Abstract
Nonischemic cardiomyopathy can complicate antineoplastic therapy and lead to irreversible heart failure. We evaluated structural changes at the time of left ventricular assist device implantation in heart failure patients who had been exposed to anthracycline, and we correlated those changes with clinical presentation.
We retrospectively studied left ventricular core samples taken at implantation of the HeartMate II left ventricular assist device in 12 heart failure patients (mean age, 46 ± 16 yr) who had histories of anthracycline exposure. We evaluated those samples for hypertrophy, myocytolysis, and fibrosis. Histopathologic findings showed moderate-to-severe myocyte hypertrophy, moderate myocytolysis, and perivascular and interstitial fibrosis with areas of replacement fibrosis. Ultrastructural studies revealed marked decreases in myofibrils, diffuse mitochondrial swelling, and disorganization of the sarcoplasmic reticulum. The interval between anthracycline therapy and heart failure was a mean of 6.8 ± 5.7 years; duration of heart failure symptoms, 38 ± 47 months; and duration of device support, 414 ± 266 days. Four patients are continuing on device support, 3 have undergone transplantation, 3 have undergone device explantation, and 2 have died. The time of heart failure onset and the duration of symptoms did not correlate with the severity and extent of the histopathologic changes.
The histopathologic findings and the clinical course varied in heart failure patients with anthracycline exposure. No correlation was observed between anthracycline therapy and the development or duration of heart failure symptoms, severity of histopathologic changes, or outcomes.
Keywords: Anthracyclines/adverse effects; antineoplastic agents/adverse effects; arrhythmias, cardiac/chemically induced; cardiomyopathies/chemically induced; congestive heart failure/chemically induced; doxorubicin/toxicity; heart-assist devices; myocardium/pathology; nonischemic cardiomyopathy; retrospective studies; ventricular remodeling
Anthracycline agents, among the most effective of antineoplastic drugs, are used to treat many hematologic cancers and solid tumors. However, the clinical use of anthracyclines is severely limited by their cardiotoxicity,1 which manifests itself in several forms, ranging from immediate nonspecific electrocardiographic changes and arrhythmias to the development of a progressive dose-dependent cardiomyopathy that evolves into congestive heart failure (HF). Anthracycline-associated HF is usually refractory to conventional therapy.2 More than 50% of patients exposed to anthracycline agents will show some degree of cardiac dysfunction 10 to 20 years after chemotherapy, and 5% of them will develop overt HF.3 Patients with anthracycline cardiomyopathy who develop terminal HF need left ventricular assist device (LVAD) support.
In the present study, we retrospectively examined histopathologic findings in core samples of the left ventricle (LV) taken at the time of LVAD implantation in HF patients with a history of anthracycline exposure. Specifically, we examined the correlation between anthracycline therapy and the severity of the histopathologic changes and outcome. This report presents our findings, in addition to a brief review of HF associated with anthracycline exposure.
Patients and Methods
We reviewed the charts of 310 patients with severe HF who underwent implantation of a HeartMate II® Left Ventricular Assist System (Thoratec Corporation; Pleasanton, Calif) at the Texas Heart Institute at St. Luke's Episcopal Hospital between 2003 and 2011, and we identified those who had a history of anthracycline treatment.
We retrospectively examined the demographic characteristics of anthracycline-treated patients, including age, sex, race, malignancy diagnosis before anthracycline treatment, time between anthracycline treatment and development of HF symptoms, and duration of HF before LVAD implantation. Follow-up data included the duration of LVAD support, as well as LVAD exchange or explantation, heart transplantation, and death.
Histopathologic analysis comprised a blinded evaluation of the LV core sample excised at the time of LVAD implantation or exchange, or of LV tissue obtained at heart transplantation or autopsy. All histologic sections were stained with hematoxylin and eosin and with Masson's trichrome. We evaluated the extent and severity of the main characteristics of ventricular remodeling, including hypertrophy, myocytolysis, and fibrosis (perivascular, interstitial, and replacement).
To examine the histopathologic changes, we modified our grading scale to include myocytolysis.4 Histopathologic characteristics were evaluated on a 4-point basis ranging from 0 to 3. If no changes were seen, a score of 0 was given; focal, mild lesions were given a score of 1; multifocal, moderate lesions were given a score of 2; and diffuse, severe lesions were given a score of 3. The total severity of the combination of hypertrophy and total fibrosis (interstitial/perivascular + replacement) was graded by using a scale ranging from 0 to 10. When the sum of hypertrophy and total fibrosis was 1 to 3, the total score was considered mild; 4 to 7, moderate; and 8 to 10, severe. We used electron microscopy to examine myofibril architecture and preservation, micro-organelles, and the extracellular matrix.
This study was conducted with the approval of the institutional review board at St. Luke's Episcopal Hospital.
Results
Patient Characteristics. Of the 310 patients supported by the HeartMate II device at our institution from 2003 through 2011, 12 nonconsecutive patients (9 women and 3 men; mean age, 46 ± 16 yr [range, 17–65 yr]) had a history of anthracycline treatment. Table I shows the patients' demographic characteristics and the various types of cancer for which the patients had undergone anthracycline treatment. The mean time from anthracycline treatment to the development of HF symptoms was 6.8 ± 5.7 years (range, 4 mo–15 yr). The cumulative anthracycline dose was not available in all cases. The duration of HF before LVAD implantation was 38 ± 47 months (range, <1–120 mo). The average preimplantation hemodynamic values were as follows: cardiac index, 1.9 ± 0.7 L/min/m2; cardiac output, 3 ± 1.2 L/min; LV ejection fraction, 0.18 ± 0.03; creatinine level, 0.8 ± 0.3 mg/dL; and total bilirubin level, 1.5 ± 1.2 mg/dL.
TABLE I.
Characteristics of 12 Patients Who Needed LVAD Support after Anthracycline Treatment
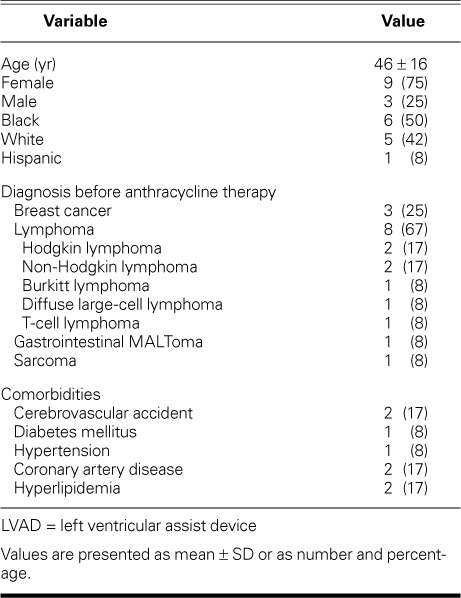
Follow-up Evaluation. The mean LVAD support duration was 414 ± 266 days (range, 102–994 d). Four patients continued to receive LVAD support, 3 needed orthotopic heart transplantation, 2 died, and 3 underwent LVAD explantation after improvement. One of the transplant patients was a 30-year-old woman with a history of diffuse large cell lymphoma who underwent an exchange from a HeartMate® XVE (after 18 mo of support) to a HeartMate II and then underwent heart transplantation 2 months later. The 2nd transplant patient was a 65-year-old woman with a history of breast cancer who had HF for 10 years after anthracycline treatment; she underwent heart transplantation after 662 days of LVAD support. The 3rd transplant patient was a 62-year-old man with a history of HF after anthracycline therapy for Hodgkin lymphoma, who had a transplant after 9 months on HeartMate II support. The 2 nonsurviving patients included a 61-year-old woman with a history of gastrointestinal MALToma and 2 years of HF, who was supported by the LVAD for 102 days; and a 63-year-old woman with a history of breast carcinoma, who died of coagulopathic sequelae. Of the 3 patients who underwent LVAD explantation, one was a 29-year-old woman who had developed HF symptoms 15 years after treatment for Hodgkin lymphoma; her condition improved substantially with HeartMate II support, and the LVAD was removed after 526 days of support. She remained in New York Heart Association (NYHA) functional class I for 1,720 days after device explantation. The 2nd patient with an explanted device was a 47-year-old woman who developed HF after anthracycline treatment for breast cancer and was supported by the HeartMate II for 10 months. The 3rd patient was a 43-year-old woman with a history of sarcoma who was supported by the HeartMate II for 2 months.
All patients were receiving conventional medical therapy for HF, which included β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers.
Histopathologic Findings. Left ventricular core samples obtained at the time of LVAD implantation were available for all 12 patients. In all cases, the histologic sections showed evidence of ventricular remodeling, with moderate cardiomyocyte hypertrophy (8/12 patients), moderate myocytolysis (8/12 patients), and variable amounts of fibrosis (Fig. 1 and Table II). Interstitial fibrosis was identified in all 12 patients: focal, mild interstitial fibrosis (Grade 1) was observed in 6 patients; multifocal, moderate interstitial fibrosis (Grade 2) in 4 patients; and diffuse, severe interstitial fibrosis (Grade 3) in 2 patients (Table II). Replacement fibrosis was identified in 6 of the 12 patients: mild, focal areas of replacement fibrosis (Grade 1) were observed in 2 patients; multifocal, moderate replacement fibrosis (Grade 2) was seen in 3 patients; and severe, diffuse replacement fibrosis (Grade 3) was observed in 1 patient (Table II). The time of onset of HF after anthracycline therapy and the duration of HF symptoms did not correlate with the severity and extent of cardiomyocyte hypertrophy, myocytolysis, or fibrosis (interstitial and replacement).
Fig. 1.
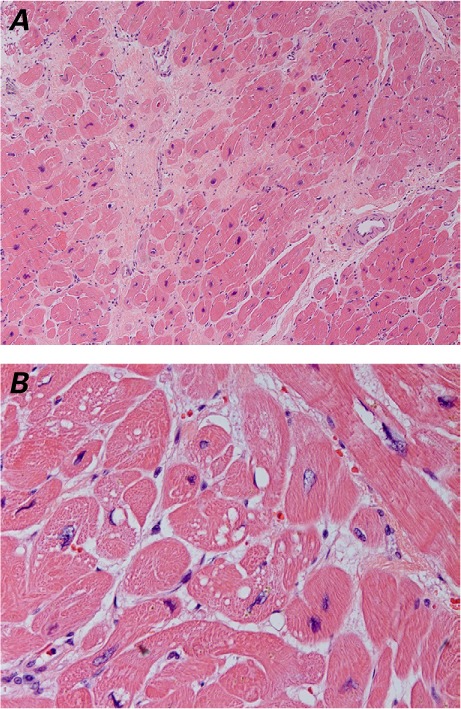
Photomicrographs of left ventricular core tissue. A) Histopathologic changes include cardiomyocyte myocytolysis and hypertrophy, perivascular and interstitial fibrosis, and focal replacement fibrosis with myocyte dropout (H & E, orig. ×10). B) At higher magnification, cardiomyocyte cytoplasmic and perinuclear vacuolization are seen (H & E, orig. ×40).
TABLE II.
Ventricular Remodeling: Semiquantitative Histopathologic Examination at LVAD Implantation
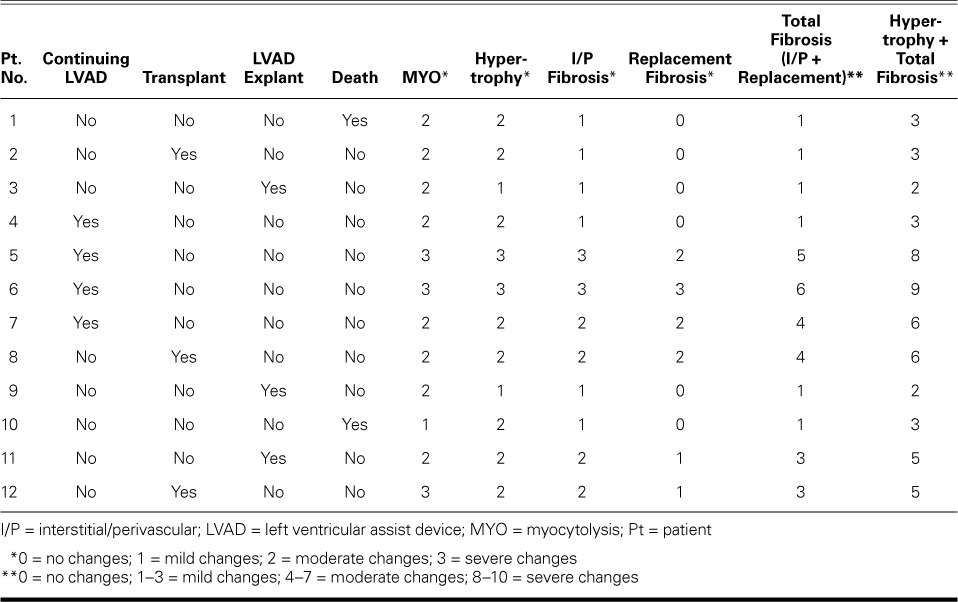
When we examined the correlation between histopathologic changes and outcome, we found that the pathology scores (the sum of the hypertrophy and total-fibrosis scores) were lowest in patients whose clinical status improved sufficiently to enable LVAD explantation, whereas the highest pathology scores were seen in patients in whom LVAD support had to be maintained because of continued impairment of heart function (Table II). In addition, high pathology scores were seen in the patients who underwent transplantation. The 2 patients who died during LVAD support had intermediate pathology scores (Table II).
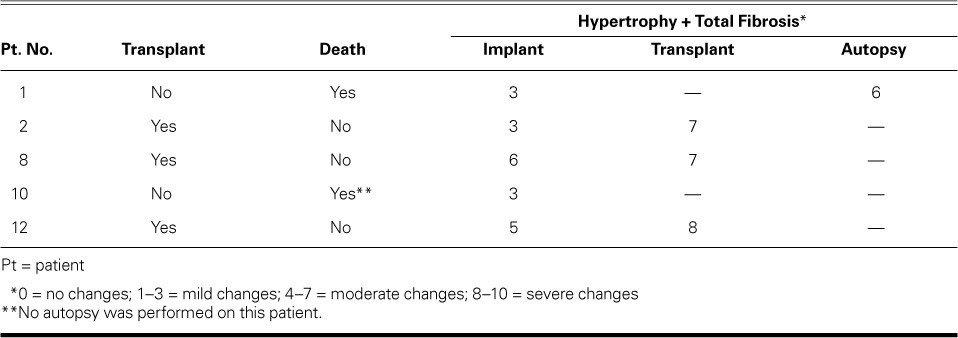
At follow-up evaluation, 4 patients had histopathologic specimens available for examination (explanted hearts from 3 patients who received a heart transplant and autopsy specimens from a patient who died). In 1 patient, an autopsy was not performed at our institution, so we did not include those specimens. All available specimens showed the same grade of hypertrophy, with an increase in total fibrosis, which raised the total histopathologic score for remodeling changes (hypertrophy + perivascular, interstitial, and replacement fibrosis) (Table III).
TABLE III.
Ventricular Remodeling: Evaluation at Follow-Up
Electron-Microscopy Findings. Electron-microscopy studies were available for 3 patients. These studies showed interruption and loss of myofibrils, changes in contraction bands, swelling and degeneration of mitochondria, and dilation of the sarcoplasmic reticulum (Fig. 2). Ultrastructural changes in cardiomyocyte nuclei included membrane irregularities and chromatin condensation.
Fig. 2.
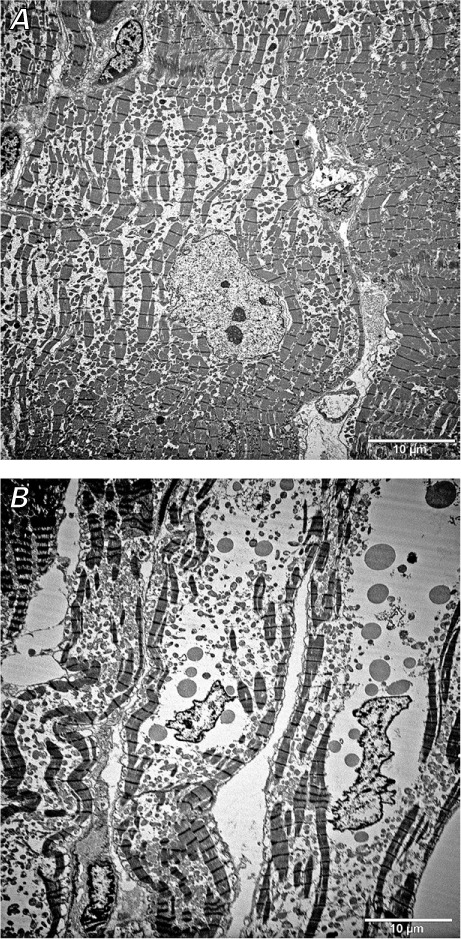
Electron-microscopy images (orig. ×3,000) of left ventricular core sections show A) interruption and loss of myofibrils and B) mitochondrial degenerative changes and swelling.
Discussion
In this study of 12 patients with a history of anthracycline and HF who were supported by the HeartMate II, we found marked variability in the clinical presentation and the histopathologic characteristics of ventricular remodeling. All LV core samples showed variable grades of cardiomyocyte hypertrophy, myocytolysis, and fibrosis—all of which are characteristic features of ventricular remodeling associated with anthracycline exposure but are not specific for anthracycline HF. Myocytolysis, a characteristic finding in myocytes of anthracycline-exposed patients, was observed in all of our patients. It was not the most prominent feature but was in all cases accompanied by myocyte hypertrophy. We did not find a correlation between the severity of histopathologic changes and the onset of HF symptoms or the duration of HF. However, we observed a trend toward more severe histopathologic changes (more extensive fibrosis and myocyte hypertrophy) in patients who continued to receive LVAD support than in patients whose LVAD was explanted because their condition had improved.
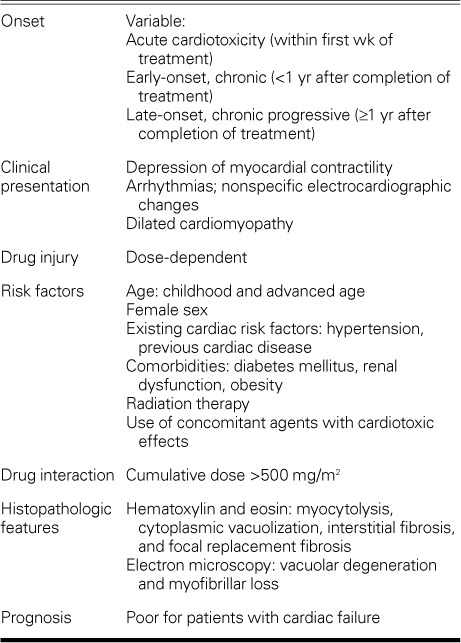
Anthracyclines are associated with 3 different forms of cardiomyopathy: acute cardiotoxicity, early-onset chronic progressive cardiomyopathy, and late-onset chronic progressive cardiomyopathy.2,5 Acute cardiotoxicity occurs in <1% of patients immediately after anthracycline infusion and manifests itself as an acute, transient decline in myocardial contractility, which is usually reversible.6 Early-onset chronic progressive cardiomyopathy arises within the first year after treatment and is seen in 1.6% to 2.1% of patients.7,8 Late-onset chronic progressive toxicity occurs at least 1 year after completion of anthracycline therapy, is seen in 1.6% to 5% of patients, and frequently progresses to severe HF.8,9
Arrhythmias, hypotension, and nonspecific ST-T electrocardiographic changes are the most frequent manifestations of anthracycline-associated cardiotoxicity, and their occurrence increases with the cumulative dose of anthracycline. Compared with lower cumulative doses, a cumulative dose higher than 550 mg/m2 is associated with a 5-fold increased risk of cardiotoxicity.10–12 Although anthracycline-induced cardiotoxicity can persist for years with no clinical symptoms, the prognosis is poor after overt HF develops.10 The 2-year mortality rate among patients who develop late cardiotoxicity has been estimated to be as high as 60%, but the prognosis can be greatly improved by early detection and prompt treatment.13
Risk factors for anthracycline-induced cardiotoxicity include high cumulative doses, higher single doses, age (elderly persons and children), female sex, and a history of cardiac disease, radiation therapy, or the use of concomitant agents with known cardiotoxic effects, such as cyclophosphamide, trastuzumab, and paclitaxel.14–17
Pathophysiology. The pathophysiology of anthracycline-induced cardiomyopathy is incompletely understood. Most evidence shows that myocyte toxicity is mediated primarily through the generation of free radicals via increased myocardial oxidative stress and lipid peroxidation.18–20 Even in the absence of continued anthracycline exposure, depletion of mitochondrial DNA occurs, and free radicals accumulate over time, leading to the delayed presentation of cardiomyopathy. Intramyocardial formation of secondary alcohol metabolites might play a key role in promoting the development of end-stage HF.21–23 Additional pathogenetic mechanisms currently under investigation include apoptosis, iron metabolism interference, and calcium dysregulation.24,25
Pathology. The fundamental lesion of anthracycline cardiomyopathy involves the myocyte. The main histologic alterations seen in the myocyte are cytoplasmic vacuolization and hypertrophy. In the extracellular matrix, a mild increase in interstitial fibrosis with occasional replacement fibrosis is seen.10,26,27 Vacuolated myocytes, called adria cells, are usually found adjacent to areas of fibrosis. In addition, rare foci of necrotic myocytes might be seen. Electron-microscopy studies have revealed 2 types of injury: vacuolar degeneration and a marked decrease in the number of myofibrils.28 Myofibrillar loss is characterized by partial or total loss of myofibrils and disarray of sarcomere myofibrils.29 In vacuolar degeneration, the earliest change is distention of the sarcoplasmic reticulum and T tubular system. The distended vacuoles swell and eventually coalesce, forming large membrane-bound spaces.30–32 As we observed in this study, histologic alterations frequently do not correlate with the severity of the disease or the development of advanced HF.8,18,33 Because these observations apply to a very small number of patients, we cannot draw any definitive conclusions about the correlation between the histopathologic findings and improvement in LV function, or about the correlation between those findings and the possibility of explantation.
Table IV delineates the main characteristics (especially onset, clinical presentation, drug injury, risk factors, and histopathologic features) that differentiate anthracycline-induced cardiotoxicity from other causes of HF.
TABLE IV.
Characteristics of Anthracycline-Induced Cardiotoxicity
LVAD and Recovery. Only a few reports have been published about LVAD implantation for severe HF due to anthracycline use.34–37 Recovery of ventricular function in LVAD patients who have anthracycline-induced cardiomyopathy is rare. Castells and colleagues35 described the case of a patient with end-stage HF due to anthracycline and trastuzumab cardiomyopathy, who underwent implantation of an LV axial pump; the patient's condition improved, and the pump was explanted at 135 days. In addition, Freilich and colleagues36 described the case of a 16-year-old girl who recovered from doxorubicin-induced cardiomyopathy after long-term LVAD support. She had presented in severe cardiogenic shock 6 months after chemotherapy and had needed LVAD implantation. After the LVAD was explanted, the patient survived and continues to survive. At the time of publication (28 mo after explantation), she remains alive, in NYHA class I, and on conventional medical therapy for HF. Other authors have reported unsuccessful explantation37 or failure to wean in this group of patients.34 In our series, the 3 patients with LV functional improvement that enabled LVAD explantation are progressing satisfactorily: 1 patient underwent transplantation and is doing well, and the other 2 are in NYHA class I.
Study Limitations. This study includes a small number of patients with a history of anthracycline treatment, who are part of our large overall series of LVAD patients. Because the number of anthracycline recipients is so small, we were able to perform only a descriptive study of the clinical history and structural remodeling changes. Findings in the 3 patients whose LVAD was explanted are only observational and do not enable us to draw definitive conclusions about the predictive value of the histologic or clinical characteristics that would indicate better possibilities of recovery. In addition, all the patients in this series were terminally ill and were referred to us from other institutions; therefore, the cumulative anthracycline doses were unavailable.
Acknowledgments
The authors thank Ralph Nichols of the Texas Heart Institute for performing electron-microscopy studies, and Rebecca A. Bartow, PhD, and Virginia Fairchild, of the Texas Heart Institute, for editorial assistance.
Footnotes
From: Departments of Cardiovascular Pathology Research (Drs. Buja and Segura) and Cardiopulmonary Transplantation (Drs. Demirozu, Frazier, and Radovancevic), Texas Heart Institute, Houston, Texas 77030; and Department of Pathology and Laboratory Medicine (Dr. Buja), The University of Texas Health Science Center, Houston, Texas 77030
Dr. Radovancevic is now at the Center for Advanced Heart Failure, The University of Texas Health Science Center, Houston, Texas. Dr. Demirozu is now at the Department of Cardiovascular Surgery, Koç School of Medicine, Istanbul, Turkey.
References
- 1.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32(2):302–14. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C. et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 3.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266(12):1672–7. [PubMed] [Google Scholar]
- 4.Segura AM, Frazier OH, Demirozu Z, Buja LM. Histopathologic correlates of myocardial improvement in patients supported by a left ventricular assist device. Cardiovasc Pathol. 2011;20(3):139–45. doi: 10.1016/j.carpath.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Simmons A, Vacek JL, Meyers D. Anthracycline-induced cardiomyopathy. Postgrad Med. 2008;120(4):67–72. doi: 10.3810/pgm.2008.11.1940. [DOI] [PubMed] [Google Scholar]
- 6.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 8.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131(5):561–78. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 9.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira AL, Matsubara LS, Matsubara BB. Anthracyclineinduced cardiotoxicity. Cardiovasc Hematol Agents Med Chem. 2008;6(4):278–81. doi: 10.2174/187152508785909474. [DOI] [PubMed] [Google Scholar]
- 11.Jensen BV. Cardiotoxic consequences of anthracycline-containing therapy in patients with breast cancer. Semin Oncol. 2006;33(3 Suppl 8):S15–21. doi: 10.1053/j.seminoncol.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15(4):1544–52. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 13.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE. Exposure to anthracyclines during childhood causes cardiac injury. Semin Oncol. 2006;33(3 Suppl 8):S8–14. doi: 10.1053/j.seminoncol.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 16.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M., Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 17.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70(22):9287–97. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23(1):15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 21.Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108(19):2423–9. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 22.Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):108–13. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 23.Mordente A, Meucci E, Silvestrini A, Martorana GE, Giardina B. New developments in anthracycline-induced cardiotoxicity. Curr Med Chem. 2009;16(13):1656–72. doi: 10.2174/092986709788186228. [DOI] [PubMed] [Google Scholar]
- 24.Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234–235(1–2):119–24. [PubMed] [Google Scholar]
- 25.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–25. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen S., Perez-Bouza A, Schalte G, Hilgers RD, Autschbach R. Selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transplant. 2008;27(1):86–92. doi: 10.1016/j.healun.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Christiansen S, Redmann K, Scheld HH, Jahn UR, Stypmann J, Fobker M et al. Adriamycin-induced cardiomyopathy in the dog--an appropriate model for research on partial left ventriculectomy? J Heart Lung Transplant. 2002;21(7):783–90. doi: 10.1016/s1053-2498(02)00402-3. [DOI] [PubMed] [Google Scholar]
- 28.Buja LM, Ferrans VJ, Mayer RJ, Roberts WC, Henderson ES. Cardiac ultrastructural changes induced by daunorubicin therapy. Cancer. 1973;32(4):771–88. doi: 10.1002/1097-0142(197310)32:4<771::aid-cncr2820320407>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53(2):105–13. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62(6):865–72. [PubMed] [Google Scholar]
- 31.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125(1):47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010;19(5):308–11. doi: 10.1016/j.carpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116(19):2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 34.Casarotto D, Bottio T, Gambino A, Testolin L, Gerosa G. The last to die is hope: prolonged mechanical circulatory support with a Novacor left ventricular assist device as a bridge to transplantation. J Thorac Cardiovasc Surg. 2003;125(2):417–8. doi: 10.1067/mtc.2003.131. [DOI] [PubMed] [Google Scholar]
- 35.Castells E, Roca J, Miralles A, Manito N, Ortiz D, Gonzalez J et al. Recovery of ventricular function with a left ventricular axial pump in a patient with end-stage toxic cardiomyopathy not a candidate for heart transplantation: first experience in Spain. Transplant Proc. 2009;41(6):2237–9. doi: 10.1016/j.transproceed.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Freilich M, Stub D, Esmore D, Negri J, Salamonsen R, Bergin P et al. Recovery from anthracycline cardiomyopathy after long-term support with a continuous flow left ventricular assist device. J Heart Lung Transplant. 2009;28(1):101–3. doi: 10.1016/j.healun.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Swartz MF, Fink GW, Carhart RL., Jr. Use of a biventricular assist device in the treatment of acute doxorubicin-induced cardiotoxicity. Congest Heart Fail. 2004;10(4):197–9. doi: 10.1111/j.1527-5299.2004.02662.x. [DOI] [PubMed] [Google Scholar]


