||||||||
| None | 77 | 32.6 (5.1) | 72.6 (13.8)a | 23.4 | 89.6 | 97 | 51.6 | 26.8 | 21.7 | 12.4 (5.6) |
| Any | 78 | 32.8 (7.4) | 77.0 (12.0) | 20.5 | 84.6 | 96 | 47.9 | 25.0 | 27.1 | 12.4 (6.4) |
Note: n = 193 for life stress variables; n = 165 for work stress variables (employed men only). BMI = body mass index; SD = standard deviation; STI = sexually transmitted infection.
P< .05, chi-square test.
P< .0001, chi-square test.
P< .1, chi-square test.
Hazardous exposures include solvents, lead, paint, pesticides, metal fumes, anesthetic gases, chemotherapeutic drugs, excess heat, vibration, radiation, electromagnetic fields, and other.
In bivariate analyses of potential confounders and semen parameters, current smokers had a lower percentage of motile and morphologically normal sperm, as reported previously elsewhere (10). Current smokers compared with nonsmokers had a lower percentage of motile sperm (30.8% vs. 41.2%) and lower percentage of morphologically normal sperm (6.3% vs. 7.9%). Men with a lower income had a lower percentage of motile sperm (34.0% for those in the lowest quartile of income, compared with 40.3%, 45.0%, and 41.3% for the 2nd, 3rd, and 4th quartiles of income, respectively). Black men had both a lower percentage of motile and lower percentage of morphologically normal sperm than white men and men of other races/ethnicities (32.8% vs. 41.0% and 43.3%, respectively). Men who biked at least 5 hours per week had a lower percentage of motile sperm than those who did not (12.1% vs. 16.4%). Other potential confounders were not statistically significantly associated with semen parameters.
Work Stress
Work stress measures were not associated with semen parameters in unadjusted or covariate-adjusted analyses (Table 2). Estimates additionally adjusted for perceived stress score or Life Events Inventory did not alter the results (data not shown). Job strain calculated using the two alternative methods also showed no association with semen parameters (data not shown).
Table 2.
Unadjusted and covariate-adjusted regression coefficients for work stress measures and semen quality, California, 2005–2008.
| Square root of sperm concentration (103/mL)a | % Motilea | % Normal morphologyb | ||||
|---|---|---|---|---|---|---|
| Variable | Unadjusted b (95% CI) | Adjusted b (95% CI)c | Unadjusted b (95% CI) | Adjusted b (95% CI)c | Unadjusted b (95% CI) | Adjusted b (95% CI)c |
| Job demands | –0.01 (–0.09, 0.07) | 0.00 (–0.09, 0.08) | –0.24 (–0.63, 0.15) | –0.22 (–0.64, 0.219) | –0.02 (–0.13, 0.08) | –0.06 (–0.17, 0.05) |
| Job control | –0.01 (–0.05, 0.03) | 0.03 (–0.02, 0.08) | 0.07 (–0.13, 0.26) | 0.11 (–0.11, 0.33) | 0.00 (–0.05, 0.05) | 0.03 (–0.03, 0.09) |
| Job strain | ||||||
| Lowd | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| High | –0.06 (–1.30, 1.18) | –0.45 (–1.74, 0.84) | –3.27 (–9.26, 2.73) | –2.99 (–9.10, 3.11) | –0.62 (–2.17, 0.94) | –0.93 (–2.59, 0.72) |
| Job security | ||||||
| Secured | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Insecure | 0.08 (–1.44, 1.60) | 0.17 (–1.41, 1.76) | 3.25 (–4.09, 10.59) | 4.02 (–3.43, 11.48) | 0.62 (–1.30, 2.54) | 0.79 (–1.25, 2.83) |
n = 165 for unadjusted model, 155 for covariate-adjusted models (demands, strain); n = 166 for unadjusted model, 156 for adjusted models (control, security).
n = 161 for unadjusted model, 151 for covariate-adjusted models (demands, strain); n = 162 for unadjusted model, 152 for adjusted models (control, security).
Adjusted for time since last ejaculation, education, income, age, race, body mass index, smoking, sexually transmitted infection, and hazardous exposures (occupational and recreational).
Reference category.
Life Stress
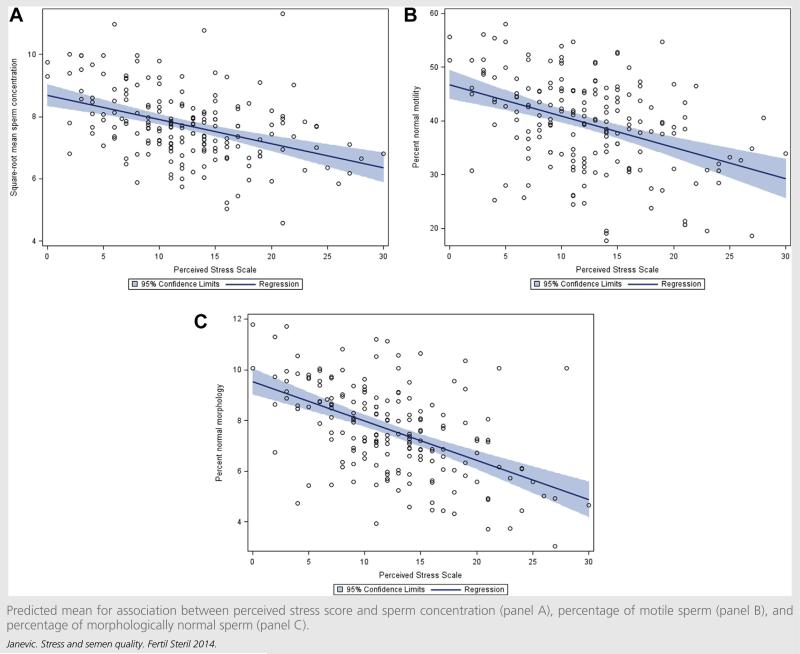
In unadjusted analyses of perceived stress, a 1 point increase in perceived stress score was associated with a decrease in mean of square root transformed sperm concentration (× 103/mL) (b = –0.06; 95% CI, –0.14, 0.02), a decrease in the percentage of motile sperm (b = –0.58; 95% CI, –0.97, –0.20), and a decrease in the percentage of morphologically normal sperm (b = –0.13; 95% CI, –0.23, –0.03) Table 3). After adjusting for potential confounders, the coefficients relating the perceived stress score to semen measures were similar (see Table 3). The scatter plots in Figure 1 show the covariate-adjusted regression line for perceived stress score predicting semen measures.
Table 3.
Unadjusted and covariate-adjusted regression coefficients for life stress measures and semen quality, California, 2005–2008.
| Square root sperm concentration (103/mL)a | % Motilea | % Normal morphologyb | ||||
|---|---|---|---|---|---|---|
| Scale | Unadjusted b (95% CI) | Adjusted b (95% CI) | Unadjusted b (95% CI) | Adjusted b (95% Cl)c | Unadjusted b (95% CI) | Adjusted b (95% Cl)c |
| PSS LEIf |
–0.06 (–0.14, 0.02) | –0.09 (–0.18, –0.01)d | –0.58 (–0.97, –0.20)d | –0.39 (–0.79, 0.01)e | –0.13 (–0.23, –0.03)d | –0.14 (–0.25, –0.04)d |
| 1 adverse event | 0.51 (–0.64, 1.65) | 0.26 (–0.98, 1.49) | –0.81 (–6.37, 4.76) | 0.34 (–5.28, 5.95) | –0.84 (–2.28, 0.61) | –0.68 (–2.24, 0.87) |
| ≥2 adverse events | –0.31 (–1.48, 0.86) | –0.74 (–2.08, 0.60) | –9.59 (–15.27, –3.91)d | –8.22 (–14.31, –2.13)d | –1.83 (–3.30, 0.35)d | –1.66 (–3.35, 0.03)e |
Note: LEI = Life Events Inventory; PSS = Perceived Stress Scale.
n = 193 for unadjusted models, 181 for covarlate-adjusted models.
n = 189 for unadjusted models, 177 covarlate-adjusted models.
Adjusted for time since last ejaculation, education, Income, age, race, BMI, smoking, STI, and hazardous exposures (occupational and recreational).
P<.05.
P<.1.
Reference category Is no stressful life events.
FIGURE 1.
Predicted mean for association between perceived stress score and sperm concentration (panel A), percentage of motile sperm (panel B), and percentage of morphologically normal sperm (panel C).
The final set of models explored stressful life events and semen parameters. Two or more stressful life events compared with none was associated with a decline in the percentage of motile sperm (b = –9.59; 95% CI, –15.27, –3.91) and in the percentage of morpholocgically normal sperm (b = –1.83; 95% CI, –3.30, 0.35) (see Table 3). After the adjustment for potential confounders, these associations were similar (b = –8.22; 95% CI, –14.31, –2.13; and b = –21.66; 95% CI, –3.35, 0.03). Two or more stressful life events compared with none was not associated with sperm concentration; one stressful life event compared with none was not associated with any semen parameter. Models including both work and life stress as independent variables did not substantially alter the results for any of the semen parameters (data not shown). Interaction terms examining the additive joint effects of work and life stress on semen parameters were not statistically significant.
Employed men compared with unemployed men had higher measures of sperm concentration, percentage of motile sperm, and percentage of morphologically normal sperm, adjusting for potential confounders (b = 2.04, 95% CI, 0.51, 3.58; b = 8.07, 95% CI, 0.89, 15.24; and b = 2.18, 95% CI, 0.2, 4.13, respectively). After additionally adjusting for the first perceived stress scale and second Life Events Inventory, the coefficients were similar.
Repeated measures analyses produced nearly identical results. For example, the coefficient for job strain was –0.45 (95% CI –1.74, 0.84) in the original model for mean measures and –0.55 (95% CI, –1.74, 0.84) in the model for repeated measures; for PSS was in –0.09 (95% CI, –0.18, –0.01) in the original model and –0.09 (95% CI, –0.19, 0.00) in the model for repeated measures; and for LEI was –0.74 (95% CI, –2.08, 0.60) in the original model and –0.72 (95% CI, –2.09, 0.65) in the model for repeated measures. We also performed a sensitivity analysis by removing three men with a measure of 0 for sperm parameters; again, the results were very similar.
Because job strain has been associated with reduced study participation in previous research, we examined whether men who gave a semen sample had lower job strain than those who did not (18). Men who gave a semen sample had similar levels of job strain (22%) than those who did not (19%), suggesting that participation bias is not a likely explanation for modest or null associations between job strain and semen quality. However, we do not know the levels of job strain in the men who did not participate in either segment of the study, so participation bias cannot be ruled out entirely.
Neuroendocrine Mediators of Stress and Semen Quality
In covariate-adjusted models examining associations between both FSH and total testosterone with semen quality, FSH was inversely associated with sperm concentration (b = –0.37; 95% CI, –0.50, –0.24), motility (b = –0.17; 95% CI, –1.80, –0.55), and morphology (b = –1.17; 95% CI, –0.37, 0.02). Testosterone was only weakly associated with motility (b = –0.01; 95% CI, –0.02, 0.01) morphology (b = –0.003, –0.007, 0.001). Life stress measures were not associated with FSH or total testosterone in covariate-adjusted models. High job strain was associated with lower levels of testosterone (b = –81.81; 95% CI, –145.37, –18.24) but not FSH (b = –0.57, –2.08, 0.94). Other work stress variables were not associated with total testosterone or FSH.
Influence of Previous Reproductive Health Problems or Comorbidities
To explore the alternative explanation that men who were concerned about their fertility reported higher levels of stress, we conducted an analysis in which we excluded men who answered yes to the question “Have you ever been concerned about a possible problem with your fertility?” (n = 13) or who had reported a history of reproductive health problems, including erectile dysfunction (n = 10), varicose veins around the testis (n = 1), surgery in genital or pelvic area (n = 10), or other urologic conditions or genital injuries (n = 4). The resulting estimates increased very slightly in magnitude, and the overall patterns were similar. Because other health conditions that might influence semen quality might also influence the reporting of stress, we additionally conducted an analysis excluding 80 men who reported having been previously diagnosed with diabetes, stroke, hypertension, anxiety, or depression, or were taking medications for these conditions. Again, the results were similar, thus providing no support to reverse causality as an alternative explanation for our findings.
DISCUSSION
In this first study of semen quality to measure three dimensions of stress, we found that life stress measured both subjectively (Perceived Stress Scale) and objectively (Life Events Inventory) was associated with sperm concentration, motility, and morphology. The exception was life events and sperm concentration, for which there was no association. Work stress was not associated with semen measures, although the coefficients comparing men with high job strain to low job strain were in the hypothesized direction.
Our study is consistent with some but not all previous studies. A recent study of U.S. men recruited from five prenatal clinics that examined associations between stressful live events and semen quality found that two or more stressful life events was associated with reduction in sperm concentration and the percentage of motile sperm, but was not associated with a continuous measure of the percentage of morphologically normal sperm (6). In a recent population-based study of stress and semen quality in China, higher perceived stress was associated with decreased sperm concentration and a decreased percentage of morphologically normal sperm but was not associated with motility (19). The findings of an earlier study in California reported an association between one particular life event (death of a family member) and one semen parameter (motility) (3). In Auger et al. (5), perceived stress was associated with poorer morphology, but other semen parameters were not studied. In contrast to these studies in which stress was associated with one or more semen parameters, Hjollund et al. (2) found no association at all.
Inconsistent results in studies examining perceived life stress and semen quality might be explained by the measures of stress that were used. Three previous studies measured perceived stress; of these, two did so using only one interview question (4, 5), and the third used the General Health Questionnaire (2), a scale not designed specifically to measure perceived stress (6). We used the Perceived Stress Scale and found associations with all semen parameters, suggesting it may be the most valid measure of life stress in etiologic studies of semen quality. The inconsistent results in previous studies points to the need to use comprehensive measures that measure different aspects of stress, as we did in our study.
In contrast with the life stress measure, we found no association between work stress and semen quality. The literature regarding work stress and semen quality has also had mixed findings. One study using only a single measure of work stress found an association with poorer sperm motility and morphology (7), but two others using Karasek's Job Content Questionnaire, similar to the measure used in our study, did not (2, 3). An additional case-control study found an association between job “burn-out” and infertility (20). However, just as we found that men with high job strain had a not statistically significant trend toward poorer semen quality, Hjollund et al. (2) similarly found not statistically significant trends. It may be that job strain in the populations studied does not result in a high enough level of stress to have a deleterious effect on semen quality. In addition, we did find an association between high job strain and decreased levels of total testosterone, consistent with previous research (21). Work stress is an important dimension of stress to measure that re-flects a separate domain than life events and perceived stress and has implications for workplace wellness programs as well as how to best measure stress in studies of male reproductive health. Taken together, our results for analyses associating job strain, total testosterone, and semen quality suggest that job strain may be important to male reproductive health, but our measures or study design have not sufficiently modeled the relationship. Other measures of job stress, such as effort-reward imbalance, long work hours, or organizational injustice, need to be examined in relation to semen quality (22–24).
Because our study included some unemployed men, we were curious as to whether our data would suggest that unemployment, itself a stressor, is associated with poor semen quality. In a secondary analysis, we found that employed men compared with unemployed men consistently had higher measures of sperm concentration, percentage of motile sperm, and percentage of morphologically normal sperm, adjusting for potential confounders and independent of perceived stress and other stressful life events. These findings are particularly of interest given that the data collection of this study partially overlapped the economic crisis in California (25). The finding that unemployment is associated with reduced semen quality is consistent with previous literature demonstrating that male unemployment and economic recession generally are associated with reduced fertility at the population level (26).
The proposed mechanism by which stress influences semen quality occurs via neuroendocrine factors influencing spermatogenesis. Stress leads to increased levels of glucocorticoids, leading to decreased rates of testosterone excretion from Leydig cells, responsible for spermatogenesis (27). Glucocorticoids may also induce apoptosis of Leydig cells, thus reducing the total number of cells (28). In exploratory analyses, we found expected associations FSH and total testosterone with semen measures, but we found no associations between stress measures and either FSH or total testosterone, and thus were not able to produce evidence to support a neuroendocrine mechanism for associations between life stress and semen measures. However, given the exploratory nature of this analysis, a neuroendocrine mechanism should not be ruled out.
Several other mechanisms may also explain our study findings. It has been proposed that stress results in increased levels of seminal plasma reactive oxygen species, which in turn results in oxidative stress, which has been shown to in-fluence semen quality and fertility (29, 30). In addition, animal studies suggest that exposure to maternal stress in utero may result in a reduced testosterone level in adult males exposed to stress, possibly even apoptosis of Leydig cells (28). Although this area of research needs further development, it suggests the importance of stress across the life course and intergenerational stress to male reproductive health.
Our study had several strengths. Our study sample, recruited from the CHDS cohort, is a diverse sample of men in which we measured a wide range of both stress exposures and semen quality parameters. Our survey included both perceived and objective validated measures of stress. The overlap of the data collection with a period of increasing unemployment allowed us to observe effects of this stressor. The semen collection and evaluation followed a validated protocol in an experienced laboratory that also participated in the Fertile Male Study, reducing outcome misclassification (6).
One limitation of our study is that the cross-sectional design does not allow us to establish temporality between stress and semen quality. However, in analyses in which we excluded men who had concerns about their fertility, had a history of reproductive health problems, or had other comorbidities, we found similar results, thus providing no support to reverse causality as an alternative explanation for our findings. The issue of reverse causality does highlight one strength of objective measures of stress, such as the LEI, because reporting on major life events is less likely to be influenced by worries regarding fertility than is the subjective measure of the PSS.
Our study has several other limitations, some of which are common among studies of semen quality. First, due to the expense and difficulty in recruiting men to provide semen samples, our sample size was limited and did not allow us the power to examine dichotomous outcomes such as the World Health Organization (WHO) thresholds for normal criteria, which may be of clinical interest (7). Next, there are three critical periods important to semen quality: in utero, puberty, and 72 days before ejaculation (1). Future analyses could incorporate a life-course perspective by examining the effects of stress at each of these periods on semen quality. Finally, although our study measured most major known risk factors for decreased semen quality, we cannot rule out that our findings are due to the influence of unmeasured variables.
We found that life stress measured both objectively and subjectively was associated with sperm concentration, motility, and morphology. The Perceived Stress Scale, not previously used in studies of semen quality, best captured the association between life stress and semen quality. Work stress was not similarly associated with these measures, and unemployed men had poorer semen quality than employed men. Exploratory analyses of a neuroendocrine mechanism produced unclear results. Future research should examine the influence of life course psychosocial stress on male reproductive health.
Acknowledgments
Supported by from the National Institutes of Health, National Institute of Environmental Health Sciences (Grant R01 ES 12231).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
T.J. has nothing to disclose. L.G.K. has nothing to disclose. P.L. has nothing to disclose. P.M.C. has nothing to disclose. B.A.C. has nothing to disclose. X.L. has nothing to disclose. P.F.-L. has nothing to disclose.
REFERENCES
- 1.Louis GB, Platt RW, editors. Reproductive and perinatal epidemiology. Oxford University Press; New York: 2011. [Google Scholar]
- 2.Hjollund NH, Bonde JP, Henriksen TB, Giwercman A, Olsen J. Reproductive effects of male psychologic stress. Epidemiology. 2004;15:21–7. doi: 10.1097/01.ede.0000100289.82156.8b. [DOI] [PubMed] [Google Scholar]
- 3.Fenster L, Katz D, Wyrobek A, Pieper C, Rempel D, Oman D, et al. Effects of psychological stress on human semen quality. J Androl. 1997;18:194–202. [PubMed] [Google Scholar]
- 4.Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–23. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Auger J, Eustache F, Andersen A, Irvine D, Jørgensen N, Skakkebaek N, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod. 2001;16:2710–7. doi: 10.1093/humrep/16.12.2710. [DOI] [PubMed] [Google Scholar]
- 6.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, Overstreet JW, et al. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–11. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Bigelow PL, Jarrell J, Young MR, Keefe TJ, Love EJ. Association of semen quality and occupational factors: comparison of case-control analysis and analysis of continuous variables. Fertil Steril. 1998;69:11–8. doi: 10.1016/s0015-0282(97)00437-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Kessler R, Underwood Gordon L, editors. Measuring stress: a guide for health and social scientists. Oxford University Press; New York: 1995. [Google Scholar]
- 9.Susser E, Buka S, Schaefer C, Andrews H, Cirillo P, Factor-Litvak P, et al. The Early Determinants of Adult Health Study. J Dev Orig Health Dis. 2011;2:311–21. doi: 10.1017/S2040174411000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirillo P, Cohn B, Krigbaum N, Lee M, Brazil C, Factor-Litvak P. Effect of maternal coffee, smoking and drinking behavior on adult son's semen quality: prospective evidence from the Child Health and Development Studies. J Dev Orig Health Dis. 2011;2:375–86. doi: 10.1017/S2040174411000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amick B. The Job Content Questionnaire (JCQ): an instrument for internationally comparative assessments of psychosocial job characteristics. J Occup Health Psychol. 1998;3:322–55. doi: 10.1037//1076-8998.3.4.322. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 13.Cochrane R, Robertson A. The Life Events Inventory: a measure of the relative severity of psycho-social stressors. J Psychosom Res. 1973;17:135–40. doi: 10.1016/0022-3999(73)90014-7. [DOI] [PubMed] [Google Scholar]
- 14.Spurgeon A, Jackson C, Beach J. The Life Events Inventory: re-scaling based on an occupational sample. Occup Med. 2001;51:287–93. doi: 10.1093/occmed/51.4.287. [DOI] [PubMed] [Google Scholar]
- 15.Overstreet JW, Brazil C. Semen analysis. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. Mosby; St. Louis, MO: 1997. pp. 487–90. [Google Scholar]
- 16.Brazil C, Swan SH, Tollner CR, Treece C, Drobnis EZ, Wang C, et al. Quality control of laboratory methods for semen evaluation in a multicenter research study. J Androl. 2004;25:645. doi: 10.1002/j.1939-4640.2004.tb02836.x. [DOI] [PubMed] [Google Scholar]
- 17.Landsbergis PA, Schnall PL, Warren K, Pickering TG, Schwartz JE. Association between ambulatory blood pressure and alternative formulations of job strain. Scand J Work Environ Health. 1994;20:349–63. doi: 10.5271/sjweh.1386. [DOI] [PubMed] [Google Scholar]
- 18.Cifuentes M, Boyer J, Gore R, d'Errico A, Scollin P, Tessler J, et al. Job strain predicts survey response in healthcare industry workers. Am J Ind Med. 2008;51:281–9. doi: 10.1002/ajim.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Li Y, Zhou N, Han X, Ma M, Li L, et al. Socio-psycho-behavioural factors associated with male semen quality in China: results from 1346 healthy men in Chongqing. J Fam Plann Reprod Health Care. 2013;39:102–10. doi: 10.1136/jfprhc-2011-100276. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner EK, Sheiner E, Carel R, Potashnik G, Shoham-Vardi I. Potential association between male infertility and occupational psychological stress. J Occup Environ Med. 2002;44:1093–9. doi: 10.1097/00043764-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Theorell T, Karasek R, Eneroth P. Job strain variations in relation to plasma testosterone fluctuations in working men—a longitudinal study. J Intern Med. 1990;227:31–6. doi: 10.1111/j.1365-2796.1990.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 22.Siegrist J, Starke D, Chandola T, Godin I, Marmot M, Niedhammer I, et al. The measurement of effort–reward imbalance at work: European comparisons. Soc Sci Med. 2004;58:1483–99. doi: 10.1016/S0277-9536(03)00351-4. [DOI] [PubMed] [Google Scholar]
- 23.Kivimäki M, Ferrie JE, Brunner E, Head J, Shipley MJ, Vahtera J, et al. Justice at work and reduced risk of coronary heart disease among employees: the Whitehall II Study. Arch Intern Med. 2005;165:2245–51. doi: 10.1001/archinte.165.19.2245. [DOI] [PubMed] [Google Scholar]
- 24.Virtanen M, Heikkila K, Jokela M, Ferrie JE, Batty GD, Vahtera J, et al. Long working hours and coronary heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2012;176:586–96. doi: 10.1093/aje/kws139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardhan A, Walker RA. California, pivot of the great recession. Working paper. University of California. Institute for Research on Labor and Employment. 2010 http://escholarship.org/uc/item/0qn3z3td.
- 26.Sobotka T, Skirbekk V, Philipov D. Economic recession and fertility in the developed world. Popul Dev Rev. 2011;37:267–306. doi: 10.1111/j.1728-4457.2011.00411.x. [DOI] [PubMed] [Google Scholar]
- 27.McGrady AV. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;13:1–7. doi: 10.3109/01485018408987495. [DOI] [PubMed] [Google Scholar]
- 28.Hardy MP, Gao H, Dong Q, Ge R, Wang Q, Chai WR, et al. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322:147–53. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 29.Eskiocak S, Gozen A, Yapar S, Tavas F, Kilic A, Eskiocak M. Glutathione and free sulphydryl content of seminal plasma in healthy medical students during and after exam stress. Hum Reprod. 2005;20:2595–600. doi: 10.1093/humrep/dei062. [DOI] [PubMed] [Google Scholar]
- 30.Kao S, Chao H, Chen H, Hwang T, Liao T, Wei Y. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008;89:1183–90. doi: 10.1016/j.fertnstert.2007.05.029. [DOI] [PubMed] [Google Scholar]