Abstract
A 56-year-old man with ischemic cardiomyopathy, a biventricular implantable cardioverter-defibrillator (ICD), and a left ventricular assist device (LVAD) developed a pocket hematoma and infection after an ICD generator change. The biventricular ICD was extracted, and the patient was given a full course of antibiotics. Because he had no indications for bradycardia pacing or biventricular pacing, he was implanted with a subcutaneous ICD under full anticoagulation. There was no interference in sensing or shock delivery from the ICD. The LVAD readings were unchanged during and after the procedure. The patient had an uneventful postoperative course, and both devices were functioning normally.
To our knowledge, this is the first reported case of the implantation of a subcutaneous ICD in the presence of an LVAD. This report illustrates that both devices can be implanted successfully in the same patient. In addition, the subcutaneous ICD minimizes the risk of bloodstream infections, which can be fatal in patients who have life-supporting devices such as an LVAD.
Keywords: Arrhythmias, cardiac/therapy; cardiac pacing, artificial/methods; combined modality therapy; defibrillators, implantable; equipment design; heart-assist devices/trends; prosthesis-related infections; treatment outcome
Implantable cardioverter-defibrillators (ICDs) are the standard of care for the prevention of sudden cardiac death in patients who are at high risk for ventricular tachyarrhythmias.1 Traditional transvenous ICDs carry a substantial risk of periprocedural sequelae, including pneumothorax, pericardial effusion or tamponade, and hemothorax, as well as long-term sequelae, such as thrombosis, lead failure, and infection.2,3 In 2010, Bardy and colleagues4 reported the first successful use of a completely subcutaneous ICD (S-ICD) (Boston Scientific Corporation; Natick, Mass). The U.S. Food and Drug Administration has approved the S-ICD for use in the United States; however, appropriate patient selection is crucial when implantation of this device is contemplated. Approximately 1,900 S-ICDs have been implanted worldwide, about 500 of these in the U.S.* Three patients have been given HeartWare® left ventricular assist devices (LVADs) (HeartWare Inc.; Framingham, Mass) after undergoing S-ICD implantation in Europe,* and one of these cases has been reported.5 We report our implantation of an S-ICD in a patient who already had a HeartMate II® LVAD (Thoratec Corporation; Pleasanton, Calif). To our knowledge, this is the first report of S-ICD implantation in the presence of an LVAD.
Case Report
In October 2012, a 56-year-old man underwent replacement of his biventricular ICD pulse generator because of elective-replacement indicator status. He had a history of coronary artery disease, coronary artery bypass grafting, ischemic cardiomyopathy (left ventricular ejection fraction, 0.05–0.10), type 2 diabetes mellitus, and obstructive sleep apnea on a continuous positive airway pressure of 10 mmHg. His biventricular ICD was a D274TRK Concerto II CRT-D DF-1 (Medtronic, Inc.; Minneapolis, Minn). In May 2012, the patient had undergone HeartMate II LVAD implantation as destination therapy for refractory heart failure. At that time, his early postoperative course was notable for episodes of sustained ventricular tachycardia, which were terminated by ICD shocks; thereafter, his condition returned to New York Heart Association functional class II, and he needed no further hospitalizations.
Two days after the most recent generator replacement, the patient developed a pocket hematoma. Anticoagulation was withheld at first and was then gradually restarted as the hematoma resolved. However, the patient developed a fever of 102.4 °F 2 days thereafter. Blood cultures grew methicillin-resistant Staphylococcus aureus (MRSA). A transesophageal echocardiogram revealed no vegetation on the cardiac valves or on the ICD leads. The patient underwent complete extraction of the ICD without sequelae. A total of 350 mL of serosanguineous fluid was drained from the pocket site during extraction, and the cultures grew MRSA. The patient was treated with vancomycin intravenously for 6 weeks, and negative-pressure wound therapy was used at the pocket site to promote healing. He was supported with a Life-Vest® (Zoll Medical Corporation, Chelmsford, Mass) while awaiting the reimplantation of a defibrillator.
The patient recovered uneventfully and was evaluated for ICD reimplantation 3 months later. Problems surrounding the reimplantation of an intravenous ICD included the inability to stop anticoagulation for the procedure, the patient's recent MRSA infection, and the high risk of infecting the LVAD if the infection were to recur. The patient had shown no indications for bradycardia pacing or biventricular pacing after the LVAD placement, so we evaluated him for S-ICD placement by reviewing cutaneous electrograms in the sensing configurations of the S-ICD system, in 2 postures. The patient gave written informed consent for S-ICD implantation under general anesthesia with full anticoagulation.
Surgical Technique
In our electrophysiology laboratory, fluoroscopic images of the patient's LVAD were obtained and the S-ICD incision site was marked, to ensure that the LVAD components were not in the path of the sensing and defibrillation vectors. The LVAD pump speed, governed by heart failure status, was maintained at 9,800 rpm, and the LVAD readings were closely monitored during the procedure. The patient was prepared and draped in accordance with standard sterile techniques. An incision was made in the left inframammary crease at the level of the 5th–6th intercostal space, and dissection was carried to the fascia of the serratous anterior muscle. Blunt posterolateral dissection on the anterior surface of the serratous anterior muscle fascia created a pocket anterior to that muscle and posterior to the latissimus dorsi muscle (Fig. 1A). We chose this approach to minimize bleeding by making a bloodless fascial plane, with consideration of the prior hematoma formation upon our changing the ICD pulse generator, together with the patient's intraoperative therapeutic anticoagulation status. Two small incisions were then made: a 2-cm horizontal incision 1 cm superior to the xiphoid process, to accommodate a suture sleeve; and a vertical 1-cm incision on the left upper parasternal margin, where the distal tip of the electrode was calculated to emerge. The S-ICD electrode was tunneled by means of standard insertion techniques, with use of a tunneling tool (Figs. 1B–C). An SQ-RX® pulse generator, model 1010 (Boston Scientific), was then placed. Sensing in all 3 vectors was found to be appropriate and without any interference from the continuous-flow LVAD (Fig. 2). The system chose the secondary vector. Ventricular fibrillation was induced on 2 occasions by means of a 200-mA burst at 50 Hz for 3 s from the S-ICD. The device sensed the ventricular fibrillation with no interference from the LVAD, and 65-J shocks were delivered successfully, resulting in sinus rhythm. Times to therapy, defined as time from end of induction plus a 2-s refractory period to shock therapy, were 15 s and 17 s, respectively, with impedance of 68 Ω both times. Finally, the pocket and the 2 incisions for lead placement were closed in layers, and a pressure dressing was applied to the pocket site (Fig. 1D).
Fig. 1.
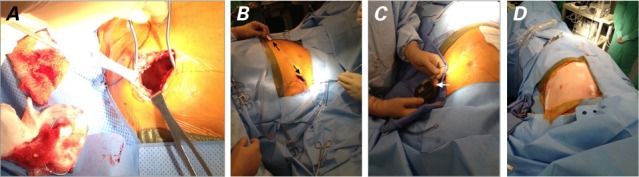
Intraoperative photographs show the procedure used to implant the subcutaneous implantable cardioverter-defibrillator: A) creation of the pocket in the left subaxillary region, B) tunneling of the lead (arrows) with use of the tunneling tool, C) insertion of the pulse generator (arrow) into the pocket, and D) the final result.
Fig. 2.
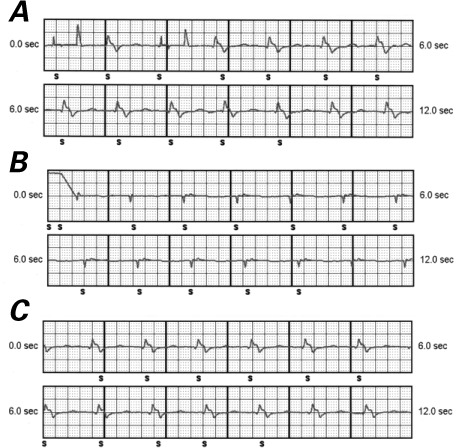
Electrograms recorded by the subcutaneous implantable cardioverter-defibrillator after implantation show appropriate sensing without any noise in the A) primary vector (ring electrode to pulse generator), B) alternate vector (tip electrode to ring electrode), and C) secondary vector (tip electrode to pulse generator).
All LVAD readings were stable during and after the implantation procedure. Automatic set-up of the S-ICD system was performed with the patient in the supine and sitting postures, after he had awakened. Sensing was appropriate in all 3 vectors, and the system chose the primary vector. The patient had an uneventful postoperative course and was discharged from the hospital after 3 days, in good condition (Fig. 3). He returned to the electrophysiology clinic after one week for an incision check, and routinely 2 months later. All the incisions healed well, and the S-ICD and LVAD were both functioning normally. He eventually underwent successful heart transplantation and was doing well.
Fig. 3.
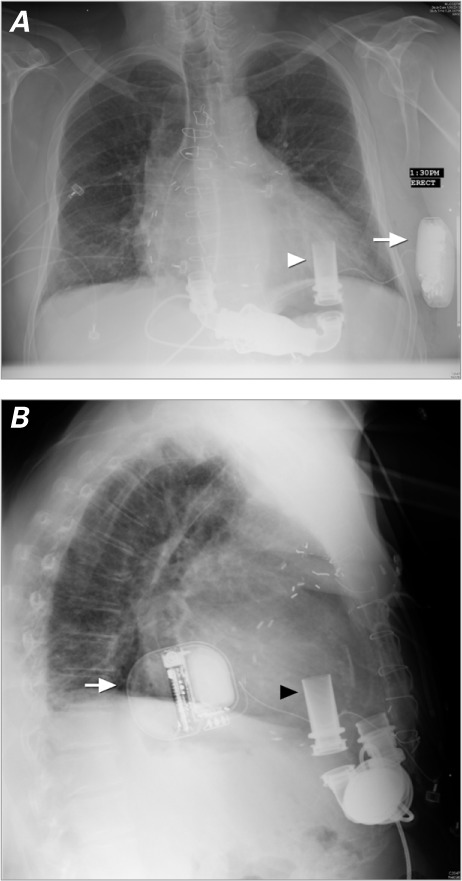
Chest radiograph after implantation shows the position of the subcutaneous implantable cardioverter-defibrillator (arrow) and its relation to the left ventricular assist device (arrowhead) in the A) posteroanterior and B) lateral views.
Discussion
To our knowledge, this is the first report of S-ICD implantation in a patient who had an LVAD already in place. The S-ICD has been effective and safe in patients at risk for sudden cardiac death, and without the need for anti-tachycardia pacing, bradycardia therapy, or cardiac resynchronization therapy.4,6–8 Traditional transvenous ICDs are associated with an approximate 5% complication rate within 30 days of implantation, a 16%-to-20% chance of lead failure over 10 years, and a 2.4% annual incidence of infection.2,3,9–11 Systemic infections and endocarditis remain a substantial concern in the use of these traditional devices.11 In addition, the risk of infections has risen along with the increasing numbers of invasive procedures, including generator changes.12 Device-associated infections have high morbidity and mortality rates and can be fatal in patients who have other life-supporting devices, such as LVADs. Accordingly, the extravascular nature of the S-ICD might reduce life-threatening infectious sequelae in these patients.
Subcutaneous ICDs can sense in 3 vectors, using 2 elements on the electrode, distal tip, and proximal ring, as well as the pulse generator.7 In 2 of the 3 patients who had an S-ICD and subsequently underwent HeartWare LVAD placement, sensing interference was observed in the primary and secondary vectors.* Both of these vectors incorporate the pulse generator, adjacent to the LVAD, as a sensing element. The HeartWare LVAD is smaller than the HeartMate II.13 In addition, the Heart-Ware system runs at pump speeds between 2,400 and 3,200 rpm—much slower than the HeartMate II's usual speed of 8,800 to 10,000 rpm. The faster speed of the HeartMate II appears less likely to be a source of interference in the S-ICD's sensing process. On the basis of our experience, we suggest that the HeartMate II LVAD might be implanted successfully in patients who already have an S-ICD, without causing interference.
Acknowledgments
We acknowledge the technical support provided by Jay Overcash, CCDS (Boston Scientific Corporation), in this case.
Footnotes
From: Division of Cardiology, Department of Internal Medicine, Drexel University College of Medicine, Philadelphia, Pennsylvania 19102
Dr. Kutalek is a consultant for The Spectranetics Corporation, Boston Scientific Corporation, and St. Jude Medical, Inc.
*Personal communication, Cameron Health, Inc. (San Clemente, Calif), 15 January 2013.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the ACC/AHA/NAPSE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons [published erratum appears in Circulation 2009;120(5):e34–5] Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 2.Borleffs CJ, van Erven L, van Bommel RJ, van der Velde ET, van der Wall EE, Bax JJ et al. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2(4):411–6. doi: 10.1161/CIRCEP.108.834093. [DOI] [PubMed] [Google Scholar]
- 3.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4(2):136–42. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 5.Saeed D, Albert A, Westenfeld R, Maxhera B, Gramsch-Zabel H, O'Connor S et al. Left ventricular assist device in a patient with a concomitant subcutaneous implantable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6(3):e32–3. doi: 10.1161/CIRCEP.113.000240. [DOI] [PubMed] [Google Scholar]
- 6.Kobe J, Reinke F, Meyer C, Shin DI, Martens E, Kaab S et al. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: a multi-center case-control study. Heart Rhythm. 2013;10(1):29–36. doi: 10.1016/j.hrthm.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 7.Cappato R, Smith WM, Hood MA, Crozier IG, Jordaens L, Spitzer SG et al. Subcutaneous chronic implantable defibrillation systems in humans. J Interv Card Electrophysiol. 2012;34(3):325–32. doi: 10.1007/s10840-012-9665-6. [DOI] [PubMed] [Google Scholar]
- 8.Dabiri Abkenari L, Theuns DA, Valk SD, van Belle Y, de Groot NM, Haitsma D et al. Clinical experience with a novel subcutaneous implantable defibrillator system in a single center. Clin Res Cardiol. 2011;100(9):737–44. doi: 10.1007/s00392-011-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellenbogen KA, Hellkamp AS, Wilkoff BL, Camunas JL, Love JC, Hadjis TA et al. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003;92(6):740–1. doi: 10.1016/s0002-9149(03)00844-0. [DOI] [PubMed] [Google Scholar]
- 10.Grimm W, Flores BF, Marchlinski FE. Complications of implantable cardioverter defibrillator therapy: follow-up of 241 patients. Pacing Clin Electrophysiol. 1993;16(1 Pt 2):218–22. doi: 10.1111/j.1540-8159.1993.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 11.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Greenspon AJ, Prutkin JM, Sohail MR, Vikram HR, Baddour LM, Danik SB et al. Timing of the most recent device procedure influences the clinical outcome of lead-associated endocarditis results of the MEDIC (Multicenter Electrophysiologic Device Infection Cohort) J Am Coll Cardiol. 2012;59(7):681–7. doi: 10.1016/j.jacc.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]


